Proteomic Analysis of Liver from Finishing Beef Cattle Supplemented with a Rumen-Protected B-Vitamin Blend and Hydroxy Trace Minerals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Liver Biopsy
2.3. Protein Extraction
2.4. Protein Digestion
2.5. Mass Spectrometry of Protein Samples
2.6. Statistical Analysis
2.7. Bioinformatics Analysis
3. Results
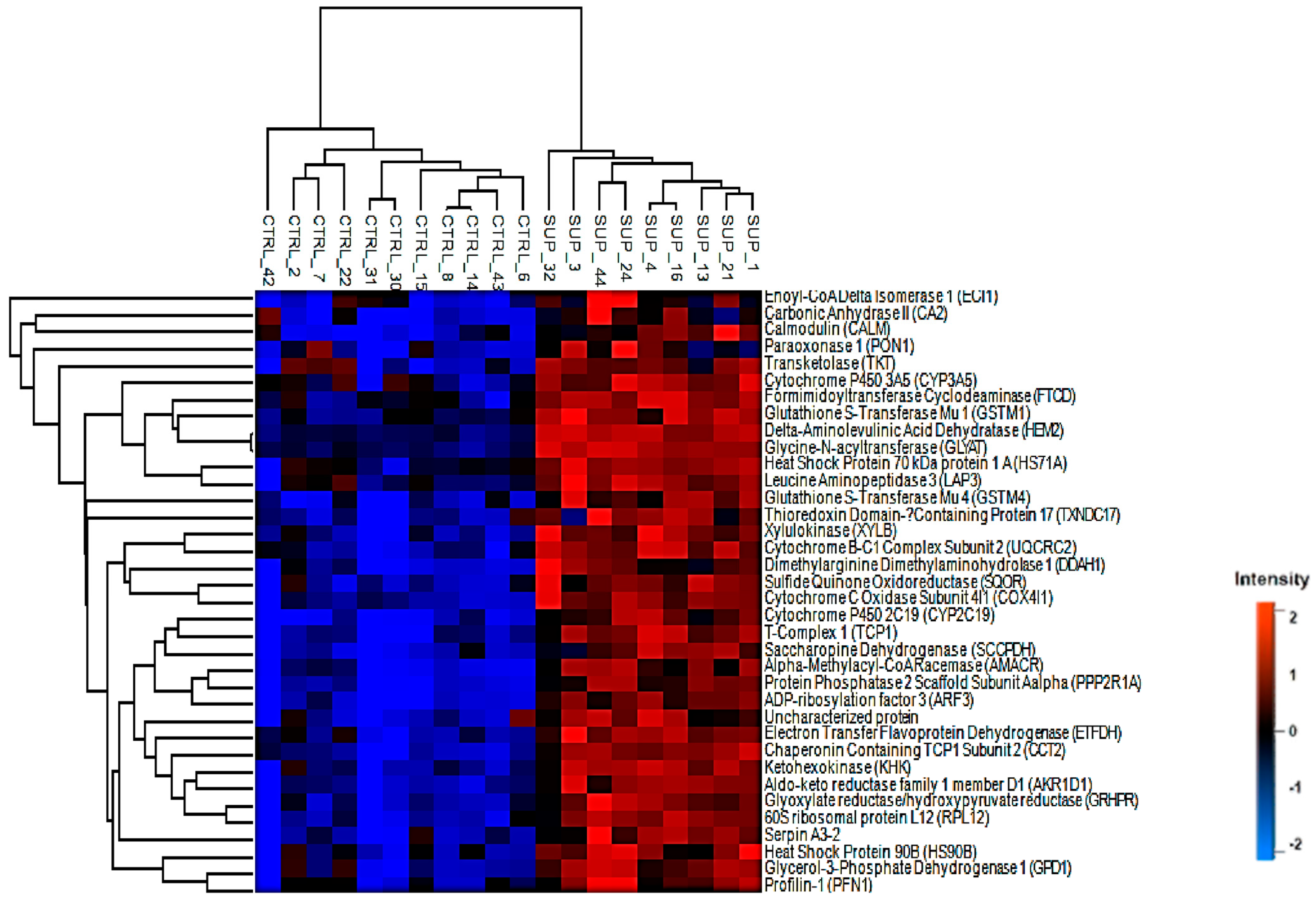
3.1. Differentially Abundant Proteins
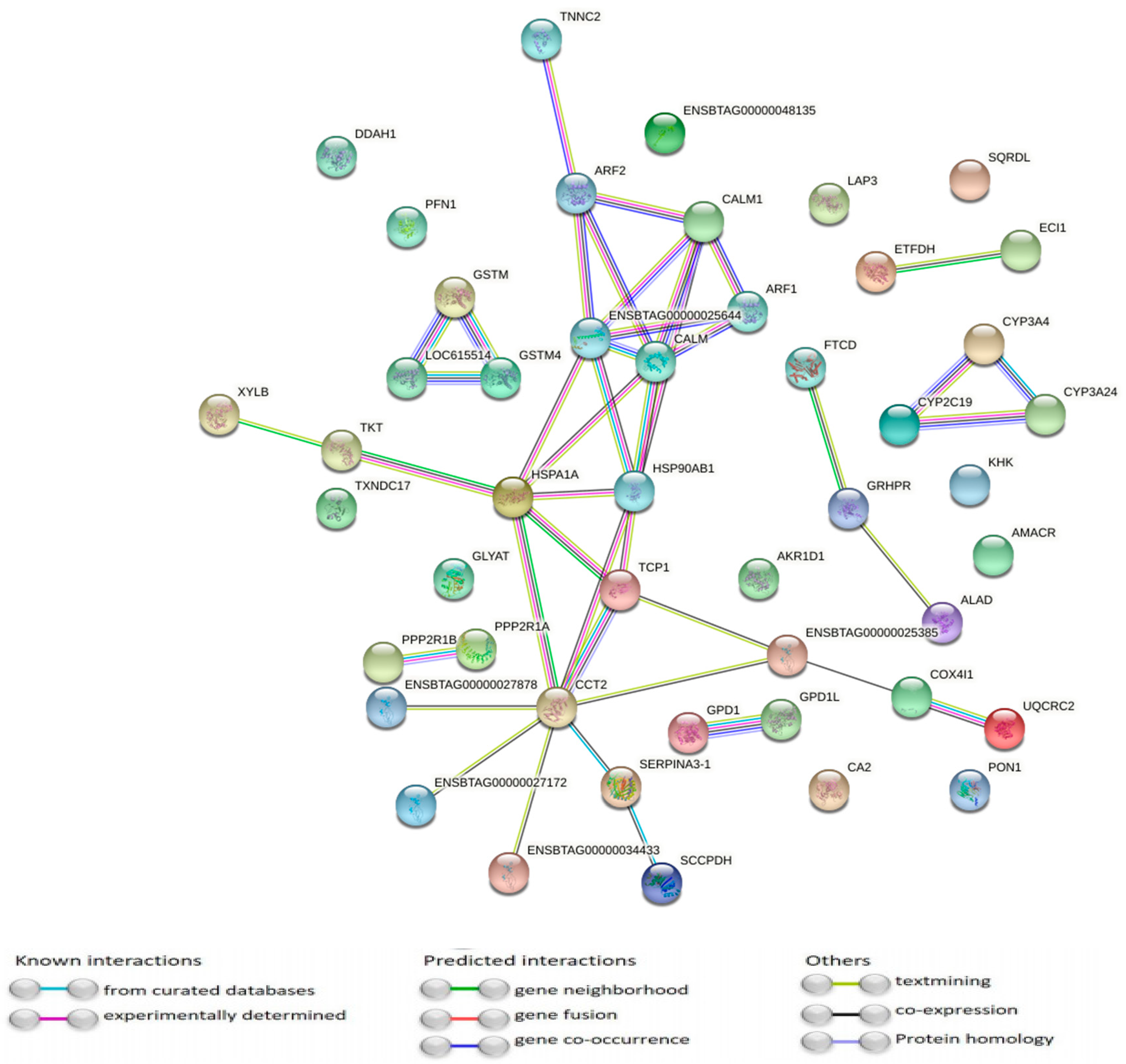
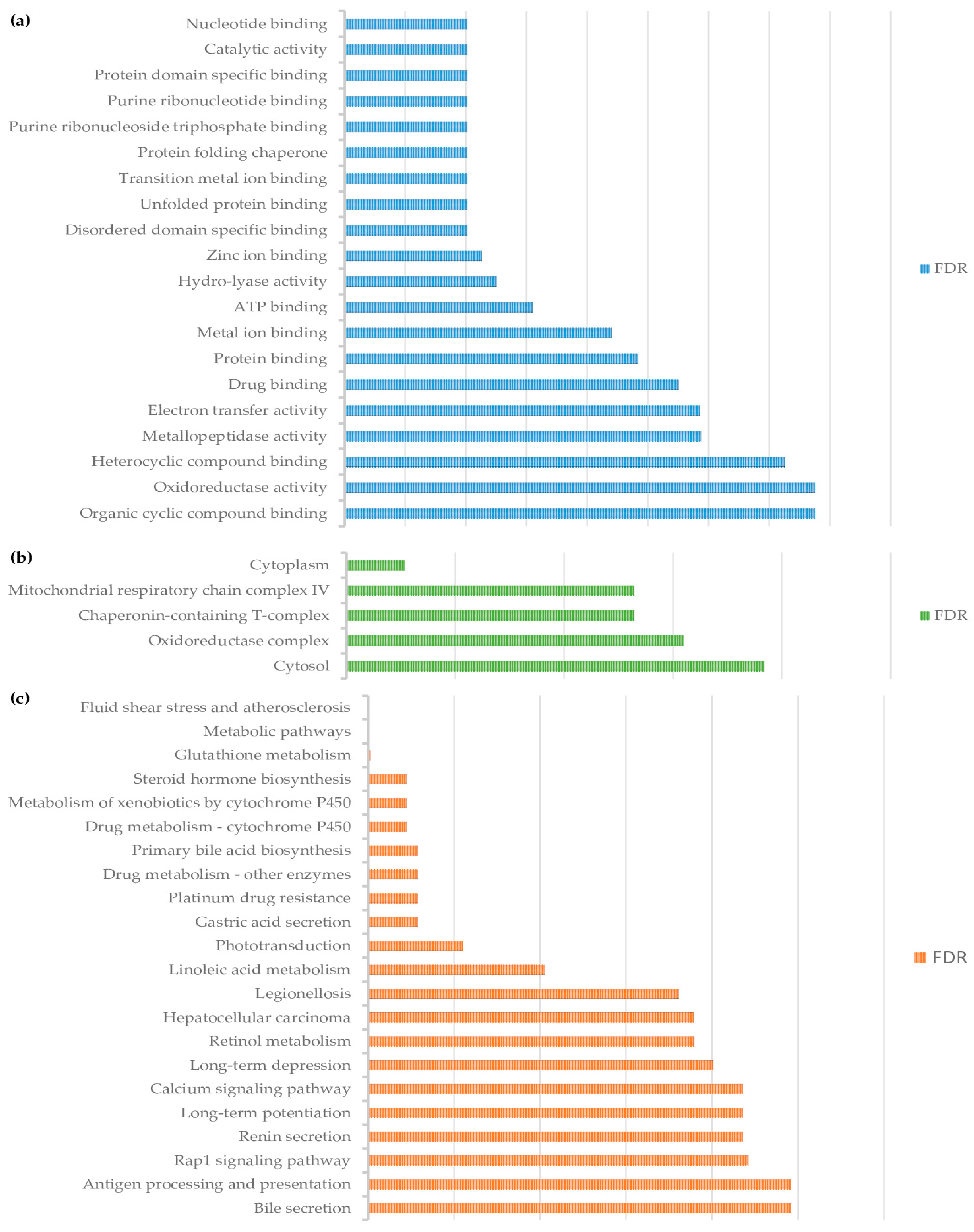
3.2. Pathway Analysis of Differentially Abundant Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- García-Cañas, V.; Simó, C.; León, C.; Cifuentes, A. Advances in Nutrigenomics research: Novel and future analytical approach-es to investigate the biological activity of natural compounds and food functions. J. Pharm. Biomed. Anal. 2010, 51, 290–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.W.; Friso, S.; Ghandour, H.; Bagley, P.J.; Selhub, J.; Mason, J.B. Vitamin B-12 deficiency induces anomalies of base substitu-tion and methylation in the DNA of rat colonic epithelium. J. Nutr. 2004, 134, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, C.L.; Brettschneider, A.-K.; Haftenberger, M.; Lehmann, F.; Frank, M.; Heide, K.; Patelakis, E.; Perlitz, H.; Krause, L.; Houben, R.; et al. Comprehensive assessment of food and nutrient intake of children and adolescents in Germany: EsKiMo II-the eating study as a KiGGS module. BMC Nutr. 2017, 3, 75–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santschi, D.; Berthiaume, R.; Matte, J.; Mustafa, A.; Girard, C. Fate of Supplementary B-Vitamins in the Gastrointestinal Tract of Dairy Cows. J. Dairy Sci. 2005, 88, 2043–2054. [Google Scholar] [CrossRef]
- Ouattara, B.; Bissonnette, N.; Duplessis, M.; Girard, C.L. Supplements of vitamins B9 and B12 affect hepatic and mammary gland gene expression profiles in lactating dairy cows. BMC Genom. 2016, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Hartling, I.; Burnett, T.A.; Polsky, L.B.; Donnan, C.R.; Leclerc, H.; Veira, D.; Cerri, R.L.A. Rumen-protected B vitamin com-plex supplementation during the transition period and early lactation alters endometrium mRNA expression on day 14 of gestation in lactating dairy cows. J. Dairy Sci. 2019, 102, 1642–1657. [Google Scholar] [CrossRef] [Green Version]
- Morrison, E.I.; Reinhardt, H.; Leclerc, H.; DeVries, T.J.; LeBlanc, S.J. Effect of rumen-protected B vitamins and choline supplemen-tation on health, production, and reproduction in transition dairy cows. J. Dairy Sci. 2018, 101, 9016–9027. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; p. 644. [Google Scholar]
- Suttle Neville, F. Mineral. Nutrition of Livestock, 4th ed.; Cabi.: Wallingford, UK, 2010. [Google Scholar]
- López-Alonso, M. Trace Minerals and Livestock: Not Too Much Not Too Little. ISRN Veter Sci. 2012, 2012, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldera, E.; Weigel, B.; Kucharczyk, V.N.; Sellins, K.S.; Archibeque, S.L.; Wagner, J.J.; Han, H.; Spears, J.W.; E Engle, T. Trace mineral source influences ruminal distribution of copper and zinc and their binding strength to ruminal digesta1,2,3. J. Anim. Sci. 2019, 97, 1852–1864. [Google Scholar] [CrossRef]
- Miles, R.D.; O’Keefe, S.F.; Henry, P.R.; Ammerman, C.B.; Luo, X.G. The effect of dietary supplementation with copper sulfate or tri-basic copper chloride on broiler performance, relative copper bioavailability, and dietary prooxidant activity. Poult. Sci. 1998, 77, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bryant, M.M.; Roland, D.A. Layer Performance and Phytase Retention as Influenced by Copper Sulfate Pentahydrate and Tribasic Copper Chloride. J. Appl. Poult. Res. 2005, 14, 499–505. [Google Scholar] [CrossRef]
- Lu, L.; Wang, R.L.; Zhang, Z.J.; Steward, F.A.; Luo, X.; Liu, B. Effect of dietary supplementation with copper sulfate or tribasic cop-per chloride on the growth performance, liver copper concentrations of broilers fed in floor pens, and stabilities of vitamin E and phytase in feeds. Biol. Trace Elem. Res. 2010, 138, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Molette, C.; Théron, L.; Marty-Gasset, N.; Fernandez, X.; Remignon, H.; Molette, C.; Théron, L.; Marty-Gasset, N.; Fernandez, X.; Remignon, H. Current advances in proteomic analysis of (fatty) liver. J. Proteom. 2012, 75, 4290–4295. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, T.; Zhang, S.; Zhang, Y. Comparative Proteomic and Bioinformatic Analysis of the Effects of a High-Grain Diet on the Hepatic Metabolism in Lactating Dairy Goats. PLoS ONE 2013, 8, e80698. [Google Scholar] [CrossRef] [Green Version]
- Mølgaard, L.; Damgaard, B.M.; Bjerre-Harpøth, V.; Herskin, M.S. Effects of percutaneous needle liver biopsy on dairy cow be-haviour. Res. Vet. Sci. 2012, 93, 1248. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and pro-teome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 28 July 2020).
- Storey, J. A direct approach to false discovery rates. J. R. Stat. Soc. Ser. B 2002, 64, 479–498. [Google Scholar] [CrossRef] [Green Version]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. qvalue: Q-value Estimation for False Discovery Rate Control. R Package Version 2.22.0. Available online: http://github.com/jdstorey/qvalue (accessed on 19 July 2020).
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Johnson, K.A.; Baldwin, R.L. Changes in liver and gastrointestinal tract energy demands in response to physio-logical workload in ruminants. J. Nutr. 1990, 120, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Lorenzi, I.; Rigoni, G.; Caicci, F.; Soriano, M.E. Regulation of Mitochondrial Electron Transport Chain Assembly. J. Mol. Biol. 2018, 430, 4849–4873. [Google Scholar] [CrossRef]
- Atamna, H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res. Rev. 2004, 3, 303–318. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Jennings, D. Role of copper in mitochondrial biogenesis via interaction with ATP synthase and cytochrome c oxidase. J. Bioenerg. Biomembr. 2002, 34, 389–395. [Google Scholar] [CrossRef]
- Zeng, H.; Saari, J.T.; Johnson, W.T. Copper Deficiency Decreases Complex IV but Not Complex I, II, III, or V in the Mitochondrial Respiratory Chain in Rat Heart. J. Nutr. 2007, 137, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Bustos, R.I.; Jensen, E.L.; Ruiz, L.M.; Rivera, S.; Ruiz, S.; Simon, F.; Riedel, C.; Ferrick, D.; Elorza, A.A. Copper deficiency alters cell bio-energetics and induces mitochondrial fusion through up-regulation of MFN2 and OPA1 in erythropoietic cells. Biochem. Biophys. Res. Commun. 2013, 437, 426–432. [Google Scholar] [CrossRef]
- Ramos, M.H.; Kerley, M.S. Mitochondrial complex I protein differs among residual feed intake phenotype in beef cattle. J. Anim. Sci. 2013, 91, 3299–3304. [Google Scholar] [CrossRef]
- Davis, M.P.; Brooks, M.A.; Kerley, M.S. Relationship between residual feed intake and lymphocyte mitochondrial complex pro-tein concentration and ratio in crossbred steers. J. Anim. Sci. 2016, 94, 1587–1591. [Google Scholar] [CrossRef] [Green Version]
- Casal, A.; Garcia-Roche, M.; Navajas, E.A.; Cassina, A.; Carriquiry, M. Hepatic mitochondrial function in Hereford steers with divergent residual feed intake phenotypes1. J. Anim. Sci. 2018, 96, 4431–4443. [Google Scholar] [CrossRef]
- Wang, Y.; Palmfeldt, J.; Gregersen, N.; Makhov, A.M.; Conway, J.F.; Wang, M.; McCalley, S.P.; Basu, S.; Alharbi, H.; Croix, C.S.; et al. Mitochondrial fatty acid oxidation and the electron transport chain comprise a multifunctional mitochondrial protein complex. J. Biol. Chem. 2019, 294, 12380–12391. [Google Scholar] [CrossRef]
- Lei, L.; Xiaoyi, S.; Fuchang, L. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr. Res. 2017, 61, 1348866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezen, T.; Rozman, D. Regulation of Hepatic Cytochromes P450 by Lipids and Cholesterol. Curr. Drug Metab. 2011, 12, 173–185. [Google Scholar] [CrossRef]
- Puntarulo, S.; I Cederbaum, A. Production of Reactive Oxygen Species by Microsomes Enriched in Specific Human Cytochrome P450 Enzymes. Free Radic. Biol. Med. 1998, 24, 1324–1330. [Google Scholar] [CrossRef]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mito-chondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid Med. Cell Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dringen, R. Glutathione (GSH) Synthesis and Metabolism. In Neural Metabolism In Vivo; Choi, I.Y., Gruet-ter, R., Eds.; Advances in Neurobiology; Springer: Boston, MA, USA, 2011; Volume 4, pp. 1029–1050. [Google Scholar]
- Huang, J.; Jia, Y.; Li, Q.; Son, K.; Hamilton, C.; Burris, W.R.; Bridges, P.J.; Stromberg, A.J.; Matthews, J.C. Glutathione content and ex-pression of proteins involved with glutathione metabolism differs in longissimus dorsi, subcutaneous adipose, and liver tis-sues of finished vs. growing beef steers. J. Anim Sci. 2018, 96, 5152–5165. [Google Scholar]
- De Maio, A. The heat-shock response. New Horiz. 1995, 3, 198–207. [Google Scholar]
- Chen, Y.; Voegeli, T.S.; Liu, P.P.; Noble, E.G.; Currie, R.W. Heat shock paradox and a new role of heat shock proteins and their re-ceptors as anti-inflammation targets. Inflamm. Allergy Drug Targets 2007, 6, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, J.K.; Fritchen, A.N.; Huff-Lonergan, E.; Dekkers, J.C.M.; Gabler, N.K.; Lonergan, S.M. Divergent genetic selection for residual feed intake impacts mitochondria reactive oxygen species production in pigs1. J. Anim. Sci. 2013, 91, 2133–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Control | Supplemented | Units | |
|---|---|---|---|
| Ca | 140 | 140 | g kg−1 |
| P | 28 | 28 | g kg−1 |
| Na | 75 | 75 | g kg−1 |
| K | 46 | 46 | g kg−1 |
| Mg | 64 | 64 | g kg−1 |
| S | 23 | 23 | mg kg−1 |
| Zn | 1150 1 | 1150 2 | mg kg−1 |
| Cu | 312 1 | 312 2 | mg kg−1 |
| F | 465 | 465 | mg kg−1 |
| Mn | 1080 | 1080 | mg kg−1 |
| Co | 31 | 31 | mg kg−1 |
| I | 22 | 22 | mg kg−1 |
| Vitamin A | 62,310 | 62,310 | UI kg−1 |
| Vitamin D3 | 8830 | 8830 | UI kg−1 |
| Vitamin E | 860 | 860 | UI kg−1 |
| Vitamin B6 | - | 161 | UI kg−1 |
| Vitamin B12 | - | 1.934 | ug kg−1 |
| Vitamin B3 | - | 20.000 | mg kg−1 |
| Vitamin B9 | - | 2.175 | mg kg−1 |
| Vitamin B7 | - | 1.615 | mg kg−1 |
| Monensin | 600 | 600 | mg kg−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, M.M.; Brito, T.R.; Lage, J.F.; Costa, T.C.; Fontes, M.M.d.S.; Serão, N.V.L.; Mendes, T.A.d.O.; Reis, R.A.; Veroneze, R.; e Silva, F.F.; et al. Proteomic Analysis of Liver from Finishing Beef Cattle Supplemented with a Rumen-Protected B-Vitamin Blend and Hydroxy Trace Minerals. Animals 2021, 11, 1934. https://doi.org/10.3390/ani11071934
Lopes MM, Brito TR, Lage JF, Costa TC, Fontes MMdS, Serão NVL, Mendes TAdO, Reis RA, Veroneze R, e Silva FF, et al. Proteomic Analysis of Liver from Finishing Beef Cattle Supplemented with a Rumen-Protected B-Vitamin Blend and Hydroxy Trace Minerals. Animals. 2021; 11(7):1934. https://doi.org/10.3390/ani11071934
Chicago/Turabian StyleLopes, Mariana Mescouto, Thaís Ribeiro Brito, Josiane Fonseca Lage, Thaís Correia Costa, Marta Maria dos Santos Fontes, Nick Vergara Lopes Serão, Tiago Antônio de Oliveira Mendes, Ricardo Andrade Reis, Renata Veroneze, Fabyano Fonseca e Silva, and et al. 2021. "Proteomic Analysis of Liver from Finishing Beef Cattle Supplemented with a Rumen-Protected B-Vitamin Blend and Hydroxy Trace Minerals" Animals 11, no. 7: 1934. https://doi.org/10.3390/ani11071934
APA StyleLopes, M. M., Brito, T. R., Lage, J. F., Costa, T. C., Fontes, M. M. d. S., Serão, N. V. L., Mendes, T. A. d. O., Reis, R. A., Veroneze, R., e Silva, F. F., & Duarte, M. d. S. (2021). Proteomic Analysis of Liver from Finishing Beef Cattle Supplemented with a Rumen-Protected B-Vitamin Blend and Hydroxy Trace Minerals. Animals, 11(7), 1934. https://doi.org/10.3390/ani11071934








