Equine Activity Time Budgets: The Effect of Housing and Management Conditions on Geriatric Horses and Horses with Chronic Orthopaedic Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses, Housing and Management Conditions
2.2. Time Budget Analysis
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Horses and Tracking
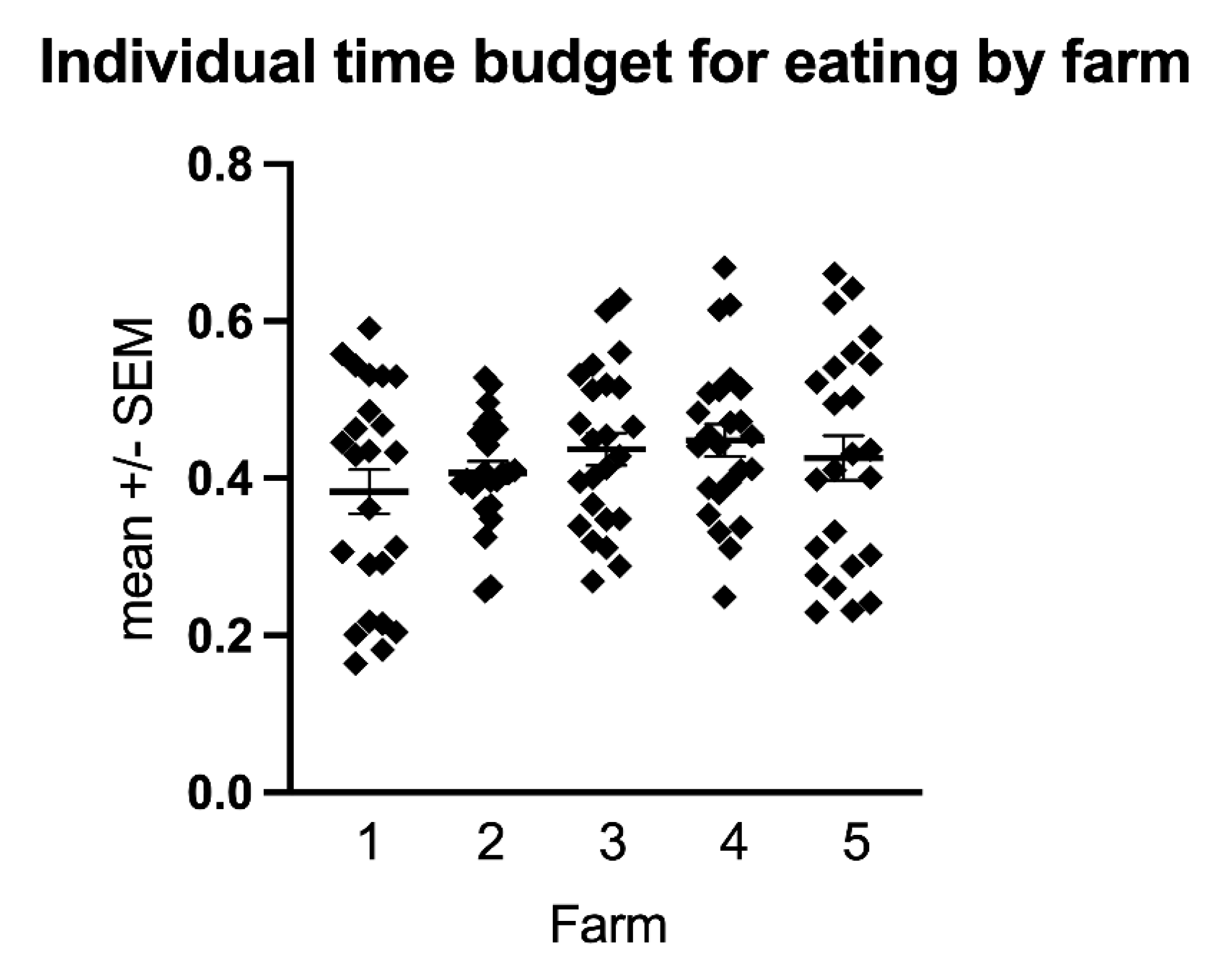
3.2. Time Budgets and Activity Counts
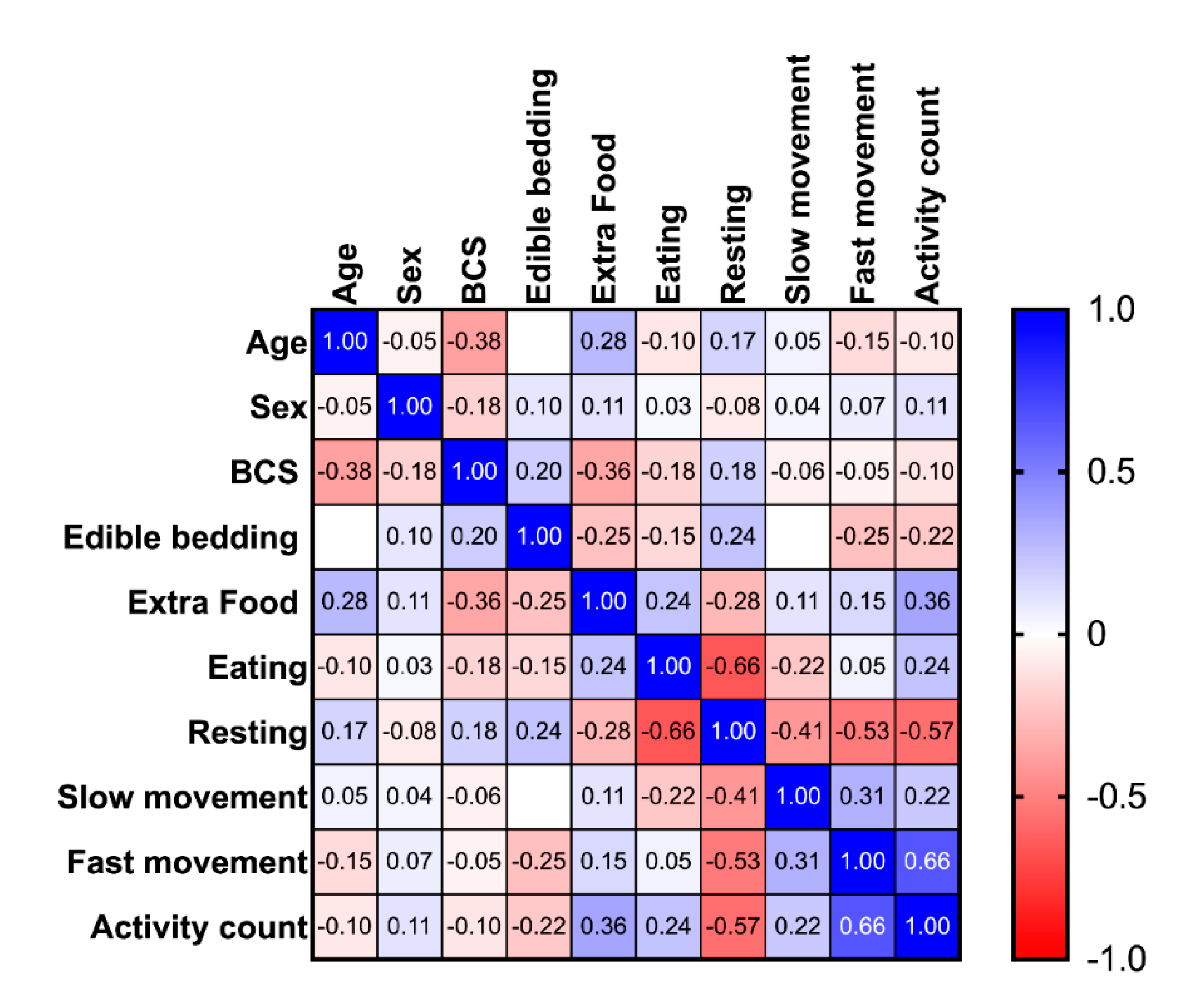
3.3. The Effect of Group, Age, Sex, BCS, Edible Bedding, Extra Food Rations and Season on Equine Time Budgets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yarnell, K.; Hall, C.; Royle, C.; Walker, S.L. Domesticated horses differ in their behavioural and physiological responses to isolated and group housing. Physiol. Behav. 2015, 143, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrafchi, A.; Blokhuis, H.J. Equine stereotypic behaviors: Causation, occurrence, and prevention. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 386–394. [Google Scholar] [CrossRef]
- Chaplin, S.; Gret, L. Effect of housing conditions on activity and lying behaviour of horses. Animal 2010, 4, 792–795. [Google Scholar] [CrossRef]
- Rose-Meierhöfer, S.; Klaer, S.; Ammon, C.; Brunsch, R.; Hoffmann, G. Activity behavior of horses housed in different open barn systems. J. Equine Vet. Sci. 2010, 30, 624–634. [Google Scholar] [CrossRef]
- Hoffmann, G.; Bentke, A.; Rose-Meierhöfer, S.; Berg, W.; Mazetti, P.; Hardarson, G.H. Influence of an active stable system on the behavior and body condition of Icelandic horses. Animal 2012, 6, 1684–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaya, L.; Cowan, E.; McGuire, B. A note on the relationship between time spent in turnout and behaviour during turnout in horses (Equus Caballus). Appl. Anim. Behav. Sci. 2006, 98, 155–160. [Google Scholar] [CrossRef]
- Hausberger, M.; Lerch, N.; Guilbaud, E.; Stomp, M.; Grandgeorge, M.; Henry, S.; Lesimple, C. On-farm welfare assessment of horses: The risks of putting the cart before the horse. Animals 2020, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Waran, N.; Randle, H. What we can manage: The importance of using robust welfare indicators in equitation science. Appl. Anim. Behav. Sci. 2017, 190, 74–81. [Google Scholar] [CrossRef]
- Thorne, J.B.; Goodwin, D.; Kennedy, M.J.; Davidson, H.P.B.; Harris, P. Foraging enrichment for individually housed horses: Practicality and effects on behaviour. Appl. Anim. Behav. Sci. 2005, 94, 149–164. [Google Scholar] [CrossRef]
- Marsden, M.D. The use of time-budgets in the assessment of animal welfare in the horse; “Are changes in time-budgeting indicative of changes in welfare?”. Proc. Br. Soc. Anim. Prod. (1972) 1993, 1993, 82. [Google Scholar] [CrossRef]
- Kiley-Worthington, M. The behavior of horses in relation to management and training—Towards ethologically sound environments. J. Equine Vet. Sci. 1990, 10, 62–75. [Google Scholar] [CrossRef]
- Broom, D.M. Indicators of poor welfare. Brit. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef]
- Auer, U.; Kelemen, Z.; Engl, V.; Jenner, F. Activity time budgets—A potential tool to monitor equine welfare? Animals 2021, 11, 850. [Google Scholar] [CrossRef]
- Howell, C.P.; Cheyne, S.M. Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. J. Appl. Anim. Welf. Sci. 2018, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.; Waran, N.; Young, R. On comparing the behaviour of zoo housed animals with wild conspecifics as a welfare indicator. Anim. Welf. 1996, 5, 13–24. [Google Scholar]
- Boy, V.; Duncan, P. Time-budgets of Camargue horses I. Developmental changes in the time-budgets of foals. Behaviour 1979, 71, 187–201. [Google Scholar] [CrossRef]
- Lesimple, C. Indicators of horse welfare: State-of-the-art. Animals 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waran, N.K. Can studies of feral horse behaviour be used for assessing domestic horse welfare? Equine Vet. J. 1997, 29, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie-Graham, T. Time budget studies in stalled horses. Ph.D. Thesis, The University of Edinburgh, Royal Dick School of Veterinary Studies, Midlothian, UK, 1994; pp. 1–260. [Google Scholar]
- Sweeting, M.P.; Houpt, C.E.; Houpt, K.A. Social facilitation of feeding and time budgets in stabled ponies. J. Anim. Sci. 1985, 60, 369–374. [Google Scholar] [CrossRef]
- Visser, E.K.; Ellis, A.D.; Reenen, C.G.V. The effect of two different housing conditions on the welfare of young horses stabled for the first time. Appl. Anim. Behav. Sci. 2008, 114, 521–533. [Google Scholar] [CrossRef]
- Mills, D.S.; Clarke, A. The welfare of horses. Anim. Welf. 2002, 77–97. [Google Scholar] [CrossRef]
- Duncan, P. Time-budgets of Camargue horses Ii. Time-budgets of adult horses and weaned sub-adults. Behaviour 1980, 72, 26–48. [Google Scholar] [CrossRef]
- Duncan, P. Time-budgets of Camargue horses Iii. Environmental influences. Behaviour 1985, 92, 188–208. [Google Scholar] [CrossRef]
- Boyd, L.E.; Carbonaro, D.A.; Houpt, K.A. The 24-hour time budget of Przewalski horses. Appl. Anim. Behav. Sci. 1988, 21, 5–17. [Google Scholar] [CrossRef]
- Berger, A.; Scheibe, K.-M.; Eichhorn, K.; Scheibe, A.; Streich, J. Diurnal and ultradian rhythms of behaviour in a mare group of Przewalski horse (Equus Ferus Przewalskii), measured through one year under semi-reserve conditions. Appl. Anim. Behav. Sci. 1999, 64, 1–17. [Google Scholar] [CrossRef]
- Green, N.F.; Green, H.D. The wild horse population of stone cabin valley, Nevada: A preliminary report. In Proceedings of the National Wild Horse Forum, University of Nevada, Reno, NV, USA, 4–7 April 1977; pp. 59–65. [Google Scholar]
- Giannetto, C.; Fazio, F.; Assenza, A.; Alberghina, D.; Panzera, M.; Piccione, G. Intrasubject and intersubject variabilities in the daily rhythm of total locomotor activity in horses. J. Vet. Behav. Clin. Appl. Res. 2016, 12, 42–48. [Google Scholar] [CrossRef]
- Rochais, C.; Henry, S.; Hausberger, M. “Hay-Bags” and “Slow Feeders”: Testing their impact on horse behaviour and welfare. Appl. Anim. Behav. Sci. 2018, 198, 52–59. [Google Scholar] [CrossRef]
- Lesimple, C.; Gautier, E.; Benhajali, H.; Rochais, C.; Lunel, C.; Bensaïd, S.; Khalloufi, A.; Henry, S.; Hausberger, M. Stall architecture influences horses’ behaviour and the prevalence and type of stereotypies. Appl. Anim. Behav. Sci. 2019, 219, 104833. [Google Scholar] [CrossRef]
- Murphy, B.A. Circadian and circannual regulation in the horse: Internal timing in an elite athlete. J. Equine Vet. Sci. 2019, 76, 14–24. [Google Scholar] [CrossRef]
- Flannigan, G.; Stookey, J.M. Day-time time budgets of pregnant mares housed in tie stalls: A comparison of draft versus light mares. Appl. Anim. Behav. Sci. 2002, 78, 125–143. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of welfare in zoo animals: Towards optimum quality of life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeantet, L.; Planas-Bielsa, V.; Benhamou, S.; Geiger, S.; Martin, J.; Siegwalt, F.; Lelong, P.; Gresser, J.; Etienne, D.; Hiélard, G.; et al. Behavioural inference from signal processing using animal-borne multi-sensor loggers: A novel solution to extend the knowledge of sea turtle ecology. Roy. Soc. Open Sci. 2020, 7, 200139. [Google Scholar] [CrossRef] [PubMed]
- Eerdekens, A.; Deruyck, M.; Fontaine, J.; Martens, L.; Poorter, E.D.; Joseph, W. Automatic equine activity detection by convolutional neural networks using accelerometer data. Comput. Electron. Agric. 2020, 168, 105139. [Google Scholar] [CrossRef]
- Maisonpierre, I.N.; Sutton, M.A.; Harris, P.; Menzies-Gow, N.; Weller, R.; Pfau, T. Accelerometer activity tracking in horses and the effect of pasture management on time budget. Equine Vet. J. 2019, 51, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ladds, M.A.; Salton, M.; Hocking, D.P.; McIntosh, R.R.; Thompson, A.P.; Slip, D.J.; Harcourt, R.G. Using accelerometers to develop time-energy budgets of wild fur seals from captive surrogates. PeerJ 2018, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Price, J.; Catriona, S.; Welsh, E.M.; Waran, N.K. Preliminary evaluation of a behaviour-based system for assessment of post-operative pain in horses following arthroscopic surgery. Vet. Anaesth. Analg. 2003, 30, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Elia, J.B.; Erb, H.N.; Houpt, K.A. Motivation for hay: Effects of a pelleted diet on behavior and physiology of horses. Physiol. Behav. 2010, 101, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Aristizabal, F.; Nieto, J.; Yamout, S.; Snyder, J. The effect of a hay grid feeder on feed consumption and measurement of the gastric PH using an intragastric electrode device in horses: A preliminary report. Equine Vet. J. 2014, 46, 484–487. [Google Scholar] [CrossRef]
- Sartori, C.; Guzzo, N.; Normando, S.; Bailoni, L.; Mantovani, R. Evaluation of behaviour in stabled draught horse foals fed diets with two protein levels. Animal 2017, 11, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.G.; Silva, C.F.R.; Dias, L.A.; Junior, S.d.S.R.; Thomes, F.R.; do Lago, L.A.; de Carvalho, A.M.; Faleiros, R.R. Welfare benefits after the implementation of slow-feeder hay bags for stabled horses. J. Vet. Behav. 2020, 38, 61–66. [Google Scholar] [CrossRef]
- Raspa, F.; Tarantola, M.; Bergero, D.; Nery, J.; Visconti, A.; Mastrazzo, C.M.; Cavallini, D.; Valvassori, E.; Valle, E. Time-budget of horses reared for meat production: Influence of stocking density on behavioural activities and subsequent welfare. Animals 2020, 10, 1334. [Google Scholar] [CrossRef]
- Baumgartner, M.; Boisson, T.; Erhard, M.H.; Zeitler-Feicht, M.H. Common feeding practices pose a risk to the welfare of horses when kept on non-edible bedding. Animals 2020, 10, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, L.M.; Cymbaluk, N.F.; Freeman, D.W.; Geor, R.J.; Graham-Thiers, P.M.; Longland, A.C.; Nielsen, B.D.; Siciliano, P.D.; Topliff, D.R.; Valdes, E.V.; et al. Nutrient Requirements of Horses, 6th ed.; The National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-48895-2. [Google Scholar]
- Henning, J.; Lawrence, L. Horse Pasture Management; Academic Press: Cambridge, MA, USA, 2019; pp. 177–208. [Google Scholar] [CrossRef]
- Lindberg, J.E. Equine applied and clinical nutrition. Sect. C Appl. Nutr. Feed. 2013, 319–331. [Google Scholar] [CrossRef]
- Glunk, E.C.; Pratt-Phillips, S.E.; Siciliano, P.D. Effect of restricted pasture access on pasture dry matter intake rate, dietary energy intake, and fecal PH in horses. J. Equine Vet. Sci. 2013, 33, 421–426. [Google Scholar] [CrossRef]
- Ellis, A.D. Biological Basis of Behaviour in Relation to Nutrition and Feed Intake in Horses; Ellis, A.D., Longland, A.C., Coenen, M., Miraglia, N., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; pp. 53–74. [Google Scholar]
- Ellis, A.D.; Fell, M.; Luck, K.; Gill, L.; Owen, H.; Briars, H.; Barfoot, C.; Harris, P. Effect of forage presentation on feed intake behaviour in stabled horses. Appl. Anim. Behav. Sci. 2015, 165, 88–94. [Google Scholar] [CrossRef]
- Bachmann, I.; Audigé, L.; Stauffacher, M. Risk factors associated with behavioural disorders of crib-biting, weaving and box-walking in swiss horses. Equine Vet. J. 2003, 35, 158–163. [Google Scholar] [CrossRef]
- Christie, J.L.; Hewson, C.J.; Riley, C.B.; McNiven, M.A.; Dohoo, I.R.; Bate, L.A. Management factors affecting stereotypies and body condition score in nonracing horses in Prince Edward Island. Can. Vet. J. La Rev. Vétérinaire Can. 2006, 47, 136–143. [Google Scholar]
- Benhajali, H.; Richard-Yris, M.-A.; Ezzaouia, M.; Charfi, F.; Hausberger, M. Foraging opportunity: A crucial criterion for horse welfare? Animal 2009, 3, 1308–1312. [Google Scholar] [CrossRef] [Green Version]
- Harris, P. Nutrition, behaviour and the role of supplements for calming horses: The veterinarian’s dilemma. Vet. J. 2005, 170, 10–11. [Google Scholar] [CrossRef]
- Hallam, S.; Campbell, E.P.; Qazamel, M.; Owen, H.; Ellis, A.D. Forages and Grazing in Horse Nutrition; Springer Science & Business Media: Berlin, Germany, 2012; pp. 319–321. [Google Scholar] [CrossRef]
- Ellis, A.D.; Redgate, S.; Zinchenko, S.; Owen, H.; Barfoot, C.; Harris, P. The effect of presenting forage in multi-layered haynets and at multiple sites on night time budgets of stabled horses. Appl. Anim. Behav. Sci. 2015, 171, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Burla, J.-B.; Rufener, C.; Bachmann, I.; Gygax, L.; Patt, A.; Hillmann, E. Space allowance of the littered area affects lying behavior in group-housed horses. Front. Vet. Sci. 2017, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, M.N.; Estvan, J.; Ladewig, J. A note on resting behaviour in horses kept on pasture: Rolling prior to getting up. Appl. Anim. Behav. Sci. 2007, 105, 265–269. [Google Scholar] [CrossRef]
- Werhahn, H.; Hessel, E.F.; Bachhausen, I.; Van den Weghe, H.F.A. Effects of different bedding materials on the behavior of horses housed in single stalls. J. Equine Vet. Sci. 2010, 30, 425–431. [Google Scholar] [CrossRef]
- Raspa, F.; Tarantola, M.; Bergero, D.; Bellino, C.; Mastrazzo, C.M.; Visconti, A.; Valvassori, E.; Vervuert, I.; Valle, E. Stocking density affects welfare indicators in horses reared for meat production. Animals 2020, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, A.; Rucklebusch, Y. Sleep and wakefulness in the housed pony under different dietary conditions. Can. J. Comp. Med. Rev. Can. Médecine Comparée. 1974, 38, 65–71. [Google Scholar]
- Dallaire, A. Rest Behavior. Vet. Clin. North. Am. Equine Pract. 1986, 2, 591–607. [Google Scholar] [CrossRef]
- McCall, C.A.; Potter, G.D.; Kreider, J.L. Locomotor, vocal and other behavioral responses to varying methods of weaning foals. Appl. Anim. Behav. Sci. 1985, 14, 27–35. [Google Scholar] [CrossRef]
- Wolfensohn, S. Too cute to kill? The need for objective measurements of quality of life. Animals 2020, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Giannetto, C.; Marafioti, S.; Casella, S.; Fazio, F.; Caola, G. Daily rhythms of rectal temperature and total locomotor activity in trained and untrained horses. J. Vet. Behav. Clin. Appl. Res. 2011, 6, 115–120. [Google Scholar] [CrossRef]
- Bertolucci, C.; Giannetto, C.; Fazio, F.; Piccione, G. Seasonal variations in daily rhythms of activity in athletic horses. Animal 2008, 2, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Giannetto, C. State of the art on daily rhythms of physiology and behaviour in horses. Biol. Rhythm. Res. 2011, 42, 67–88. [Google Scholar] [CrossRef]
- Burla, J.-B.; Ostertag, A.; Westerath, H.S.; Hillmann, E. Gait determination and activity measurement in horses using an accelerometer. Comput. Electron. Agr. 2014, 102, 127–133. [Google Scholar] [CrossRef]
- Hogan, E.S.; Houpt, K.A.; Sweeney, K. The effect of enclosure size on social interactions and daily activity patterns of the captive asiatic wild horse (Equus Przewalskii). Appl. Anim. Behav. Sci. 1988, 21, 147–168. [Google Scholar] [CrossRef]
- Mal, M.E.; Friend, T.H.; Lay, D.C.; Vogelsang, S.G.; Jenkins, O.C. Behavioral responses of mares to short-term confinement and social isolation. Appl. Anim. Behav. Sci. 1991, 31, 13–24. [Google Scholar] [CrossRef]
- Christensen, J.W.; Ladewig, J.; Søndergaard, E.; Malmkvist, J. Effects of individual versus group stabling on social behaviour in domestic stallions. Appl. Anim. Behav. Sci. 2002, 75, 233–248. [Google Scholar] [CrossRef]
- Winskill, L.C.; Waran, N.K.; Young, R.J. The effect of a foraging device (a modified ‘Edinburgh foodball’) on the behaviour of the stabled horse. Appl. Anim. Behav. Sci. 1996, 48, 25–35. [Google Scholar] [CrossRef]
- Learmonth, M.J. Dilemmas for natural living concepts of zoo animal welfare. Animals 2019, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]

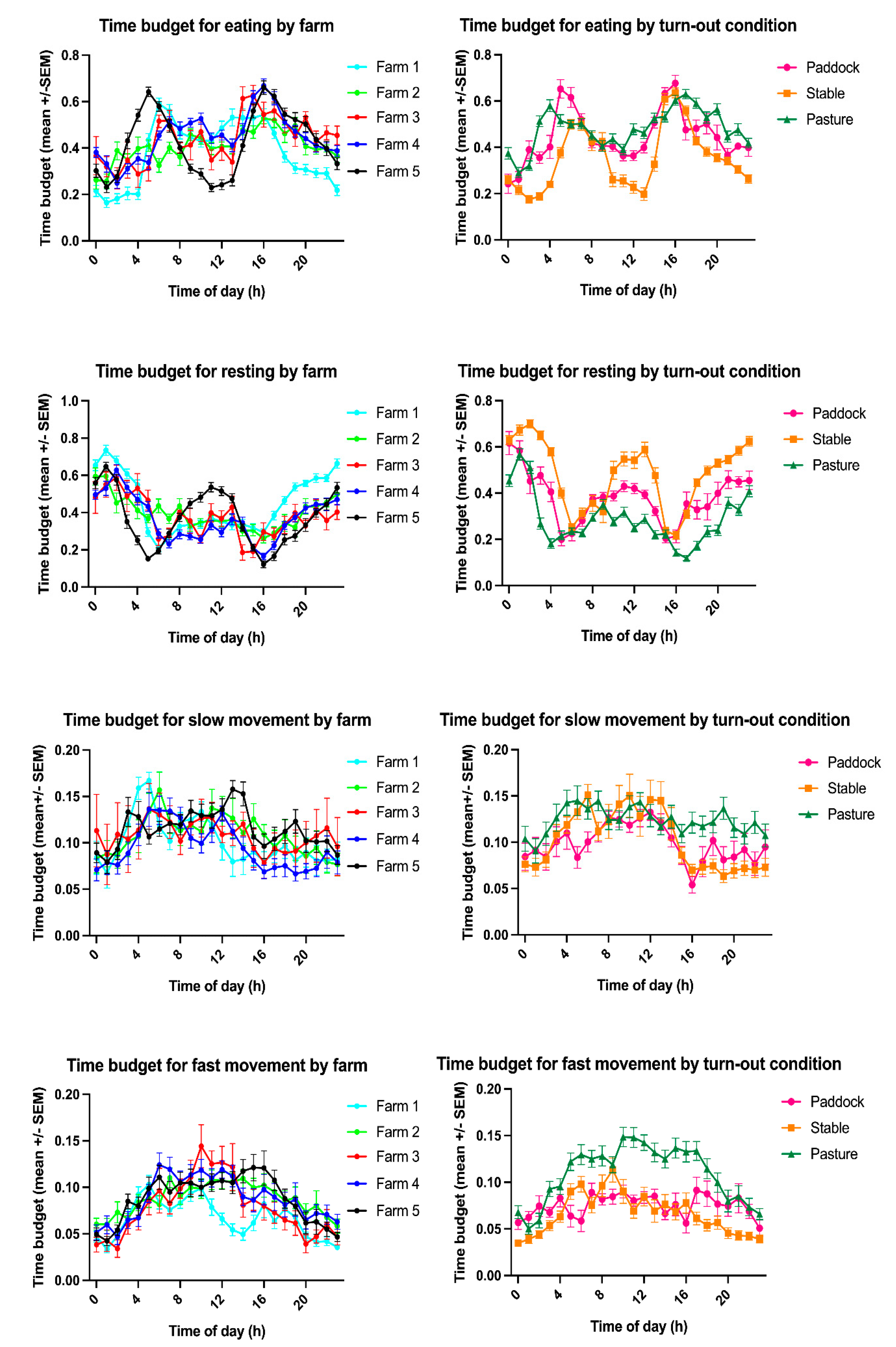

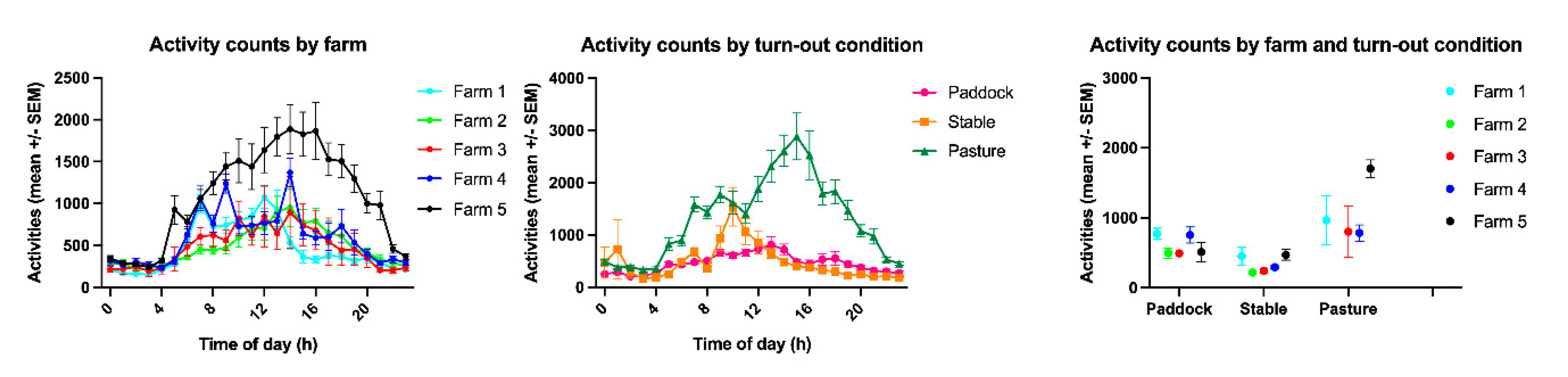
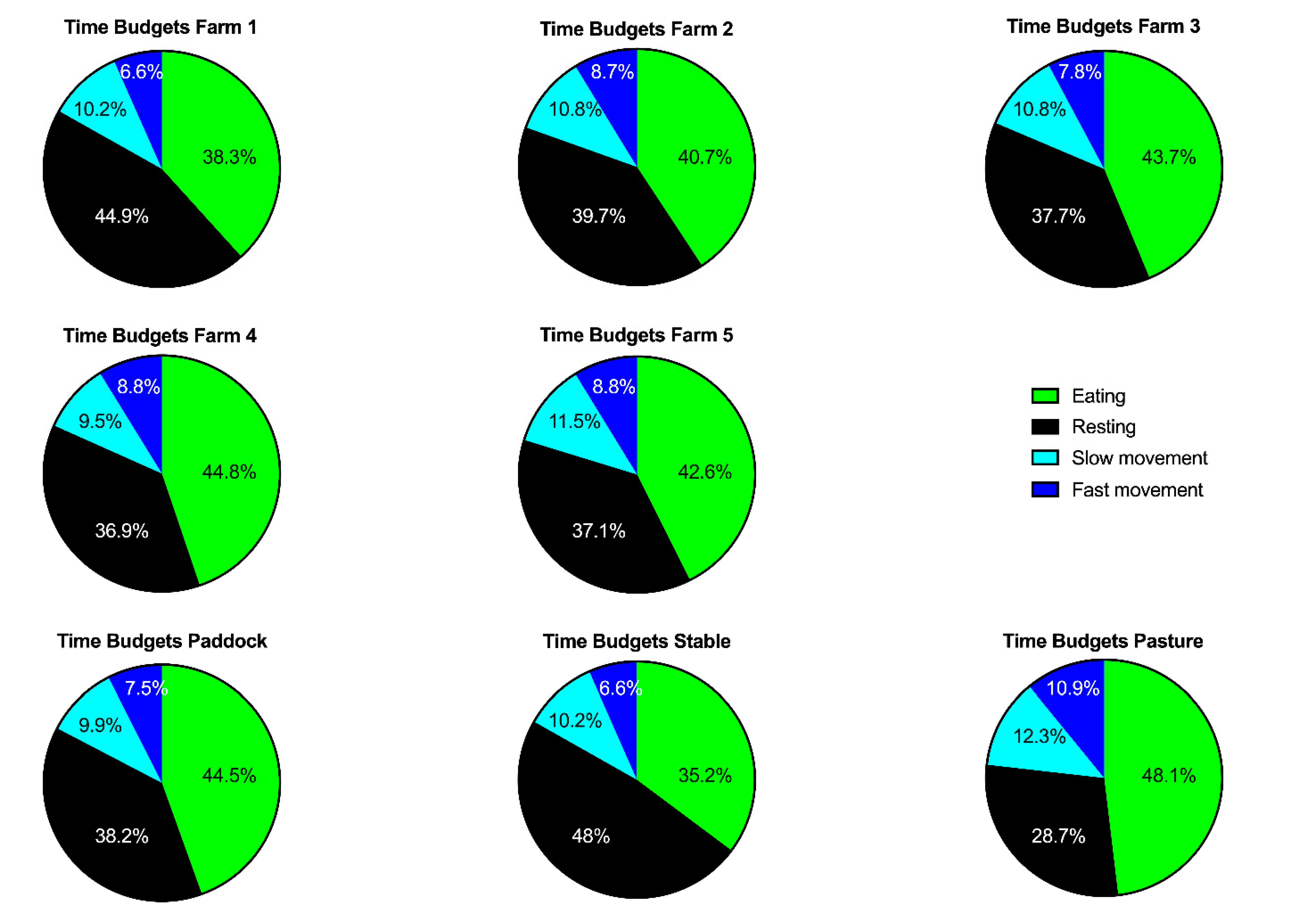
| Farm | Month | Turn-out | Time Budget for Eating | Time Budget for Resting | Time Budget for Slow Movement | Time Budget for Fast Movement | Activity Count | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | 95% Conf. Int. | Mean | s.d. | 95% Conf. Int. | Mean | s.d. | 95% Conf. Int. | Mean | s.d. | 95% Conf. Int. | Mean | s.d. | 95% Conf. Int. | |||||||||||||
| Overall | 42.0 | 29.0 | 42.3 | - | 41.6 | 39.1 | 29.3 | 39.4 | - | 38.7 | 11.0 | 12.7 | 11.1 | - | 10.8 | 8.0 | 10.2 | 8.1 | - | 7.9 | 684.6 | 2531.7 | 716.3 | - | 652.9 | ||
| 1 | Overall | 38.7 | 29.1 | 39.3 | - | 38.1 | 44.4 | 28.7 | 45.0 | - | 43.8 | 10.3 | 12.4 | 10.5 | - | 10.0 | 6.7 | 9.6 | 6.9 | - | 6.4 | 529.6 | 3721.9 | 610.0 | - | 449.2 | |
| May- June | Paddock | 47.5 | 28.1 | 48.9 | - | 46.1 | 33.6 | 25.6 | 34.9 | - | 32.3 | 10.9 | 12.8 | 11.5 | - | 10.2 | 8.0 | 9.6 | 8.5 | - | 7.5 | 873.6 | 2098.9 | 978.6 | - | 768.5 | |
| Stable | 35.9 | 28.9 | 36.8 | - | 35.0 | 47.5 | 29.3 | 48.4 | - | 46.6 | 10.0 | 12.9 | 10.4 | - | 9.6 | 6.6 | 9.6 | 6.9 | - | 6.3 | 492.2 | 5105.5 | 650.4 | - | 334.0 | ||
| Pasture | - | - | - | - | - | ||||||||||||||||||||||
| October-November | Paddock | 43.5 | 30.3 | 45.9 | - | 41.1 | 39.1 | 27.1 | 41.2 | - | 36.9 | 10.9 | 11.9 | 11.9 | - | 10.0 | 6.5 | 9.4 | 7.2 | - | 5.7 | 525.3 | 1099.7 | 611.8 | - | 438.8 | |
| Stable | 34.5 | 27.9 | 35.8 | - | 33.2 | 50.0 | 27.3 | 51.2 | - | 48.7 | 10.1 | 11.5 | 10.6 | - | 9.6 | 5.4 | 9.0 | 5.8 | - | 5.0 | 255.6 | 355.1 | 271.8 | - | 239.4 | ||
| Pasture | 50.2 | 30.2 | 54.2 | - | 46.3 | 30.3 | 26.2 | 33.8 | - | 26.9 | 10.4 | 11.2 | 11.9 | - | 8.9 | 9.1 | 12.2 | 10.7 | - | 7.5 | 1121.5 | 2672.9 | 1472.3 | - | 770.6 | ||
| 2 | Overall | 41.8 | 26.0 | 42.6 | - | 41.0 | 38.0 | 26.9 | 38.9 | - | 37.2 | 11.3 | 12.4 | 11.6 | - | 10.9 | 8.9 | 9.9 | 9.2 | - | 8.6 | 515.1 | 826.1 | 541.1 | - | 489.1 | |
| June | Paddock | 41.8 | 26.0 | 42.7 | - | 40.8 | 38.3 | 26.7 | 39.3 | - | 37.3 | 11.0 | 11.8 | 11.4 | - | 10.6 | 9.0 | 9.9 | 9.3 | - | 8.6 | 641.0 | 988.3 | 679.6 | - | 602.5 | |
| Stable | 33.4 | 24.6 | 39.6 | - | 27.2 | 56.3 | 27.2 | 63.2 | - | 49.5 | 6.1 | 10.6 | 8.8 | - | 3.5 | 4.1 | 7.2 | 5.9 | - | 2.3 | 227.0 | 183.4 | 273.0 | - | 181.0 | ||
| Pasture | - | - | - | - | - | ||||||||||||||||||||||
| November | Paddock | 42.7 | 25.9 | 44.1 | - | 41.2 | 35.8 | 26.7 | 37.3 | - | 34.3 | 12.4 | 13.6 | 13.1 | - | 11.6 | 9.1 | 10.1 | 9.7 | - | 8.6 | 284.5 | 212.1 | 296.4 | - | 272.6 | |
| Stable | 36.9 | 25.3 | 43.4 | - | 30.4 | 53.8 | 27.4 | 60.9 | - | 46.7 | 5.9 | 10.9 | 8.7 | - | 3.1 | 3.4 | 5.8 | 4.9 | - | 1.9 | 202.6 | 133.4 | 237.0 | - | 168.3 | ||
| Pasture | - | - | - | - | - | ||||||||||||||||||||||
| 3 | Overall | 44.3 | 28.8 | 45.5 | - | 43.1 | 35.2 | 28.5 | 36.4 | - | 34.0 | 12.4 | 13.7 | 13.0 | - | 11.9 | 8.0 | 9.6 | 8.4 | - | 7.6 | 436.4 | 677.8 | 473.0 | - | 399.9 | |
| August | Paddock | 49.2 | 34.4 | 58.7 | - | 39.6 | 29.2 | 27.3 | 36.8 | - | 21.6 | 10.3 | 9.0 | 12.8 | - | 7.8 | 11.3 | 14.4 | 15.3 | - | 7.3 | 368.9 | 235.3 | 434.2 | - | 303.7 | |
| Stable | 31.8 | 26.8 | 36.9 | - | 26.7 | 55.3 | 29.0 | 60.7 | - | 49.8 | 10.4 | 10.8 | 12.4 | - | 8.3 | 2.6 | 5.2 | 3.6 | - | 1.6 | 156.6 | 145.6 | 184.2 | - | 129.0 | ||
| Pasture | 49.0 | 28.5 | 50.6 | - | 47.4 | 28.2 | 26.3 | 29.7 | - | 26.7 | 13.7 | 15.3 | 14.5 | - | 12.8 | 9.2 | 9.1 | 9.7 | - | 8.6 | 738.6 | 1056.8 | 845.0 | - | 632.2 | ||
| November | Paddock | 41.6 | 26.8 | 45.3 | - | 38.0 | 33.6 | 22.9 | 36.7 | - | 30.5 | 13.2 | 10.6 | 14.6 | - | 11.8 | 11.6 | 12.7 | 13.3 | - | 9.8 | 487.8 | 388.8 | 540.5 | - | 435.1 | |
| Stable | 37.2 | 27.6 | 39.5 | - | 35.0 | 47.5 | 28.8 | 49.9 | - | 45.2 | 10.1 | 11.2 | 11.0 | - | 9.2 | 5.1 | 8.1 | 5.8 | - | 4.4 | 276.8 | 394.3 | 309.0 | - | 244.6 | ||
| Pasture | - | - | - | - | - | ||||||||||||||||||||||
| 4 | Overall | 44.8 | 27.7 | 45.6 | - | 44.0 | 36.7 | 28.0 | 37.5 | - | 35.9 | 9.8 | 12.1 | 10.2 | - | 9.5 | 8.7 | 11.2 | 9.0 | - | 8.4 | 595.3 | 1120.2 | 627.1 | - | 563.6 | |
| September | Paddock | - | - | - | - | - | |||||||||||||||||||||
| Stable | 43.1 | 26.3 | 45.5 | - | 40.7 | 41.3 | 26.5 | 43.7 | - | 38.9 | 8.7 | 11.0 | 9.7 | - | 7.7 | 6.9 | 8.7 | 7.7 | - | 6.1 | 396.1 | 416.5 | 434.4 | - | 357.8 | ||
| Pasture | 46.0 | 27.6 | 47.1 | - | 44.8 | 32.9 | 27.7 | 34.1 | - | 31.8 | 10.5 | 12.3 | 11.0 | - | 10.0 | 10.6 | 11.9 | 11.1 | - | 10.1 | 788.8 | 1345.2 | 843.8 | - | 733.7 | ||
| January | Paddock | 53.8 | 26.7 | 56.1 | - | 51.5 | 24.4 | 23.1 | 26.3 | - | 22.5 | 11.8 | 12.0 | 12.8 | - | 10.8 | 10.0 | 11.9 | 11.0 | - | 9.0 | 878.6 | 1507.8 | 1006.1 | - | 751.2 | |
| Stable | 40.3 | 27.7 | 41.7 | - | 38.9 | 45.3 | 27.9 | 46.7 | - | 43.9 | 8.4 | 11.8 | 9.0 | - | 7.8 | 6.0 | 9.8 | 6.5 | - | 5.5 | 261.8 | 414.9 | 282.7 | - | 240.9 | ||
| Pasture | |||||||||||||||||||||||||||
| 5 | Overall | 43.3 | 31.3 | 44.1 | - | 42.5 | 36.0 | 31.9 | 36.8 | - | 35.2 | 12.1 | 13.2 | 12.4 | - | 11.7 | 8.7 | 10.6 | 8.9 | - | 8.4 | 1110.8 | 2265.1 | 1166.7 | - | 1054.9 | |
| July- August | Paddock | 35.8 | 28.5 | 44.2 | - | 27.3 | 30.1 | 27.1 | 38.1 | - | 22.2 | 19.5 | 16.5 | 24.4 | - | 14.6 | 14.6 | 9.4 | 17.4 | - | 11.8 | 1574.9 | 1751.2 | 2092.4 | - | 1057.5 | |
| Stable | 26.2 | 25.0 | 28.1 | - | 24.3 | 49.1 | 30.4 | 51.4 | - | 46.8 | 14.8 | 15.6 | 16.0 | - | 13.6 | 9.9 | 11.5 | 10.8 | - | 9.0 | 841.7 | 1893.0 | 986.5 | - | 697.0 | ||
| Pasture | 50.1 | 28.4 | 51.1 | - | 49.2 | 25.6 | 27.8 | 26.6 | - | 24.7 | 13.5 | 13.0 | 13.9 | - | 13.0 | 10.8 | 10.7 | 11.1 | - | 10.4 | 1702.0 | 2783.6 | 1795.3 | - | 1608.6 | ||
| December | Paddock | 38.5 | 35.4 | 40.9 | - | 36.2 | 44.9 | 33.2 | 47.0 | - | 42.7 | 11.1 | 12.4 | 11.9 | - | 10.2 | 5.6 | 9.7 | 6.2 | - | 4.9 | 351.4 | 770.8 | 402.2 | - | 300.6 | |
| Stable | 37.5 | 33.6 | 39.3 | - | 35.7 | 50.6 | 31.8 | 52.3 | - | 48.9 | 7.5 | 11.7 | 8.1 | - | 6.8 | 4.4 | 8.3 | 4.9 | - | 4.0 | 200.0 | 199.2 | 210.8 | - | 189.2 | ||
| Pasture | - | - | - | - | - | ||||||||||||||||||||||
| Time Budget for Eating (%) | Time Budget for Resting (%) | Time Budget for Slow Movement (%) | Time Budget for Fast Movement (%) | Activity Count | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| 00:00 | 25.39 | 23.23 | 45.31 | 25.07 | 8.38 | 11.14 | 4.26 | 7.07 | 340.33 | 2201.22 |
| 01:00 | 23.30 | 22.04 | 48.74 | 23.79 | 7.44 | 11.20 | 3.85 | 6.05 | 336.82 | 2458.94 |
| 02:00 | 23.74 | 22.13 | 46.61 | 24.22 | 8.64 | 11.36 | 4.35 | 6.54 | 237.46 | 898.87 |
| 03:00 | 27.70 | 22.56 | 40.20 | 24.34 | 9.77 | 11.59 | 5.66 | 7.82 | 180.47 | 230.20 |
| 04:00 | 29.87 | 21.17 | 35.61 | 23.02 | 11.18 | 11.74 | 6.67 | 8.47 | 206.21 | 231.46 |
| 05:00 | 35.91 | 20.59 | 28.04 | 19.22 | 11.66 | 11.12 | 7.73 | 8.44 | 368.12 | 489.36 |
| 06:00 | 41.78 | 21.57 | 22.23 | 18.52 | 11.35 | 11.43 | 7.98 | 8.78 | 467.32 | 621.99 |
| 07:00 | 41.35 | 23.27 | 23.66 | 21.49 | 10.37 | 10.27 | 7.95 | 9.05 | 685.41 | 778.00 |
| 08:00 | 35.07 | 23.46 | 30.10 | 22.46 | 10.10 | 9.83 | 8.07 | 8.28 | 634.36 | 800.58 |
| 09:00 | 35.67 | 22.32 | 28.38 | 21.99 | 10.47 | 10.33 | 8.81 | 8.81 | 739.46 | 970.91 |
| 10:00 | 35.45 | 21.67 | 28.02 | 20.62 | 10.35 | 9.65 | 9.52 | 9.76 | 730.83 | 1222.96 |
| 11:00 | 31.19 | 21.45 | 32.13 | 22.04 | 11.02 | 10.08 | 9.00 | 8.78 | 714.23 | 1118.62 |
| 12:00 | 33.23 | 21.71 | 30.84 | 21.55 | 10.43 | 9.79 | 8.83 | 8.29 | 827.57 | 1462.30 |
| 13:00 | 32.09 | 21.83 | 32.26 | 21.47 | 10.25 | 10.39 | 8.74 | 8.84 | 832.82 | 1690.96 |
| 14:00 | 41.83 | 22.40 | 24.53 | 20.52 | 9.47 | 10.11 | 7.50 | 8.25 | 878.74 | 1364.45 |
| 15:00 | 46.91 | 22.96 | 20.57 | 18.76 | 8.33 | 9.51 | 7.52 | 9.09 | 715.40 | 1325.86 |
| 16:00 | 49.95 | 20.72 | 18.10 | 17.11 | 7.54 | 8.77 | 7.75 | 9.68 | 723.97 | 1549.86 |
| 17:00 | 46.54 | 21.04 | 21.60 | 19.01 | 8.03 | 9.76 | 7.16 | 8.46 | 614.44 | 1008.52 |
| 18:00 | 40.30 | 21.49 | 28.16 | 21.41 | 8.30 | 9.75 | 6.58 | 8.30 | 627.81 | 902.45 |
| 19:00 | 37.85 | 22.14 | 31.11 | 22.61 | 8.06 | 10.07 | 6.31 | 8.67 | 517.78 | 735.37 |
| 20:00 | 36.72 | 22.55 | 33.75 | 23.49 | 8.07 | 10.15 | 4.79 | 7.11 | 423.57 | 508.00 |
| 21:00 | 32.37 | 22.37 | 37.77 | 22.80 | 8.27 | 11.01 | 4.93 | 6.78 | 359.12 | 446.76 |
| 22:00 | 33.05 | 22.32 | 37.09 | 23.31 | 8.34 | 10.56 | 4.85 | 7.06 | 258.25 | 293.40 |
| 23:00 | 29.69 | 22.22 | 41.91 | 23.92 | 7.65 | 10.29 | 4.07 | 6.13 | 230.49 | 218.50 |
| Time Budget | Farm | Sex | Age | Lame | Season | Horse | R2 |
|---|---|---|---|---|---|---|---|
| Eating | 0.09 *** | 0.01 | 0.00 | 0.01 * | 0.04 *** | 0.90 *** | 0.61 |
| Resting | 0.15 *** | 0.00 | 0.00 | 0.01 | 0.27 *** | 0.66 | 0.26 |
| Active | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 * | 0.31 | 0.10 |
| H Act. | 0.01 ** | 0.00 | 0.01 ** | 0.00 | 0.05 *** | 0.21 *** | 0.20 |
| Farm | Turn-Out | Time of Day | Health/Age Group | ||
|---|---|---|---|---|---|
| Eating | p-value | 0.0004 | 0.0001 | 0.0001 | 0.09 |
| Dfn | 4 | 2 | 23 | 3 | |
| Dfd | 240 | 240 | 2298 | 131 | |
| F | 5.332 | 35.7 | 44.7 | 2.21 | |
| Resting | p-value | 0.013 | 0.001 | 0.0001 | 0.106 |
| Dfn | 4 | 2 | 23 | 3 | |
| Dfd | 251 | 251 | 2298 | 131 | |
| F | 3.23 | 89.3 | 71.78 | 2.077 | |
| Slow movement | p-value | 0.4798 | 0.0056 | 0.0001 | 0.925 |
| Dfn | 2 | 4 | 23 | 3 | |
| Dfd | 240 | 240 | 2298 | 131 | |
| F | 5.293 | 0.8746 | 11.03 | 0.157 | |
| Fast movement | p-value | 0.6772 | 0.0001 | 0.0001 | 0.601 |
| Dfn | 4 | 4 | 23 | 3 | |
| Dfd | 240 | 240 | 2298 | 131 | |
| F | 0.5802 | 18.6 | 24.36 | 0.624 | |
| Activity Count | p-value | 0.0239 | 0.0001 | 0.0001 | 0.504 |
| Dfn | 2 | 4 | 23 | 3 | |
| Dfd | 236 | 236 | 2272 | 129 | |
| F | 34.1 | 2869 | 22.78 | 0.785 | |
| Age | Sex | BCS | Edible Bedding | Extra Food | Eating | Resting | Slow Movement | Fast Movement | Activity Count | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | −0.05 | −0.38 *** | 0 | 0.28 *** | −0.1 | 0.17 * | 0.05 | −0.15 * | −0.1 |
| Sex | −0.05 | 1 | −0.18 * | 0.1 | 0.11 | 0.03 | −0.08 | 0.04 | 0.07 | 0.11 |
| BCS | −0.38 *** | −0.18 * | 1 | 0.2 ** | −0.36 *** | −0.18 ** | 0.18 * | −0.06 | −0.05 | −0.1 |
| Edible Bedding | 0 | 0.1 | 0.2 ** | 1 | −0.25 *** | −0.15 * | 0.24 ** | 0 | −0.25 *** | −0.22 ** |
| Extra Food | 0.28 *** | 0.11 | −0.36 *** | −0.25 *** | 1 | 0.24 ** | −0.28 *** | 0.11 | 0.15 * | 0.36 *** |
| Eating | −0.1 | 0.03 | −0.18 ** | −0.15 * | 0.24 ** | 1 | −0.66 *** | −0.22 ** | 0.05 | 0.24 ** |
| Resting | 0.17 * | −0.08 | 0.18 * | 0.24 ** | −0.28 *** | −0.66 *** | 1 | −0.41 *** | −0.53 *** | −0.57 *** |
| Slow Movement | 0.05 | 0.04 | −0.06 | 0 | 0.11 | −0.22 ** | −0.41 *** | 1 | 0.31 *** | 0.22 ** |
| Fast Movement | −0.15 * | 0.07 | −0.05 | −0.25 *** | 0.15 * | 0.05 | −0.53 *** | 0.31 *** | 1 | 0.66 *** |
| Activity count | −0.1 | 0.11 | −0.1 | −0.22 ** | 0.36 *** | 0.24 ** | −0.57 *** | 0.22 ** | 0.66 *** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelemen, Z.; Grimm, H.; Vogl, C.; Long, M.; Cavalleri, J.M.V.; Auer, U.; Jenner, F. Equine Activity Time Budgets: The Effect of Housing and Management Conditions on Geriatric Horses and Horses with Chronic Orthopaedic Disease. Animals 2021, 11, 1867. https://doi.org/10.3390/ani11071867
Kelemen Z, Grimm H, Vogl C, Long M, Cavalleri JMV, Auer U, Jenner F. Equine Activity Time Budgets: The Effect of Housing and Management Conditions on Geriatric Horses and Horses with Chronic Orthopaedic Disease. Animals. 2021; 11(7):1867. https://doi.org/10.3390/ani11071867
Chicago/Turabian StyleKelemen, Zsofia, Herwig Grimm, Claus Vogl, Mariessa Long, Jessika M. V. Cavalleri, Ulrike Auer, and Florien Jenner. 2021. "Equine Activity Time Budgets: The Effect of Housing and Management Conditions on Geriatric Horses and Horses with Chronic Orthopaedic Disease" Animals 11, no. 7: 1867. https://doi.org/10.3390/ani11071867
APA StyleKelemen, Z., Grimm, H., Vogl, C., Long, M., Cavalleri, J. M. V., Auer, U., & Jenner, F. (2021). Equine Activity Time Budgets: The Effect of Housing and Management Conditions on Geriatric Horses and Horses with Chronic Orthopaedic Disease. Animals, 11(7), 1867. https://doi.org/10.3390/ani11071867






