Effects of Slaughter Positions on Catecholamine, Blood Biochemical and Electroencephalogram Changes in Cattle Restrained Using a Modified Mark IV Box
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
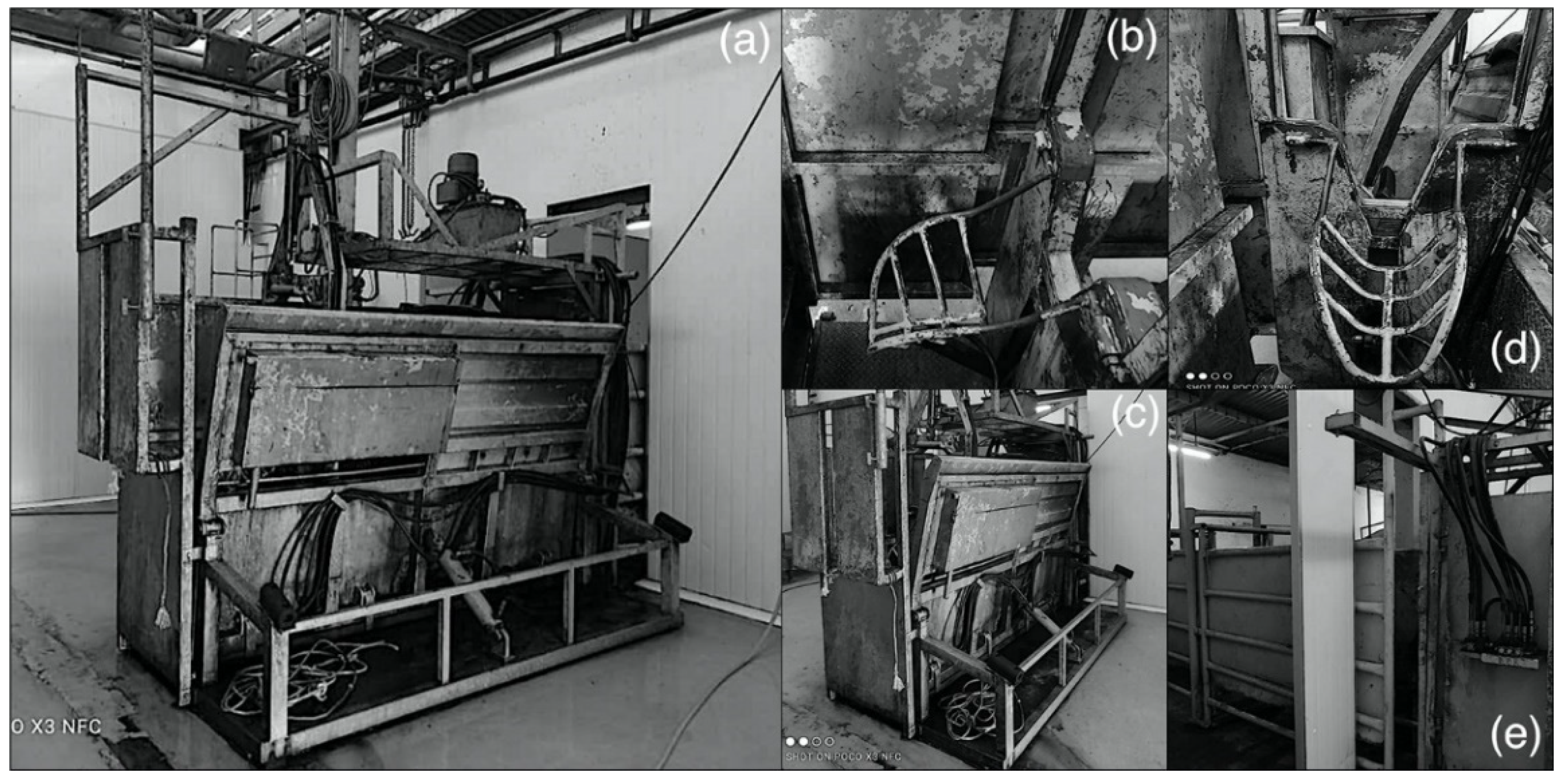
2.1. Animals
2.2. Electroencephalography
2.3. Blood Sampling
2.4. Determination of Blood Biochemical Parameters
2.5. Determination of Adrenaline
2.5.1. Sample Preparation, Extraction and Acylation
2.5.2. Enzymatic Conversion
2.5.3. Adrenaline Evaluation
2.5.4. Determination of Noradrenaline
2.5.5. Statistical Analysis
3. Results
3.1. Blood Biochemical Parameters
3.2. Influence of Slaughter Positions on Hormonal Parameters
3.3. Influence of Slaughter Positions on EEG Recording
4. Discussion
4.1. Blood Biochemical Parameters
4.2. Influence of Slaughter Positions on Catecholamines
4.3. Influence of Slaughter Positions on EEG Recording
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, N.; Schuster, P.; Mirabito, L.; Kolesar, R.; McManus, T. Arrested blood flow during false aneurysm formation in the carotid arteries of cattle slaughtered with and without stunning. Meat Sci. 2012, 90, 368–372. [Google Scholar] [CrossRef]
- Grandin, T. Improving religious slaughter practices in the U.S. Anthr. Food 2006. [Google Scholar] [CrossRef]
- Anil, M.H. Effects of Slaughter Method on Carcass and Meat Characteristics in the Meat of Cattle and Sheep; EBLEX—A Divison of the Agriculture and Horticulture Development Board: Stoneleigh, UK, 2012; pp. 1–73. [Google Scholar]
- CFIA. Guidelines for Ritual Slaughter of Food Animals Without Pre-Slaughter Stunning. Canadian Food Inspection Agency 2020, Canada. Available online: https://inspection.canada.ca/food-safety-for-industry/food-specific-requirements-and-guidance/meat-products-and-food-animals/ritual-slaughter/eng/1519849364873/1519849365434 (accessed on 20 August 2020).
- Gregory, N.; von Wenzlawowicz, M.; von Holleben, K. Blood in the respiratory tract during slaughter with and without stunning in cattle. Meat Sci. 2009, 82, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Making Slaughterhouses More Humane for Cattle, Pigs, and Sheep. Annu. Rev. Anim. Biosci. 2013, 1, 491–512. [Google Scholar] [CrossRef]
- Gerritzen, M.; Reimert, H.; van der Werf, J.; Hindle, V.; Visser, E.; van Dixhoorn, I. Progress Report Restraining Ruminants; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2014; pp. 1570–8616. [Google Scholar]
- Velarde, A.; Rodriguez, P.; Dalmau, A.; Fuentes, C.; Llonch, P.; von Holleben, K.; Anil, M.; Lambooij, J.; Pleiter, H.; Yesildere, T.; et al. Religious slaughter: Evaluation of current practices in selected countries. Meat Sci. 2014, 96, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.A.A.; Borilova, G.; Steinhauserova, I. Halal Criteria Versus Conventional Slaughter Technology. Animals 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A. Religion and Animal Welfare—An Islamic Perspective. Animals 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Awan, J.A.; Sohaib, M. Halal and humane slaughter; Comparison between Islamic teachings and modern methods. Pak. J. Food Sci. 2016, 26, 234–240. [Google Scholar]
- Johnson, C.; Mellor, D.; Hemsworth, P.; Fisher, A. A scientific comment on the welfare of domesticated ruminants slaughtered without stunning. N. Z. Vet. J. 2015, 63, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, A.; Knowles, T.; Lines, J.; Hadley, P.; Wotton, S. The stunning and slaughter of cattle within the EU: A review of the current situation with regard to the halal market. Anim. Welf. 2016, 25, 365–376. [Google Scholar] [CrossRef]
- Gregory, N. Recent concerns about stunning and slaughter. Meat Sci. 2005, 70, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Evaluation of Methods of Restraint for Holding (Fixation) of Cattle, Calves, and Sheep for Kosher and Halal Slaughter. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2009. [Google Scholar]
- Von Holleben, K.; Von Wenzlawowicz, M.; Gregory, N.; Anil, H.; Velarde, A.; Rodriguez, P.; Cenci Goga, B.; Catanese, B.; Lambooij, B. Report on Good and Adverse Practices: Animal Welfare Concerns in Relation to Slaughter Practices from the Viewpoint of Veterinary Sciences; Dialrel Deliverable Schwarzenbek: Schwarzenbek, Germany, 2010; p. 24. [Google Scholar]
- Tagawa, M.; Okano, S.; Sako, T.; Orima, H.; Steffey, E.P. Effect of Change in Body Position on Cardiopulmonary Function and Plasma Cortisol in Cattle. J. Veter Med. Sci. 1994, 56, 131–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA. Scientific report of the scientific panel for animal health and welfare on a request from the commission related to welfare aspects of animal stunning and killing methods. EFSA J. 2004, 45, 1–29. [Google Scholar]
- Gibson, T.; Johnson, C.; Murrell, J.; Hulls, C.; Mitchinson, S.; Stafford, K.; Johnstone, A.; Mellor, D. Electroencephalographic responses of halothane-anaesthetized calves to slaughter by ventral-neck incision without prior stunning. N. Z. Vet. J. 2009, 57, 77–83. [Google Scholar] [CrossRef]
- Imlan, J.C.; Kaka, U.; Goh, Y.-M.; Idrus, Z.; Awad, E.A.; Abubakar, A.A.; Ahmad, T.; Nizamuddin, H.N.Q.; Sazili, A.Q. Effects of Slaughter Knife Sharpness on Blood Biochemical and Electroencephalogram Changes in Cattle. Animals 2020, 10, 579. [Google Scholar] [CrossRef]
- Jakim Halal Food—Production, Preparation, Handling and Storage—General Guidelines, 2nd ed.; Department of Standards Malaysia: Kuala Lumpur, Malaysia, 2009.
- Grandin, T. The feasibility of using vocalization scoring as an indicator of poor welfare during cattle slaughter. Appl. Anim. Behav. Sci. 1998, 56, 121–128. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Software, Version 9.4; SAS Inst. Inc.: Cary, NC, USA, 2007. [Google Scholar]
- O’Neill, H.; Webb, E.; Frylinck, L.; Strydom, P. The stress reponsiveness of three different beef breed types and the effect on ultimate pH and meat colour. In Proceedings of the 52nd International Congress on Meat Science and Technology, Dublin, Ireland, 13–18 August 2006; Volume 82, pp. 1401–1409. [Google Scholar]
- Adenkola, A.; Ayo, J. Physiological and behavioural responses of livestock to road transportation stress: A review. Afr. J. Biotechnol. 2010, 9, 4845–4856. [Google Scholar]
- Pollard, J.; Littlejohn, R.; Asher, G.; Pearse, A.; Stevenson-Barry, J.; McGregor, S.; Manley, T.; Duncan, S.; Sutton, C.; Pollock, K. A comparison of biochemical and meat quality variables in red deer (Cervus elaphus) following either slaughter at pasture or killing at a deer slaughter plant. Meat Sci. 2002, 60, 85–94. [Google Scholar] [CrossRef]
- Shaw, F.; Tume, R. The assessment of pre-slaughter and slaughter treatments of livestock by measurement of plasma constituents—A review of recent work. Meat Sci. 1992, 32, 311–329. [Google Scholar] [CrossRef]
- Tarrant, V.; Grandin, T. Cattle transport. In Livestock Handling and Transport; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Gruber, S.L.; Tatum, J.D.; Engle, T.E.; Chapman, P.L.; Belk, K.E.; Smith, G.C. Relationships of behavioral and physiological symptoms of preslaughter stress to beef longissimus muscle tenderness1. J. Anim. Sci. 2010, 88, 1148–1159. [Google Scholar] [CrossRef]
- Coombes, S.; Gardner, G.; Pethick, D.; McGilchrist, P. The impact of beef cattle temperament assessed using flight speed on muscle glycogen, muscle lactate and plasma lactate concentrations at slaughter. Meat Sci. 2014, 98, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Anil, M.; TYesildere, H.A.; Matur, E.; McKinstry, J.; Erdogan, O.; Hughest, S.; Mason, C. Comparison of religious slaughter of sheep with methods that include pre-slaughter stunning, and the lack of differences in exsanguination. Anim. Welfare 2004, 13, 387–392. [Google Scholar]
- Warriss, P.; Brown, S. Bem-Estar de Suinos e Qualidade da Carne: Uma Visão Britânica. Proceedings of Conferência Internacional Virtual Sobre Qualidade de Carne Suína 16 de novembro a 16 de dezembro de 2000, Concórdia, Brazil, 16 November 2000–16 December 2000; Volume 1, pp. 17–20. [Google Scholar]
- Hambrecht, E.; Eissen, J.; Newman, D.; Smits, C.; Den Hartog, L.; Verstegen, M. Negative effects of stress immediately be-fore slaughter on pork quality are aggravated by suboptimal transport and lairage conditions. J. Anim. Sci. 2005, 83, 440–448. [Google Scholar] [CrossRef]
- Edwards, L.; Grandin, T.; Engle, T.; Porter, S.; Ritter, M.; Sosnicki, A.; Anderson, D. Use of exsanguination blood lactate to assess the quality of pre-slaughter pig handling. Meat Sci. 2010, 86, 384–390. [Google Scholar] [CrossRef]
- Warriss, P. The handling of cattle pre-slaughter and its effects on carcass and meat quality. Appl. Anim. Behav. Sci. 1990, 28, 171–186. [Google Scholar] [CrossRef]
- Lima, M.L.P.; Negrão, J.A.; de Paz, C.C.P.; Trindade, P.H.E.; Grandin, T. Exit speed score and serum lactate can be used as tools to assess improved cattle handling practices. Livest. Res. Rural Dev. 2018, 30, 151. [Google Scholar]
- Peres, L.M.; Bridi, A.M.; Da Silva, C.A.; Andreo, N.; Tarsitano, M.A.; Stivaletti, E.L.T. Effect of low or high stress in pre-slaughter handling on pig carcass and meat quality. Rev. Bras. Zootec. 2014, 43, 363–368. [Google Scholar] [CrossRef]
- Grandin, T. Auditing animal welfare at slaughter plants. Meat Sci. 2010, 86, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Minka, N.; Ayo, J. Physiological responses of food animals to road transportation stress. Afr. J. Biotechnol. 2010, 9, 6601–6613. [Google Scholar]
- Tackett, J.; Reynolds, A.S.; Dickerman, R.D. Enzyme elevations with muscle injury: Know what to look for! Br. J. Clin. Pharmacol. 2008, 66, 725. [Google Scholar] [CrossRef] [PubMed]
- Wickham, S.; Collins, T.; Barnes, A.; Miller, D.; Beatty, D.; Stockman, C.; Blache, D.; Wemelsfelder, F.; Fleming, P. Qualitative behavioral assessment of transport-naïve and transport-habituated sheep. J. Anim. Sci. 2012, 90, 4523–4535. [Google Scholar] [CrossRef]
- Adzitey, F.; Nurul, H. Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these inci-dences—A mini review. Int. Food Res. J. 2011, 18, 11–20. [Google Scholar]
- Mota-Rojas, D.; Roldan-San, P.; Gonzalez-L, M.; Flores-Pei, S.; Camacho-Mo, D.; Concepción, M.; Morfin-Loy, L.; Mora-Medin, P.; Ramirez-Ne, R.; Cardona, A. Physiological Response and Welfare of Ducks During Slaughter. Asian J. Anim. Veter Adv. 2011, 6, 1256–1263. [Google Scholar] [CrossRef][Green Version]
- Nakyinsige, K.; Man, Y.C.; Aghwan, Z.A.; Zulkifli, I.; Goh, Y.-M.; Abu Bakar, F.; Al-Kahtani, H.; Sazili, A. Stunning and animal welfare from Islamic and scientific perspectives. Meat Sci. 2013, 95, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, E.E.; Yalcintan, H. Comparison of Certain haematological and biochemical parameters regarding pre-slaughter stress in Saanen, Maltese, Gokceada and hair goat kids. J. Fac. Vet. Med. Istanbul Univ. 2013, 39, 189–196. [Google Scholar]
- Ekiz, B.; Ekiz, E.E.; Kocak, O.; Yalcintan, H.; Yilmaz, A. Effect of pre-slaughter management regarding transportation and time in lairage on certain stress parameters, carcass and meat quality characteristics in Kivircik lambs. Meat Sci. 2012, 90, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.; Stafford, K. Acute castration and/or tailing distress and its alleviation in lambs. N. Z. Veter J. 2000, 48, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.D.; Carroll, J.A.; Sanchez, N.C.B.; Richeson, J.T. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 2014, 20, 888–896. [Google Scholar] [CrossRef]
- Young, J.B.; Rosa, R.M.; Landsberg, L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am. J. Physiol. Metab. 1984, 247, E35–E40. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, F.; Chen, Y.; Tsao, T.; Nouri, P.; Rabkin, R. Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J. Clin. Invest. 2001, 108, 467–475. [Google Scholar] [CrossRef]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifying some responses to pain as a stressor. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Egham, UK, 2009; pp. 171–198. [Google Scholar]
- Anil, M.H. Religious slaughter: A current controversial animal welfare issue. Anim. Front. 2012, 2, 64–67. [Google Scholar] [CrossRef]
- Sabow, A.; Goh, Y.; Zulkifli, I.; Sazili, A.; Ab Kadir, M.; Kaka, U.; Khadijah, N.; Adeyemi, K.; Ebrahimi, M. Electroencepha-lographic responses to neck cut and exsanguination in minimally anaesthetized goats. S. Afr. J. Anim. Sci. 2017, 47, 34–40. [Google Scholar] [CrossRef][Green Version]
- Zulkifli, I.; Goh, Y.; Norbaiyah, B.; Sazili, A.; Lotfi, M.; Soleimani, A.; Small, A. Changes in blood parameters and electro-encephalogram of cattle as affected by different stunning and slaughter methods in cattle. Anim. Prod. Sci. 2014, 54, 187–193. [Google Scholar] [CrossRef]
- Gibson, T.; Johnson, C.; Murrell, J.; Mitchinson, S.; Stafford, K.; Mellor, D. Electroencephalographic responses to concussive non-penetrative captive-bolt stunning in halothane-anaesthetized calves. N. Z. Vet. J. 2009, 57, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Veter Pharmacol. Ther. 2006, 29, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.K. Examination stress and its effect on EEG. Int. J. Med. Sci. Public Health 2015, 4, 1493–1497. [Google Scholar] [CrossRef]
| Parameter | Treatment | Sampling Period | Trt * Period | |
|---|---|---|---|---|
| T1 | T2 | |||
| Glucose | Lateral | 4.50 ± 0.07 ay | 4.78 ± 0.12 ay | |
| (mmol/L) | Upright | 5.17 ± 0.04 bx | 5.52 ± 0.05 ax | 0.7008 |
| p-value | <0.0001 | <0.0001 | ||
| Creatine kinase | Lateral | 198.90 ± 8.08 ay | 229.60 ± 15.7 ay | |
| (U/L) | Upright | 248.77 ± 5.75 bx | 275.44 ± 7.76 ax | 0.8474 |
| p-value | 0.0001 | 0.0219 | ||
| Lactate dehydrogenase | Lateral | 1274.60 ± 68.0 bx | 1415.00 ± 39.7 bx | |
| (U/L) | Upright | 1556.44 ± 91.29 ax | 1728.67 ± 50.93 ax | 0.8071 |
| p-value | 0.0226 | 0.0001 | ||
| Calcium | Lateral | 2.10 ± 0.07 ax | 2.20 ± 0.03 ax | |
| (mmol/L) | Upright | 2.18 ± 0.10 ax | 2.14 ± 0.05 ax | 0.3231 |
| p-value | 0.4983 | 0.4059 | ||
| Total Protein | Lateral | 75.55 ± 1.95 bx | 83.19 ± 1.50 ax | |
| (g/L) | Upright | 78.83 ± 3.35 ax | 78.51 ± 3.06 ax | 0.1224 |
| p-value | 0.3983 | 0.1745 | ||
| Creatinine | Lateral | 178.50 ± 7.91 ax | 164.80 ± 5.75 ax | |
| (µmol/L) | Upright | 176.66 ± 11.8 ax | 186.67 ± 12.80 ax | 0.2320 |
| p-value | 0.8973 | 0.1250 | ||
| Lactate | Lateral | 3.89 ± 0.33 ay | 4.74 ± 0.45 ax | |
| (mmol/L) | Upright | 5.22 ± 0.42 ax | 5.51 ± 0.11 ax | 0.446 |
| p-value | 0.0237 | 0.1353 | ||
| Parameter | Treatment | Sampling Period | |||
|---|---|---|---|---|---|
| T1 | T2 | p-Value | Trt * Period | ||
| Adrenaline | Lateral | 1524.11 ± 7.75 by | 2736.47 ± 6.95 ay | <0.0001 | <0.0001 |
| (pg/mL) | Upright | 1625.87 ± 7.36 bx | 3211.56 ± 28.21 ax | <0.0001 | |
| p-value | <0.0001 | <0.0001 | |||
| Noradrenaline | Lateral | 244.85 ± 13.71 by | 287.83 ± 1.03 ay | 0.0108 | 0.5070 |
| (pg/mL) | Upright | 317.64 ± 7.77 bx | 349.61 ± 4.05 ax | 0.0045 | |
| p-value | 0.0001 | <0.0001 | |||
| Parameter | Treatment | Sampling Period | |||
|---|---|---|---|---|---|
| T1 | T2 | p-Value | Trt * Period | ||
| Alpha (µv) | Lateral | 1.44 ± 0.05 bx | 5.03 ± 0.23 ax | <0.0001 | 0.2085 |
| Upright | 1.25 ± 0.04 by | 5.37 ± 0.34 ax | <0.0001 | ||
| p-value | 0.0059 | 0.4187 | |||
| Beta (µv) | Lateral | 2.35 ± 0.07 bx | 9.31 ± 0.42 ax | <0.0001 | 0.2511 |
| Upright | 2.35 ± 0.08 bx | 8.59 ± 0.44 ax | <0.0001 | ||
| p-value | 0.9925 | 0.2422 | |||
| Delta (µv) | Lateral | 8.18 ± 0.56 bx | 43.41 ± 2.01 ay | <0.0001 | 0.0265 |
| Upright | 7.35 ± 0.46 bx | 50.84 ± 3.03 ax | <0.0001 | ||
| p-value | 0.2616 | 0.0419 | |||
| Theta (µv) | Lateral | 1.81 ± 0.09 bx | 7.53 ± 0.43 ax | <0.0001 | 0.0357 |
| Upright | 1.42 ± 0.04 by | 8.72 ± 0.60 ax | <0.0001 | ||
| p-value | 0.0002 | 0.1109 | |||
| Ptot (µv) | Lateral | 12.04 ± 0.46 bx | 58.60 ± 2.05 ax | <0.0001 | 0.082 |
| Upright | 11.42 ± 0.43 bx | 63.49 ± 2.32 ax | <0.0001 | ||
| p-value | 0.3268 | 0.1158 | |||
| MF (µv) | Lateral | 12.45 ± 0.38 by | 15.06 ± 0.37 ay | <0.0001 | 0.5478 |
| Upright | 15.27 ± 0.52 bx | 18.52 ± 0.74 ax | <0.0001 | ||
| p-value | <0.0001 | <0.0001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imlan, J.C.; Kaka, U.; Goh, Y.-M.; Idrus, Z.; Awad, E.A.; Abubakar, A.A.; Ahmad, T.; Quaza Nizamuddin, H.N.; Sazili, A.Q. Effects of Slaughter Positions on Catecholamine, Blood Biochemical and Electroencephalogram Changes in Cattle Restrained Using a Modified Mark IV Box. Animals 2021, 11, 1979. https://doi.org/10.3390/ani11071979
Imlan JC, Kaka U, Goh Y-M, Idrus Z, Awad EA, Abubakar AA, Ahmad T, Quaza Nizamuddin HN, Sazili AQ. Effects of Slaughter Positions on Catecholamine, Blood Biochemical and Electroencephalogram Changes in Cattle Restrained Using a Modified Mark IV Box. Animals. 2021; 11(7):1979. https://doi.org/10.3390/ani11071979
Chicago/Turabian StyleImlan, Jurhamid Columbres, Ubedullah Kaka, Yong-Meng Goh, Zulkifli Idrus, Elmutaz Atta Awad, Ahmed Abubakar Abubakar, Tanbir Ahmad, Hassan N. Quaza Nizamuddin, and Awis Qurni Sazili. 2021. "Effects of Slaughter Positions on Catecholamine, Blood Biochemical and Electroencephalogram Changes in Cattle Restrained Using a Modified Mark IV Box" Animals 11, no. 7: 1979. https://doi.org/10.3390/ani11071979
APA StyleImlan, J. C., Kaka, U., Goh, Y.-M., Idrus, Z., Awad, E. A., Abubakar, A. A., Ahmad, T., Quaza Nizamuddin, H. N., & Sazili, A. Q. (2021). Effects of Slaughter Positions on Catecholamine, Blood Biochemical and Electroencephalogram Changes in Cattle Restrained Using a Modified Mark IV Box. Animals, 11(7), 1979. https://doi.org/10.3390/ani11071979








