Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Populations
2.2. Species Ecology
2.3. Amphibian Husbandry
2.4. Observational Methods and Sampling Schedule
2.5. Data Analysis
2.5.1. Assessing Ecological Differences
2.5.2. Comparing Pool Frog Populations
3. Results
3.1. Assessing Ecological Differences
3.2. Comparing Pool Frog Populations
4. Discussion
4.1. Ecological and Evolutionary Explanations for an Amphibian Visitor Effect
4.2. Potential Extraneous Influences on Amphibian Enclosure Usage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, D.M.; Watters, J.V. The evolution of the animal welfare movement in US zoos and aquariums. Zool. Gart. 2017, 86, 219–234. [Google Scholar] [CrossRef]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; Lemos de Figueiredo, R.; Riley, L.M. What’s new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Proctor, H. Animal sentience: Where are we and where are we heading? Animals 2012, 2, 628–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of animal welfare research in zoos and aquariums: Where are we, where to next? J. Zoo Aquar. Res. 2020, 8, 166–174. [Google Scholar]
- Benn, A.L.; McLelland, D.J.; Whittaker, A.L. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality protocol to the pygmy blue-tongue skink, Tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Rudh, A.; Qvarnström, A. Adaptive colouration in amphibians. Semin. Cell Dev. Biol. 2013, 24, 553–561. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of welfare in zoo animals: Towards optimum quality of life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.E.; Evans, C.; Coffin, R.C.; Miller, R.; Nash, S.M. Using student-centred research to evidence-base exhibition of reptiles and amphibians: Three species-specific case studies. J. Zoo Aquar. Res. 2014, 2, 25–32. [Google Scholar]
- Michaels, C.J.; Downie, J.R.; Campbell-Palmer, R. The importance of enrichment for advancing amphibian welfare and conservation goals. Amphib. Reptile Conserv. 2014, 8, 7–23. [Google Scholar]
- Silla, A.J.; Calatayud, N.E.; Trudeau, V.L. Amphibian reproductive technologies: Approaches and welfare considerations. Conserv. Physiol. 2021, 9, coab011. [Google Scholar] [CrossRef]
- Tapley, B.; Bradfield, K.S.; Michaels, C.J.; Bungard, M. Amphibians and conservation breeding programmes: Do all threatened amphibians belong on the ark? Biodivers. Conserv. 2015, 24, 2625–2646. [Google Scholar] [CrossRef]
- Graham, C.; von Keyserlingk, M.A.G.; Franks, B. Zebrafish welfare: Natural history, social motivation and behaviour. Appl. Anim. Behav. Sci. 2018, 200, 13–22. [Google Scholar] [CrossRef]
- Rose, P.E.; Nash, S.M.; Riley, L.M. To pace or not to pace? A review of what abnormal repetitive behavior tells us about zoo animal management. J. Vet. Behav. 2017, 20, 11–21. [Google Scholar] [CrossRef]
- Godinez, A.M.; Fernandez, E.J. What is the zoo experience? How zoos impact a visitor’s behaviors, perceptions, and conservation efforts. Front. Psychol. 2019, 10, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.J. Visitor reaction to pacing behavior: Influence on the perception of animal care and interest in supporting zoological institutions. Zoo Biol. 2012, 31, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Razal, C.B.; Miller, L.J. Examining the impact of naturalistic and unnaturalistic environmental enrichment on visitor perception of naturalness, animal welfare, and conservation. Anthrozoös 2019, 32, 141–153. [Google Scholar] [CrossRef]
- Rose, P.E.; Badman-King, A.; Hurn, S.; Rice, T. Visitor presence and a changing soundscape, alongside environmental parameters, can predict enclosure usage in captive flamingos. Zoo Biol. 2021, 1–13. [Google Scholar] [CrossRef]
- Rose, P.E.; Scales, J.S.; Brereton, J.E. Why the “visitor effect” is complicated. Unraveling individual animal, visitor number, and climatic influences on behavior, space use and interactions with keepers- a case study on captive hornbills. Front. Vet. Sci. 2020, 7, 236. [Google Scholar] [CrossRef]
- Goodenough, A.E.; McDonald, K.; Moody, K.; Wheeler, C. Are “visitor effects” overestimated? Behaviour in captive lemurs is mainly driven by co-variation with time and weather. J. Zoo Aquar. Res. 2019, 7, 59–66. [Google Scholar]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Impacts of COVID-19 on animals in zoos: A longitudinal multi-species analysis. J. Zool. Bot. Gard. 2021, 2, 130–145. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.; Passos, L.; Vecci, M.A.M.; Young, R.J. Zoo visitor effect on mammal behaviour: Does noise matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.C.; Soltis, J.; Leeds, A. Evaluating the Effect of Visitor Presence on Nile Crocodile (Crocodylus niloticus) Behavior. J. Zool. Bot. Gard. 2021, 2, 115–129. [Google Scholar] [CrossRef]
- Bloomfield, R.C.; Gillespie, G.R.; Kerswell, K.J.; Butler, K.L.; Hemsworth, P.H. Effect of partial covering of the visitor viewing area window on positioning and orientation of zoo orangutans: A preference test. Zoo Biol. 2015, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sherwen, S.L.; Hemsworth, P.H. The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arntzen, J.W.; Kuzmin, S.; Beebee, T.; Papenfuss, T.; Sparreboom, M.; Ugurtas, I.H.; Anderson, S.; Anthony, B.; Andreone, F.; Tarkhnishvili, D.; et al. Lissotriton Vulgaris. Available online: https://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T59481A11932252.en (accessed on 1 June 2021).
- AmphibiaWeb. Lissotriton Vulgaris: Smooth Newt. Available online: https://amphibiaweb.org/species/4303 (accessed on 1 June 2021).
- Kuzmin, S.; Ishchenko, V.; Tuniyev, B.; Beebee, T.; Andreone, F.; Nyström, P.; Anthony, B.P.; Schmidt, B.; Ogrodowczyk, A.; Ogielska, M.; et al. Rana Temporaria (Errata Version Published in 2016). Available online: https://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T58734A11834246.en (accessed on 1 June 2021).
- AmphibiaWeb. Rana Temperoria: Common Frog. Available online: https://amphibiaweb.org/species/5168 (accessed on 1 June 2021).
- Agasyan, A.; Avisi, A.; Tuniyev, B.; Isailovic, J.C.; Lymberakis, P.; Andrén, C.; Cogalniceanu, D.; Wilkinson, J.; Ananjeva, N.; Üzüm, N.; et al. Bufo Bufo. Available online: https://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T54596A11159939.en (accessed on 1 June 2021).
- AmphibiaWeb. Bufo Bufo: Common Toad. Available online: https://amphibiaweb.org/species/127 (accessed on 1 June 2021).
- Kuzmin, S.; Beebee, T.; Andreone, F.; Nyström, P.; Anthony, B.P.; Schmidt, B.; Ogrodowczyk, A.; Ogielska, M.; Cogalniceanu, D.; Kovács, T.; et al. Pelophylax Lessonae (Errata Version Published in 2016). Available online: https://dx.doi.org/10.2305/IUCN.UK.2009.RLTS.T58643A11818386.en (accessed on 1 June 2021).
- AmphibiaWeb. Pelophylax Lessonae: Pool Frog. Available online: https://amphibiaweb.org/species/5077 (accessed on 1 June 2021).
- IUCN SSC Amphibian Specialist Group. Phyllobates Terribilis. Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T55264A85887889.en (accessed on 1 June 2021).
- AmphibiaWeb. Phyllobates Terribilis: Golden Poison Frog. Available online: https://amphibiaweb.org/species/1707 (accessed on 1 June 2021).
- IUCN SSC Amphibian Specialist Group. Mantella Aurantiaca. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T12776A508612.en (accessed on 1 June 2021).
- AmphibiaWeb. Mantella Aurantiaca: Golden Mantella. Available online: https://amphibiaweb.org/species/4559 (accessed on 1 June 2021).
- RStudio Team. RStudio: Integrated Development for R. 2021. Available online: http://www.rstudio.com (accessed on 28 March 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Minitab 19. Minitab Statistical Software; State College, PA, USA, 2020. Available online: www.minitab.com (accessed on 28 March 2021).
- Griffiths, R.A. Diel profile of behaviour in the smooth newt, Triturus vulgaris (L.): An analysis of environmental cues and endogenous timing. Anim. Behav. 1985, 33, 573–582. [Google Scholar] [CrossRef]
- Hoehfurtner, T.; Wilkinson, A.; Nagabaskaran, G.; Burman, O.H.P. Does the provision of environmental enrichment affect the behaviour and welfare of captive snakes? Appl. Anim. Behav. Sci. 2021, 239, 105324. [Google Scholar] [CrossRef]
- Blanchett, M.K.S.; Finegan, E.; Atkinson, J. The effects of increasing visitor and noise levels on birds within a free-flight aviary examined through enclosure use and behavior. Anim. Behav. Cogn. 2020, 7, 49–69. [Google Scholar] [CrossRef]
- Figueiredo Passos, L.; Garcia, G.; Young, R.J. How does captivity affect skin colour reflectance of golden mantella frogs? Herpetol. J. 2020, 30, 13–19. [Google Scholar] [CrossRef]
- Ozel, L.D.; Stynoski, J.L. Differences in escape behavior between a cryptic and an aposematic litter frog. J. Herpetol. 2011, 45, 395–398. [Google Scholar] [CrossRef]
- Woodland Trust. Common Toad. Available online: https://www.woodlandtrust.org.uk/trees-woods-and-wildlife/animals/reptiles-and-amphibians/common-toad/ (accessed on 9 June 2021).
- Touzot, M.; Teulier, L.; Lengagne, T.; Secondi, J.; Théry, M.; Libourel, P.-A.; Guillard, L.; Mondy, N. Artificial light at night disturbs the activity and energy allocation of the common toad during the breeding period. Conserv. Physiol. 2019, 7, coz002. [Google Scholar] [CrossRef] [PubMed]
- Meek, R.; Jolley, E. Body temperatures of the common toad, Bufo bufo, in the Vendée, France. Herpetol. Bull. 2006, 95, 21–24. [Google Scholar]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J.F. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, W.M.; Griffiths, R.A.; Bungard, M.J.; Rakotondrasoa, E.F.; Razafimanahaka, J.H.; Razafindraibe, P.; Andriantsimanarilafy, R.R.; Randrianantoandro, J.C. Microhabitat preference of the critically endangered golden mantella frog in Madagascar. Herpetol. J. 2019, 29, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Orban, D.A.; Soltis, J.; Perkins, L.; Mellen, J.D. Sound at the zoo: Using animal monitoring, sound measurement, and noise reduction in zoo animal management. Zoo Biol. 2017, 36, 231–236. [Google Scholar] [CrossRef]
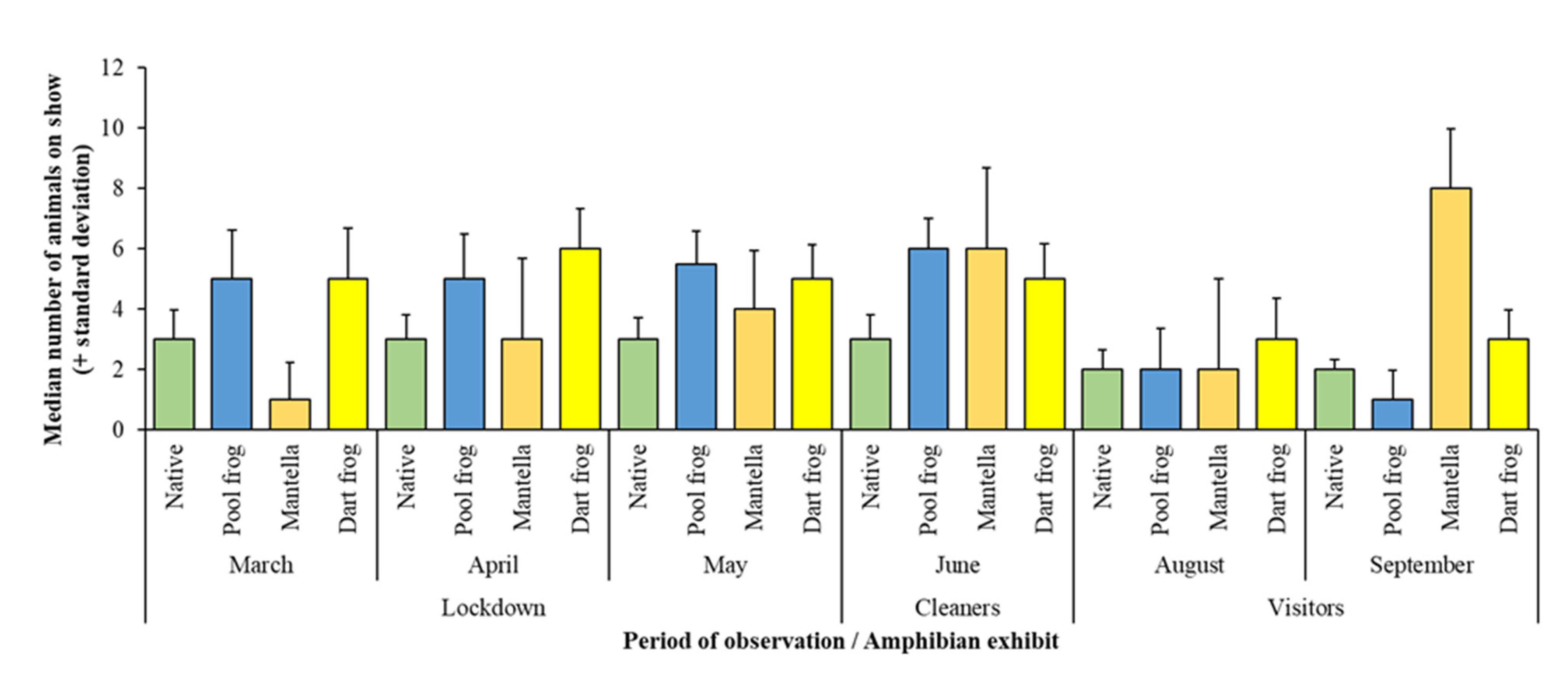
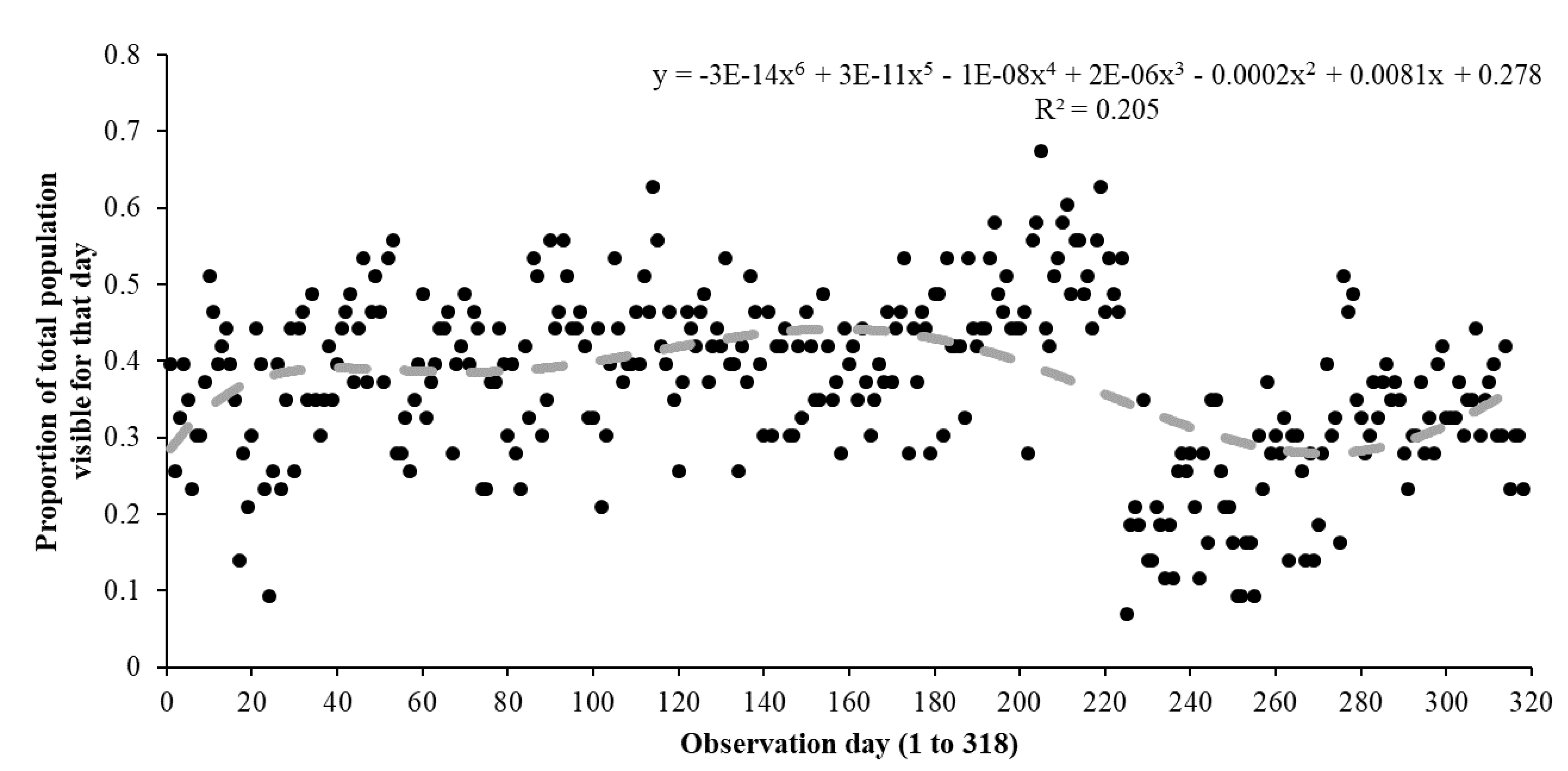
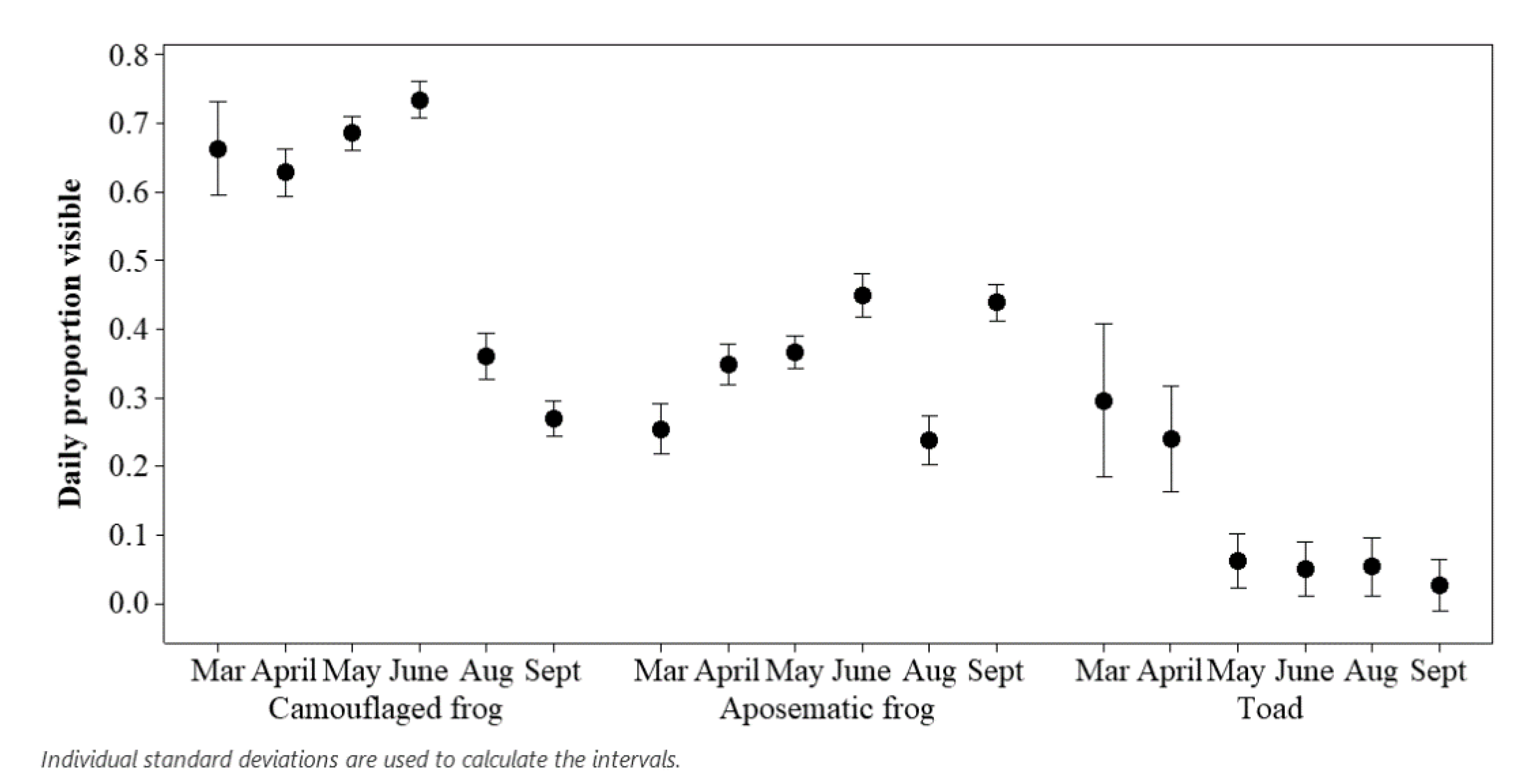

| Exhibit | Species Housed | Population | Enclosure Size | % Cover to Open Space | % Land to Water |
|---|---|---|---|---|---|
| Native Species Exhibit | Common toad (Bufo bufo) Common frog (Rana temporaria) Pool frog (Pelophylax lessonae) Smooth newt (Lissotriton vulgaris) | 2 2 2 4 | L 130 cm H 50 cm D 50 cm | 55%/45% | 80%/20% |
| Pool Frog Exhibit | Pool frog | 8 | L 100 cm H 50 cm D 50 cm | 40%/60% | 15%/85% |
| Golden Mantella Exhibit | Golden mantella (Mantella aurantiaca) | 18 | L 100 cm H 50 cm D 50 cm | 65%/35% | 90%/10% |
| Dart Frog Exhibit | Golden poison dart frog (Phyllobates terribilis) | 7 | L 100 cm H 50 cm D 50 cm | 45%/55% | 70%/30% |
| Predictor | Estimate | Standard Error | Z Value | p Value |
|---|---|---|---|---|
| Lockdown (March) | −1.28 | 0.06 | −20.6 | <0.001 |
| Lockdown (May) | 0.06 | 0.04 | 1.45 | 0.146 |
| Cleaners (June) | 0.001 | 0.04 | 0.021 | 0.984 |
| Visitors (August) | −0.71 | 0.05 | −14.1 | <0.001 |
| Visitors (September) | −0.78 | 0.05 | −15.12 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boultwood, J.; O’Brien, M.; Rose, P. Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity? Animals 2021, 11, 1982. https://doi.org/10.3390/ani11071982
Boultwood J, O’Brien M, Rose P. Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity? Animals. 2021; 11(7):1982. https://doi.org/10.3390/ani11071982
Chicago/Turabian StyleBoultwood, Jack, Michelle O’Brien, and Paul Rose. 2021. "Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity?" Animals 11, no. 7: 1982. https://doi.org/10.3390/ani11071982
APA StyleBoultwood, J., O’Brien, M., & Rose, P. (2021). Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity? Animals, 11(7), 1982. https://doi.org/10.3390/ani11071982







