Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Site
2.2. Behavioral Data Collection
2.3. Training Performance
2.4. Statistical Analysis
3. Results
3.1. Changes in Activity Budgets
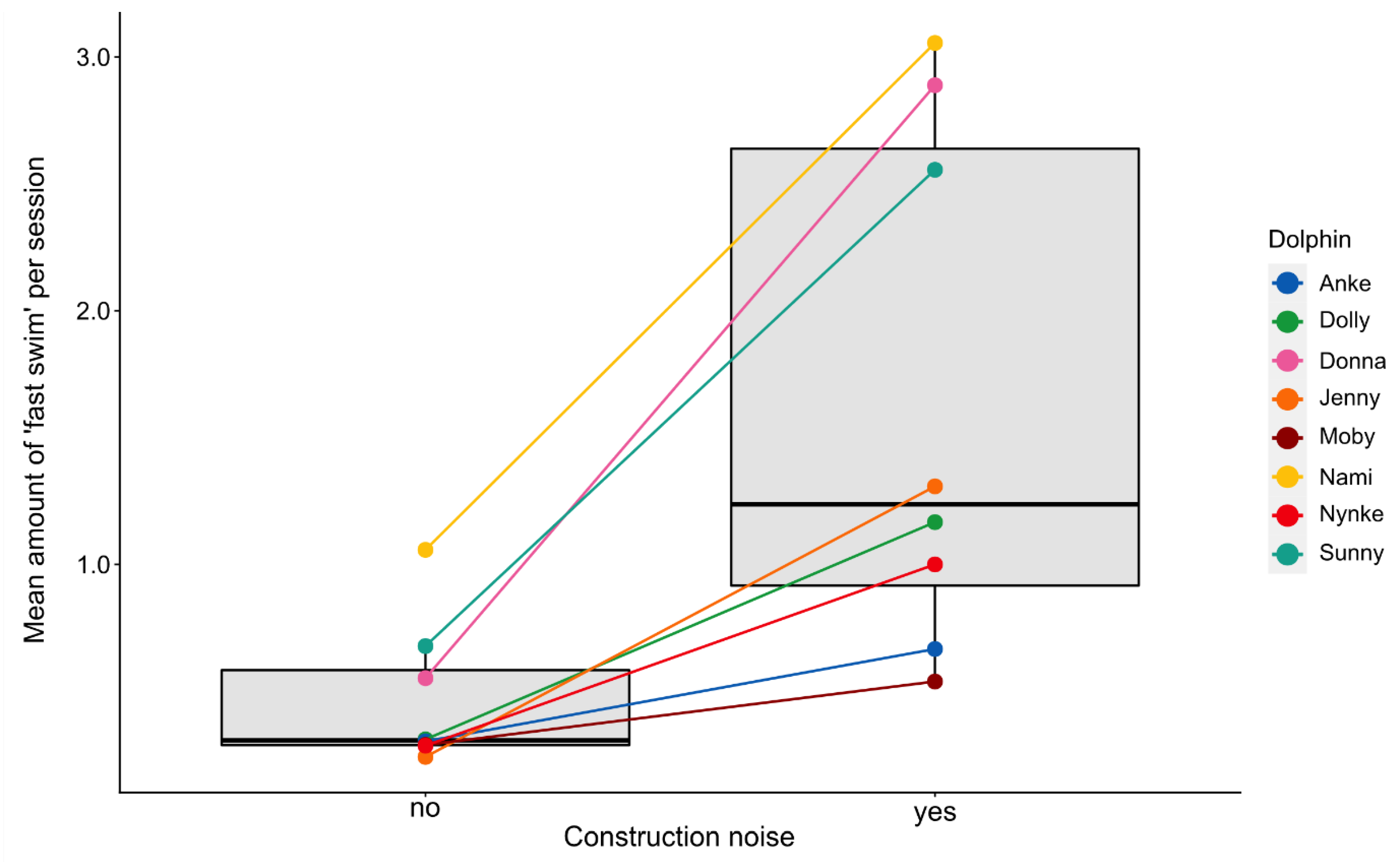
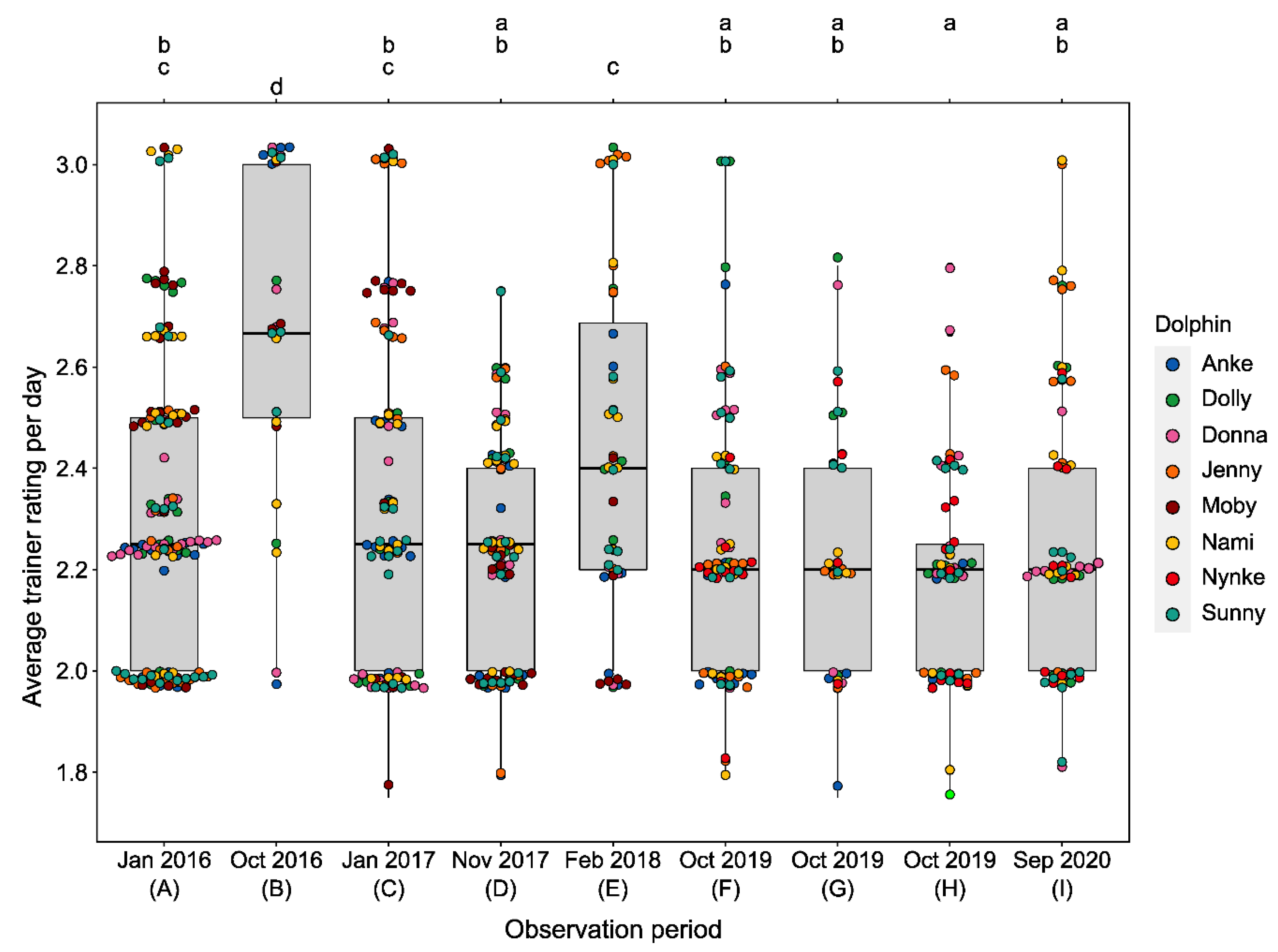
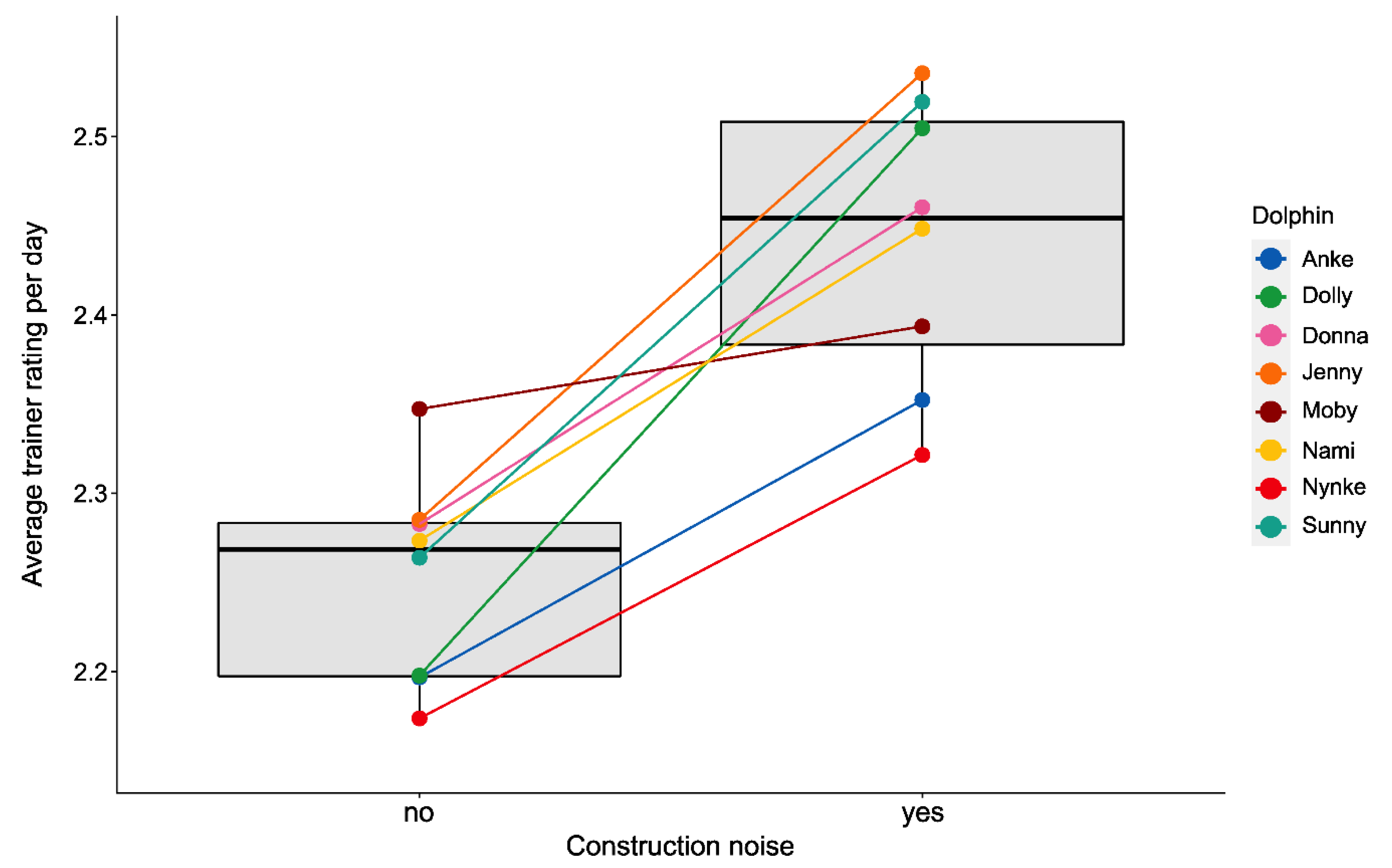
3.2. Effects of Environmental Disturbances
3.2.1. Affiliative, Play, and Solitary Behavior
3.2.2. Fast Swimming
3.2.3. Training Performance
4. Discussion
4.1. Activity Budgets
4.2. Influence of Environmental Disturbances and Welfare Implications
4.2.1. Behavioral Changes
4.2.2. Training Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers Brambell, F.W. Report of the Technical Committee to Enquire into the Welfare of Animals kept under Intensive Livestock Husbandry Systems; Her Majesty’s Stationery Office: London, UK, 1965. [Google Scholar]
- Mellor, D.J.; Reid, C.S.W. Concepts of animal well-being and predicting the impact of procedures on experimental animals. In Improving the Well-Being of Animals in the Research Environment; Baker, R.M., Jenkin, G., Mellor, D.J., Eds.; Australian and New Zealand Council for the Care of Animals in Research and Teaching: Glen Osmond, Australia, 1994; pp. 3–18. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human-animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Moberg, G.P. Influence of the adrenal axis upon the gonads. Oxf. Rev. Reprod. Biol. 1987, 9, 456–496. [Google Scholar] [PubMed]
- Terio, K.A.; Marker, L.; Munson, L. Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J. Wildl. Dis. 2004, 40, 259–266. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implication for Animal Welfare; CABI: Wallingford, UK, 2007; ISBN 9780851993591. [Google Scholar]
- Elsasser, T.H.; Klasing, K.C.; Filipov, N.; Thompson, F. The metabolic consequences of stress: Targets for stress and priorities of nutrient use. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI: Wallingford, UK, 2000; pp. 77–110. ISBN 9780851993591. [Google Scholar]
- Blecha, F. Immune system response to stress. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI: Wallingford, UK, 2000; pp. 111–121. ISBN 9780851993591. [Google Scholar]
- Colborn, D.R.; Thompson, D.L.; Roth, T.L.; Capehart, J.S.; White, K.L. Responses of cortisol and prolactin to sexual excitement and stress in stallions and geldings. J. Anim. Sci. 1991, 69, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Broom, D. Cortisol: Often not the best indicator of stress and poor welfare. Physiol. News 2017, 30–32. [Google Scholar] [CrossRef]
- Collins, P.M.; Halsey, L.G.; Arnould, J.P.Y.; Shaw, P.J.A.; Dodd, S.; Green, J.A. Energetic consequences of time-activity budgets for a breeding seabird. J. Zool. 2016, 300, 153–162. [Google Scholar] [CrossRef]
- Melfi, V.A.; Feistner, A.T.C. A comparison of the activity budgets of wild and captive Sulawesi crested black macaques (Macaca nigra). Anim. Welf. 2002, 11, 213–222. [Google Scholar]
- Howell, C.P.; Cheyne, S.M. Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. J. Appl. Anim. Welf. Sci. 2019, 22, 78–96. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Meehan, C.L.; Mench, J.A.; Carlstead, K.; Hogan, J.N. Determining connections between the daily lives of zoo elephants and their welfare: An epidemiological approach. PLoS ONE 2016, 11, e0158124. [Google Scholar] [CrossRef]
- Powell, D.M. Preliminary evaluation of environmental enrichment techniques for African lions (Panthera leo). Anim. Welf. 1995, 4, 361–370. [Google Scholar]
- Hocking, D.P.; Burville, B.; Parker, W.M.G.; Evans, A.R.; Park, T.; Marx, F.G. Percussive underwater signaling in wild gray seals. Mar. Mammal Sci. 2020, 169, 210. [Google Scholar] [CrossRef]
- Delfour, F.; Vaicekauskaite, R.; García-Párraga, D.; Pilenga, C.; Serres, A.; Brasseur, I.; Pascaud, A.; Perlado-Campos, E.; Sánchez-Contreras, G.J.; Baumgartner, K.; et al. Behavioural diversity study in bottlenose dolphin (Tursiops truncatus) groups and its implications for welfare assessments. Animals 2021, 11, 1715. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G.J. Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 2007, 102, 303–328. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Magrath, M.J.; Butler, K.L.; Hemsworth, P.H. Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors. Appl. Anim. Behav. Sci. 2015, 168, 71–76. [Google Scholar] [CrossRef]
- Bastian, M.L.; Glendinning, D.R.; Brown, J.L.; Boisseau, N.P.; Edwards, K.L. Effects of a recurring late-night event on the behavior and welfare of a population of zoo-housed gorillas. Zoo Biol. 2020, 39, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.T.; Miller, L.J.; Kuczaj II, S.A.; Solangi, M. Seasonal, diel, and age differences in activity budgets of a group of bottlenose dolphins (Tursiops truncatus) under professional care. Int. J. Comp. Psychol. 2017, 30. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Swimming features in captive odontocetes: Indicative of animals’ emotional state? Behav. Process. 2020, 170, 103998. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Rödel, H.G.; Cellier, M.; Vink, D.; Michaud, I.; Mercera, B.; Böye, M.; Hausberger, M.; Lemasson, A.; Delfour, F. Schedule of human-controlled periods structures bottlenose dolphin (Tursiops truncatus) behavior in their free-time. J. Comp. Psychol. 2017, 131, 214–224. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Contextual impacts on individual and synchronous breathing rate variations in three captive odontocete groups. Zoo Biol. 2020. [Google Scholar] [CrossRef]
- Williams, L.J.; Finch, K.; Agnew, R.; Holmes, L. Effects of nearby construction work on the behavior of asiatic lions (Panthera leo persica). JZBG 2021, 2, 5. [Google Scholar] [CrossRef]
- Delfour, F.; Beyer, H. Assessing the effectiveness of environmental enrichment in bottlenose dolphins (Tursiops truncatus). Zoo Biol. 2012, 31, 137–150. [Google Scholar] [CrossRef]
- Vaicekauskaite, R.; Schneider, J.N.; Delfour, F. Does enrichment improve well being in animals under human care? A case study of two harbor seals (Phoca Vitulina). J. Appl. Anim. Welf. Sci. 2019, 22, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.P.; Litchfield, C.A. An empirical case study examining effectiveness of environmental enrichment in two captive Australian Sea Lions (Neophoca cinerea). J. Appl. Anim. Welf. Sci. 2010, 13, 103–122. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Rödel, H.G.; Mercera, B.; van der Heul, S.; Schrijvers, T.; Laender, P.; de Gojceta, R.; Zimmitti, M.; Verhoeven, E.; Burger, J.; et al. Dolphins’ willingness to participate (WtP) in positive reinforcement training as a potential welfare indicator, where WtP predicts early changes in health status. Front. Psychol. 2019, 10, 2112. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Highfill, L.E.; Kuczaj, S.A., II. How studies of wild and captive dolphins contribute to our understanding of individual differences and personality. Int. J. Comp. Psychol. 2010, 23. [Google Scholar] [CrossRef]
- Hill, H.M.; Woodruff, M.J.; Noonan, M. Individual differences in the behavioral characteristics of beluga whales (Dephinapterus leucas). Behav. Process. 2019, 166, 103885. [Google Scholar] [CrossRef] [PubMed]
- Bagley, K.C.; Winship, K.A.; Bolton, T.T. Personality and affiliation in a cooperative task for bottlenose dolphin (Tursiops truncatus) dyads. Int. J. Comp. Psychol. 2020, 33. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Rödel, H.G.; Delfour, F. Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behav. Brain Res. 2017, 322, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I.L.; Borger-Turner, J.L.; Eskelinen, H.C. C-Well: The development of a welfare assessment index for captive bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2015, 24, 267–282. [Google Scholar] [CrossRef]
- Dudzinski, K.M.; Danaher-García, N.; Gregg, J.D. Pectoral fin contact between dolphin dyads at zoo duisburg, with comparison to other dolphin study populations. Aquat. Mamm. 2013, 39, 335–343. [Google Scholar] [CrossRef]
- Cappiello, B.M.; Hill, H.M.; Bolton, T.T. Solitary, observer, parallel, and social object play in the bottlenose dolphin (Tursiops truncatus). Behav. Process. 2018, 157, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Delfour, F.; Monreal-Pawlowsky, T.; Vaicekauskaite, R.; Pilenga, C.; Garcia-Parraga, D.; Rödel, H.G.; García Caro, N.; Perlado Campos, E.; Mercera, B. Dolphin welfare assessment under professional care: ‘Willingness to Participate’, an indicator significantly associated with six potential ‘alerting factors’. JZBG 2020, 1, 4. [Google Scholar] [CrossRef]
- Dudzinski, K.M.; Ribic, C. Pectoral fin contact as a mechanism for social bonding among dolphins. ABC 2017, 4, 30–48. [Google Scholar] [CrossRef][Green Version]
- Kuczaj, S.A.; Highfill, L.E.; Makecha, R.N.; Byerly, H.C. Why do dolphins smile? A comparative perspective on dolphin emotions and emotional expressions. In Emotions of Animals and Humans: Comparative Perspectives; Watanabe, S., Kuczaj, S., Eds.; Springer: Tokyo, Japan, 2013; pp. 63–85. ISBN 978-4-431-54122-6. [Google Scholar]
- Fellner, W.; Bauer, G.B.; Stamper, S.A.; Losch, B.A.; Dahood, A. The development of synchronous movement by bottlenose dolphins (Tursiops truncatus). Mar. Mammal Sci. 2013, 29, E203–E225. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Body contacts and social interactions in captive odontocetes are influenced by the context: An implication for welfare assessment. Animals 2020, 10, 924. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. The frequency of solitary behaviours in captive odontocetes is modulated by environmental and social factors. Int. J. Comp. Psychol. 2019, 32. [Google Scholar] [CrossRef]
- Serres, A.; Delfour, F. Environmental changes and anthropogenic factors modulate social play in captive bottlenose dolphins (Tursiops truncatus). Zoo Biol. 2017, 36, 99–111. [Google Scholar] [CrossRef]
- Themelin, M.; Ribic, C.A.; Melillo-Sweeting, K.; Dudzinski, K.M. A new approach to the study of relationship quality in dolphins: Framework and preliminary results. Behav. Process. 2020, 181, 104260. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. Animal-based welfare monitoring: Using keeper ratings as an assessment tool. Zoo Biol. 2009, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- McMillan, F.D. Quality of life in animals. J. Am. Vet. Med. Assoc. 2000, 216, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780521535632. [Google Scholar]
- Shane, S.H. Comparison of bottlenose dolphin behavior in Texas and Florida, with a critique of methods for studying dolphin behavior. In The Bottlenose Dolphin; Leatherwood, S., Ed.; Academic Press: San Diego, CA, USA, 1990; pp. 541–558. ISBN 9780124402805. [Google Scholar]
- Hanson, M.T.; Defran, R.H. The behavior and feeding ecology of the Pacific coast bottlenose dolphin, Tursiops truncatus. Aquat. Mamm. 1993, 19, 127. [Google Scholar]
- Bearzi, G.; Politi, E.; Sciara, G.N. Diurnal behavior of free-ranging bottlenose dolphins in the Kvarnerić (Northern Adriatic sea)1. Mar. Mammal Sci. 1999, 15, 1065–1097. [Google Scholar] [CrossRef]
- Steiner, A. Activity budget of inshore Indo-Pacific bottlenose dolphins (Tursiops aduncus): A critical evaluation of methods and comparison among other populations. Mar. Mammal Sci. 2011, 27, 20–38. [Google Scholar] [CrossRef]
- Harvey, B.S.; Dudzinski, K.M.; Kuczaj, S.A. Associations and the role of affiliative, agonistic, and socio-sexual behaviors among common bottlenose dolphins (Tursiops truncatus). Behav. Process. 2017, 135, 145–156. [Google Scholar] [CrossRef]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin: Social relationships in a fission-fusion society. In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Ed.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. ISBN 0-226-50341-0. [Google Scholar]
- Scott, E.M.; Mann, J.; Watson-Capps, J.J.; Sargeant, B.L.; Connor, R.C. Aggression in bottlenose dolphins: Evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 2005, 142, 21–44. [Google Scholar] [CrossRef]
- Von Streit, C.; Ganslosser, U.; von Fersen, L. Ethogram of two captive mother-calf dyads of bottlenose dolphins (Tursiops truncatus): Comparison with field ethograms. Aquat. Mamm. 2011, 37, 193–197. [Google Scholar] [CrossRef]
- Noren, S.R. Infant carrying behaviour in dolphins: Costly parental care in an aquatic environment. Funct. Ecol. 2008, 22, 284–288. [Google Scholar] [CrossRef]
- Noren, S.R.; Edwards, E.F. Infant position in mother-calf dolphin pairs: Formation locomotion with hydrodynamic benefits. Mar. Ecol. Prog. Ser. 2011, 424, 229–236. [Google Scholar] [CrossRef]
- Dudzinski, K.M. Overlap between information gained from complementary and comparative studies of captive and wild dolphins. Int. J. Comp. Psychol. 2010, 23, 566–586. [Google Scholar]
- Janik, V.M. Play in dolphins. Curr. Biol. 2015, 25, R7–R8. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.M.; Dietrich, S.; Cappiello, B. Learning to play: A review and theoretical investigation of the developmental mechanisms and functions of cetacean play. Learn. Behav. 2017, 45, 335–354. [Google Scholar] [CrossRef]
- Moreno, K.R.; Macgregor, R.P. Bubble trails, bursts, rings, and more: A review of multiple bubble types produced by cetaceans. ABC 2019, 6, 105–126. [Google Scholar] [CrossRef]
- Pace, D.S. Fluke-made bubble rings as toys in bottlenose dolphin calves (Tursiops truncatus). Aquat. Mamm. 2000, 26, 57–64. [Google Scholar]
- Mann, J. Establishing trust: Socio-sexual behaviour and the development of male-male bonds among Indian Ocean bottlenose dolphins. In Homosexual Behaviour in Animals: An Evolutionary Perspective, 1st ed.; Sommer, V., Vasey, P.L., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 107–130. ISBN 0521864461. [Google Scholar]
- Weaver, A. Conflict and reconciliation in captive bottlenose dolphins, Tursiops truncatus. Mar. Mammal Sci. 2003, 19, 836–846. [Google Scholar] [CrossRef]
- Walsh, M.T.; Friday, R.B.; Johnson, A.B.; Messinger, D. Regurgitation in cetaceans: Medical implications. In Proceedings of the 27th Annual IAAAM Conference, Chattanooga, TN, USA, 11–15 May 1996. [Google Scholar]
- Mizrahi, N.; Kerem, D.; Goffman, O.; Lernau, O.; Spanier, E. Identified fish remains regurgitated by a solitary Indian Ocean Bottlenose Dolphin, Tursiops aduncus, in the Gulf of Aqaba (Mammalia: Delphinidae). Zool. Middle East 2009, 46, 19–28. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319242774. [Google Scholar]
- Kassambra, A. ggpubr: ‘ggplot2′ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 15 July 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Agonistic interactions and dominance relationships in three groups of captive odontocetes: Method of assessment and inter-species/group comparison. Aquat. Mamm. 2019, 45, 478–499. [Google Scholar] [CrossRef]
- Fury, C.A.; Ruckstuhl, K.E.; Harrison, P.L. Spatial and social sexual segregation patterns in indo-pacific bottlenose dolphins (Tursiops aduncus). PLoS ONE 2013, 8, e52987. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.C.; Heithaus, M.R.; Barre, L.M. Superalliance of bottlenose dolphins. Nature 1999, 397, 571–572. [Google Scholar] [CrossRef]
- Connor, R.C.; Smolker, R.A.; Richards, A.F. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl. Acad. Sci. USA 1992, 89, 987–990. [Google Scholar] [CrossRef]
- Oliveira, A.F.S.; Rossi, A.O.; Silva, L.F.R.; Lau, M.C.; Barreto, R.E. Play behaviour in nonhuman animals and the animal welfare issue. J. Ethol. 2010, 28, 1. [Google Scholar] [CrossRef]
- Paulos, R.D.; Trone, M.; Kuczaj, S.A., II. Play in wild and captive cetaceans. Int. J. Comp. Psychol. 2010, 23, 701–722. [Google Scholar]
- Kuczaj, S.A., II; Eskelinen, H.C. Why do dolphins play? ABC 2014, 2, 113. [Google Scholar] [CrossRef]
- Greene, W.E.; Melillo-Sweeting, K.; Dudzinski, K.M. Comparing object play in captive and wild dolphins. Int. J. Comp. Psychol. 2011, 24, 292–306. [Google Scholar]
- Eskelinen, H.; Winship, K.A.; Borger-Turner, J. Sex, age, and individual differences in bottlenose dolphins (Tursiops truncatus) in response to environmental enrichment. ABC 2015, 2, 241–253. [Google Scholar] [CrossRef]
- Delfour, F.; Aulagnier, S. Bubbleblow in beluga whales (Delphinapterus leucas): A play activity? Behav. Process. 1997, 40, 183–186. [Google Scholar] [CrossRef]
- Gewalt, W. Orinoco freshwater dolphins (Inia geoffrensis) using self-produced air bubble rings as toys. Aquat. Mamm. 1989, 15, 73–79. [Google Scholar]
- Hill, H.M.M.; Kahn, M.S.; Brilliott, L.J.; Roberts, B.M.; Gutierrez, C.; Artz, S. Beluga (Delphinapterus leucas) bubble bursts: Surprise, protection, or play? Int. J. Comp. Psychol. 2011, 24, 235–243. [Google Scholar]
- Jones, B.L.; Kuczaj, S.A., II. Beluga (Delphinapterus leucas) novel bubble helix play behavior. ABC 2014, 2, 206. [Google Scholar] [CrossRef]
- Marten, K.; Shariff, K.; Psarakos, S.; White, D.J. Ring bubbles of dolphins. Sci. Am. 1996, 275, 82–87. [Google Scholar] [CrossRef]
- Connor, R.; Whitehead, H. Alliances II. Rates of encounter during resource utilization: A general model of intrasexual alliance formation in fission–fusion societies. Anim. Behav. 2005, 69, 127–132. [Google Scholar] [CrossRef]
- Bruck, J.N. Decades-long social memory in bottlenose dolphins. Proc. R. Soc. Lond. B Biol. Sci. 2013, 280, 20131726. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Smuts, B. Behavioral development in wild bottlenose dolphin newborns (Tursiops sp.). Behaviour 1999, 136, 529–566. [Google Scholar] [CrossRef]
- Mann, J. Female reproductive success in bottlenose dolphins (Tursiops sp.): Life history, habitat, provisioning, and group-size effects. Behav. Ecol. 2000, 11, 210–219. [Google Scholar] [CrossRef]
- Maple, T.L.; Perdue, B.M. Zoo Animal Welfare; Springer: New York, NY, USA, 2013; ISBN 978-3-642-35954-5. [Google Scholar]
- Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; van Bemmel, E.; Connor, R.; Descovich, K. Potential impact of construction noise on selected zoo animals. Animals 2019, 9, 504. [Google Scholar] [CrossRef]
- Powell, D.M.; Carlstead, K.; Tarou, L.R.; Brown, J.L.; Monfort, S.L. Effects of construction noise on behavior and cortisol levels in a pair of captive giant pandas (Ailuropoda melanoleuca). Zoo Biol. 2006, 25, 391–408. [Google Scholar] [CrossRef]
- Sulser, C.E.; Steck, B.L.; Baur, B. Effects of construction noise on behaviour of and exhibit use by Snow leopards Uncia uncia at Basel zoo. Int. Zoo Yearb. 2008, 42, 199–205. [Google Scholar] [CrossRef]
- Held, S.D.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, MA, USA, 2005; ISBN 9780262524698. [Google Scholar]
- Kruse, S. The interactions between killer whales and boats in Johnstone Strait, B.C. In Dolphin Societies: Discoveries and Puzzles; Pryor, K., Norris, K.S., Eds.; University of California Press: Berkeley, CA, USA, 1991; pp. 149–159. ISBN 0520216563. [Google Scholar]
- Nowacek, S.M.; Wells, R.S.; Solow, A.R. Short-term effects of boat traffic on bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mammal Sci. 2001, 17, 673–688. [Google Scholar] [CrossRef]
- Perry, C. A Review of the Impact of Anthropogenic Noise on Cetaceans. Paper Presented to the Scientific Committee at the 50th Meeting of the International Whaling Commission. SC/50/E9; 1998. Available online: http://filesrodadas.anp.gov.br/round9/arquivos_r9/guias_R9/sismica_R9/Bibliografia/Perry%201999%20-%20EIA%20-%20anthropogenic%20noise%20cetaceans.pdf (accessed on 15 July 2021).
- Gilby, I.C.; Pokempner, A.A.; Wrangham, R.W. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol. 2010, 81, 254–264. [Google Scholar] [CrossRef] [PubMed]
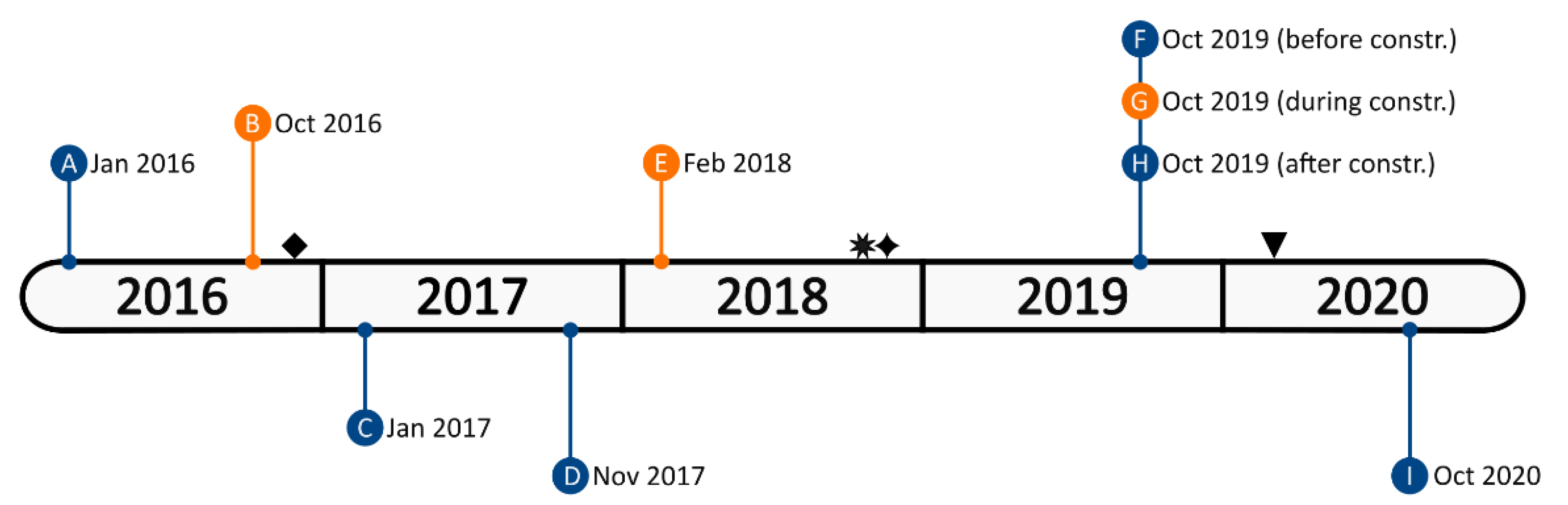
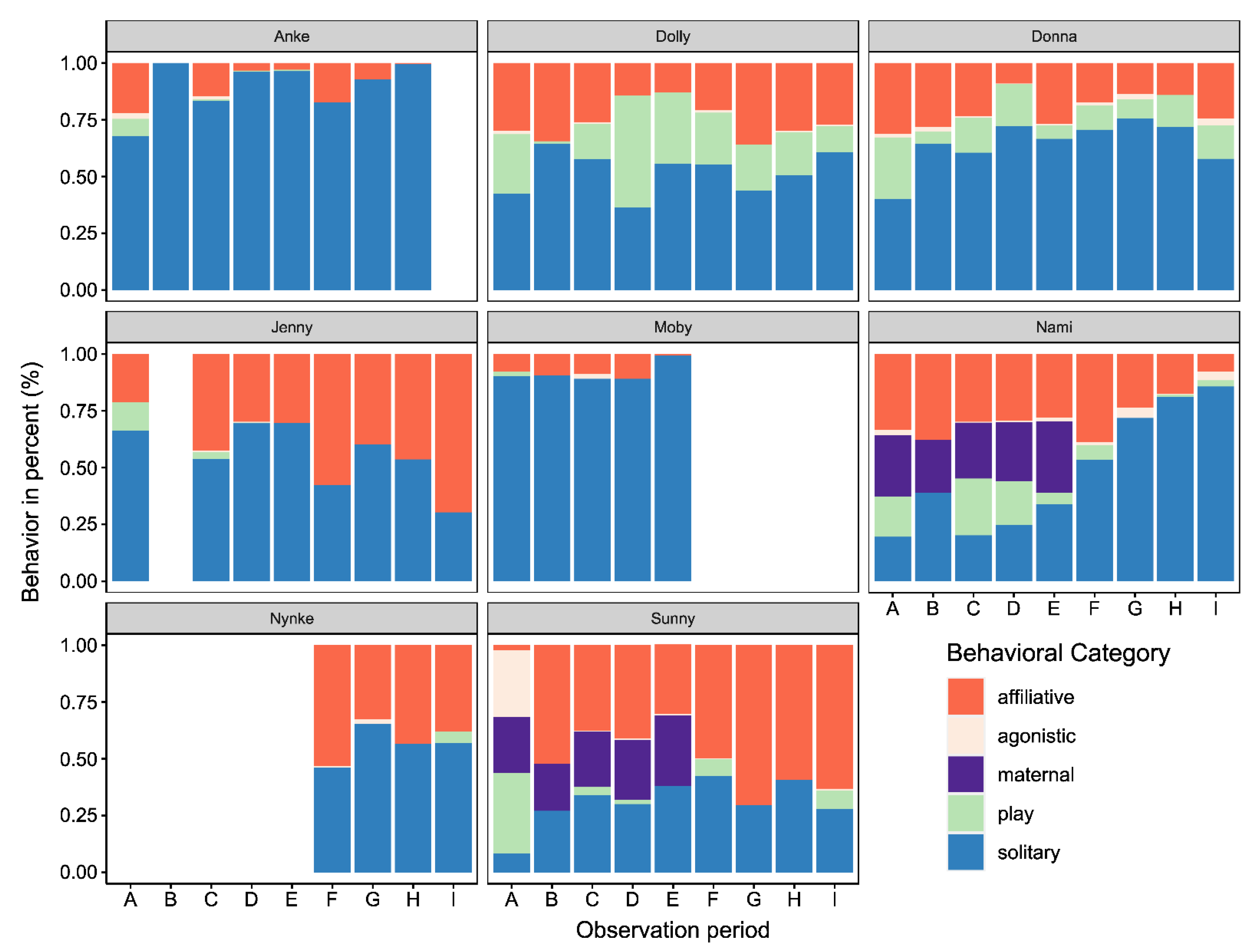
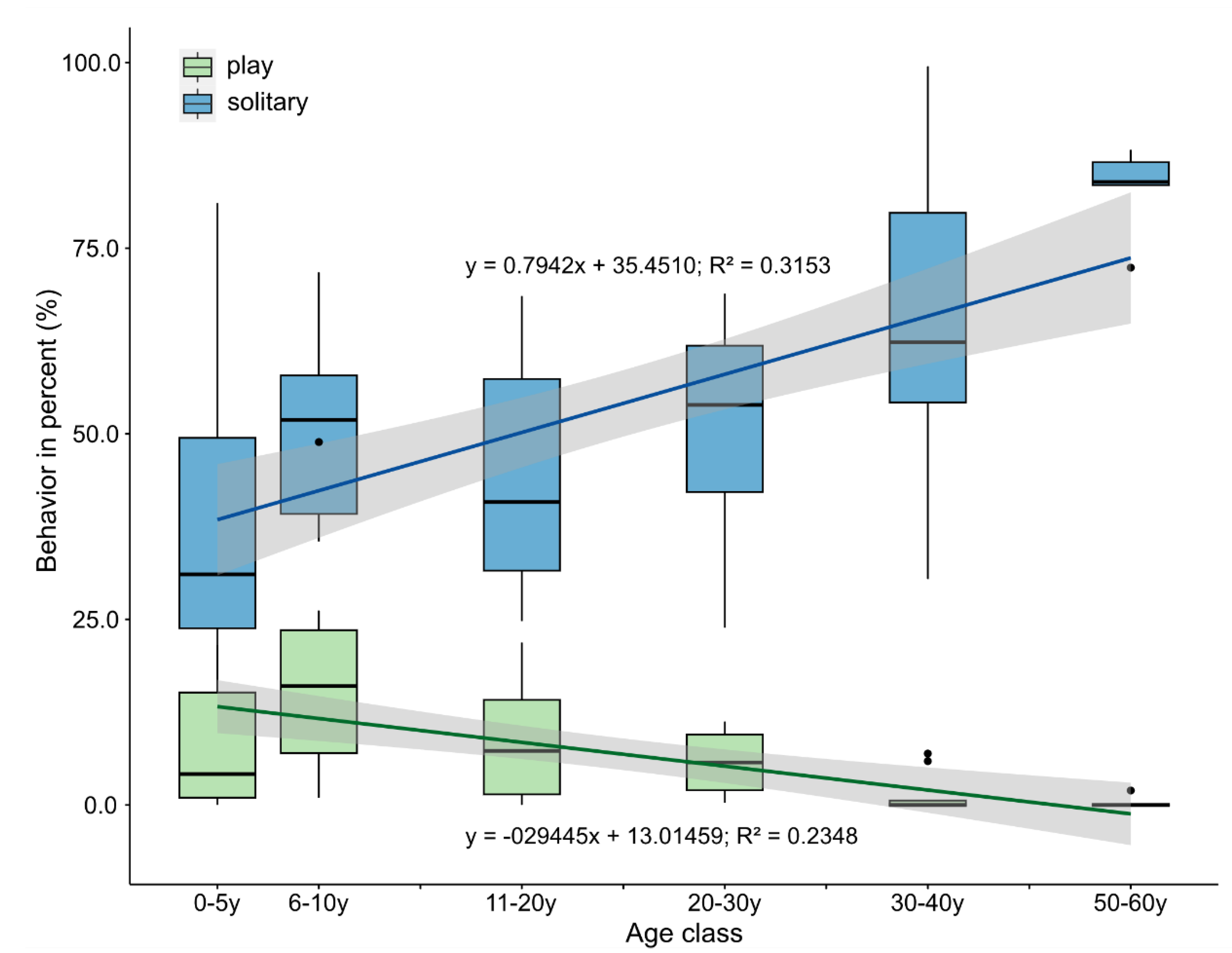
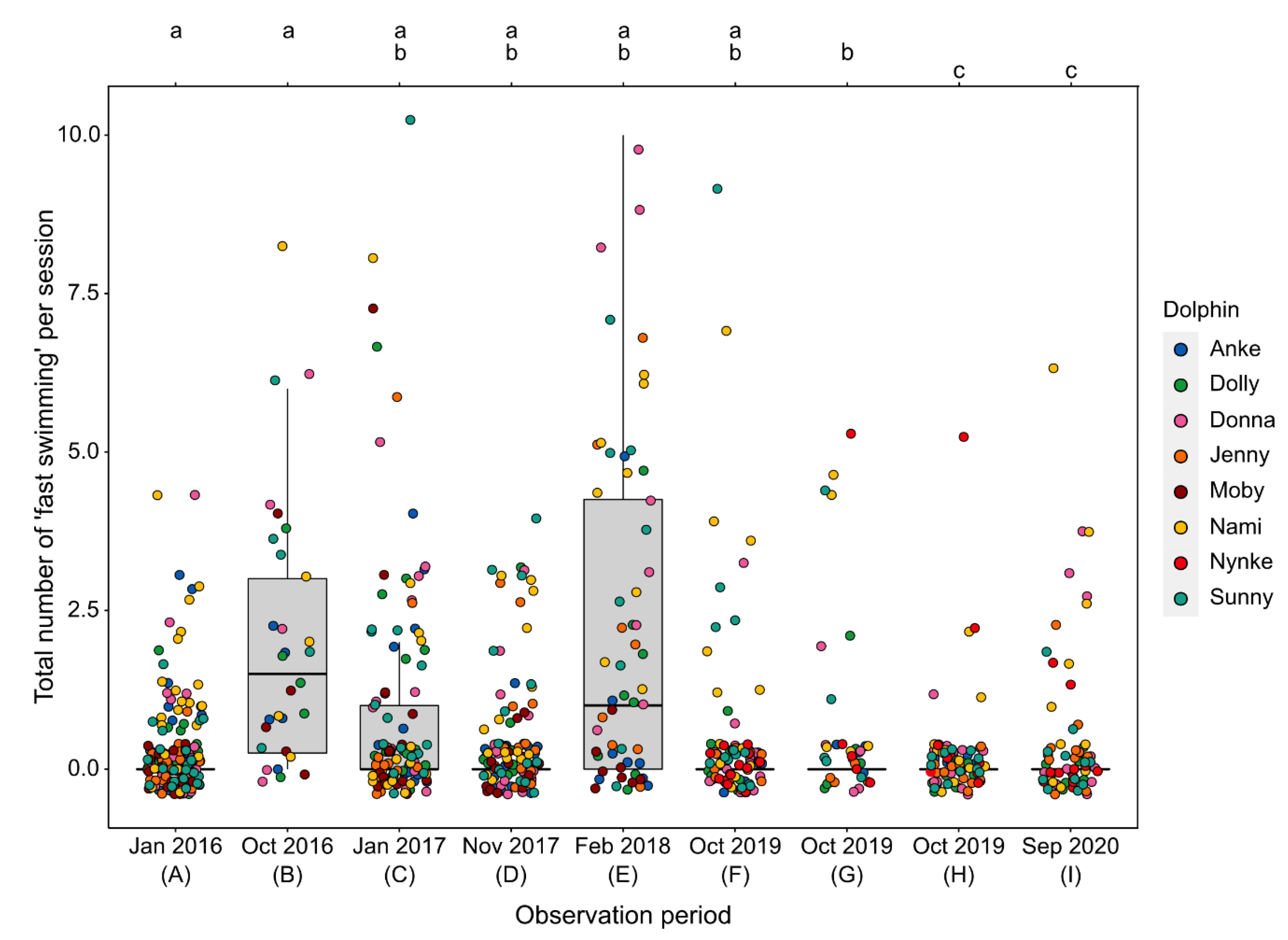
| Name | Sex | Date of Birth | Wild/Captive Born | Observation Period | Comments |
|---|---|---|---|---|---|
| Anke | F | approx. 1983 | wild |  | Died in April 2020 |
| Arnie | M | 18.06.2000 | captive |  | Transported to another facility in November 2016 |
| Dolly | F | 04.08.2007 | captive |  | Half-sister to Donna |
| Donna | F | 17.09.2007 | captive |  | Half-sister to Dolly |
| Jenny | F | approx. 1987 | wild |  | |
| Kai | M | 21.08.2010 | captive |  | Transported to another facility in November 2016 |
| Moby | M | approx. 1960 | wild |  | Died in September 2018 |
| Nami | F | 31.10.2014 | captive |  | Calf of Sunny |
| Noah | M | 16.11.1993 | captive |  | Transported to another facility in November 2016 |
| Nynke | F | approx. 1983 | wild |  | Transported to NBG in September 2018 |
| Sunny | F | 16.05.1999 | captive |  | Mother to Nami |
| Category | Code | Definition | References |
|---|---|---|---|
| Affiliative | aff | All affiliative interactions between two or more dolphins, e.g., synchronous breathing, flipper rubbing, and pair swimming. | [41,57,58] |
| Agonistic | ago | Any form of agonistic or aggressive behavior, e.g., biting, (tail) slapping, ramming, or hitting (with rostrum or fluke). | [57,59] |
| Maternal | mat | Mother–calf interactions were defined as echelon swimming, nursing, or swimming in infant position. | [60,61,62] |
| Play | play | Object-play behavior, e.g., ball play, bubble ring play, locomotor play, or social play, between two or more dolphins. | [39,63,64,65,66,67] |
| Socio-sexual | sex | All behaviors between at least two dolphins with any form of body contact in the genital area, including goosing, petting, or rubbing of the genital area. | [57,68] |
| Solitary | sol | Behaviors shown by one dolphin without interactions with another individual, e.g., resting or swimming. | [57] |
| All-Occurrence Behavior | Definition | References |
|---|---|---|
| Fast swimming | A dolphin swims at a higher speed than normal, indicated by powerful fluke strokes, riddles on its skin, or waves on the water’s surface. | [24] |
| Jumping | A dolphin performs an aerial behavior by leaping out of the water and diving headfirst. | [60] |
| Agonistic interaction | Any agonistic or aggressive behavioral events, e.g., biting, (tail) slapping, ramming, or hitting (with rostrum or fluke), between two or more individuals. | [57,69] |
| Socio-sexual interaction | All behaviors between at least two dolphins with any form of body contact in the genital area, including goosing, petting, or rubbing of the genital area. | [57,68] |
| Regurgitation | A dolphin regurgitates already swallowed fish at will. Regurgitation is indicated by the dolphin ejecting a plume of water and fish debris out of its mouth. Sometimes, whole fish are regurgitated and swallowed again. | [70,71] |
| Rating | Dolphin Performance | Definition |
|---|---|---|
| 1 | excellent | The dolphin performed all of the conditioned behaviors correctly at a high level (above the average standard), showed a high level of motivation (e.g., returned to its trainer immediately), was highly focused, and took every fish reward without problems. |
| 2 | good | The dolphin performed all behaviors well, but with some minor mistakes, meeting the trainer’s expectations (e.g., conditioned behaviors are performed according to the established standard). |
| 3 | poor | The dolphin performed poorly, e.g., did not eat well, left the trainer, interacted with other dolphins, or performed most behaviors below the average standard, refused to perform certain behaviors, or was very distant from the trainer (no or only little eye contact). |
| LMM-Model | Observation Periods | Estimate | Std. Error | Df | T-Value | p-Value |
|---|---|---|---|---|---|---|
| (a) | ||||||
| Fast swimming | Intercept | 0.36853 | 0.17284 | 18.18687 | 2.132 | 0.046873 * |
| Poisson | Observation period B | 1.63546 | 0.27633 | 709.19429 | 5.918 | <0.0001 **** |
| AIC: 2531.8 | Observation period C | 0.61732 | 0.17478 | 708.12697 | 3.532 | 0.000439 *** |
| Deviance: 2509.8 | Observation period D | 0.11255 | 0.17478 | 708.12697 | 0.644 | 0.519792 |
| Observation period E | 1.97565 | 0.2155 | 708.12697 | 9.168 | <0.0001 **** | |
| Observation period F | 0.01046 | 0.18841 | 715.93166 | 0.056 | 0.955751 | |
| Observation period G | 0.22204 | 0.26123 | 714.31994 | 0.85 | 0.395621 | |
| Observation period H | −0.29224 | 0.19627 | 715.9851 | −1.489 | 0.136935 | |
| Observation period I | 0.03387 | 0.20829 | 715.6305 | 0.163 | 0.870888 | |
| (b) | ||||||
| Average Trainer Rating | ||||||
| Poisson | Intercept | 2.31881 | 0.02379 | 64.29293 | 97.483 | <0.0001 **** |
| AIC: 181.6 | Observation period B | 0.37637 | 0.05397 | 710.44062 | 6.974 | <0.0001 **** |
| Deviance: 159.6 | Observation period C | 0.02191 | 0.03423 | 706.33135 | 0.64 | 0.52236 |
| Observation period D | −0.08809 | 0.03423 | 706.33135 | −2.574 | 0.01027 * | |
| Observation period E | 0.12611 | 0.04217 | 706.30673 | 2.991 | 0.00288 ** | |
| Observation period F | −0.07889 | 0.03641 | 701.3391 | −2.167 | 0.03058 * | |
| Observation period G | −0.06559 | 0.05076 | 713.89821 | −1.292 | 0.19672 | |
| Observation period H | −0.14983 | 0.03798 | 706.4147 | −3.945 | <0.0001 **** | |
| Observation period I | −0.04498 | 0.04015 | 690.11795 | −1.12 | 0.26295 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huettner, T.; Dollhaeupl, S.; Simon, R.; Baumgartner, K.; von Fersen, L. Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare. Animals 2021, 11, 2107. https://doi.org/10.3390/ani11072107
Huettner T, Dollhaeupl S, Simon R, Baumgartner K, von Fersen L. Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare. Animals. 2021; 11(7):2107. https://doi.org/10.3390/ani11072107
Chicago/Turabian StyleHuettner, Tim, Sandra Dollhaeupl, Ralph Simon, Katrin Baumgartner, and Lorenzo von Fersen. 2021. "Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare" Animals 11, no. 7: 2107. https://doi.org/10.3390/ani11072107
APA StyleHuettner, T., Dollhaeupl, S., Simon, R., Baumgartner, K., & von Fersen, L. (2021). Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare. Animals, 11(7), 2107. https://doi.org/10.3390/ani11072107






