Simple Summary
Mastitis remains one of dairy cattle’s most perplexing and expensive diseases. This study is the first to look into the virulence traits, antimicrobial and biocide resistance, and epidemiological typing of Streptococcus uberis (S. uberis) isolated from bovine clinical mastitis in dairy farms of diverse hygienic interventions in Egypt. The overall S. uberis infection rate was 20.59%; all were multidrug-resistant (MDR). The sua gene was the most frequent virulence gene (42.02%), followed by pauA (40.57%), cfu (21.73%), skc (20.28%), and opp (11.59%). The erm(B) gene serves as the predominant antimicrobial-resistant gene (75.36%), followed by fexA (52.63%) and tet(M), blaZ, and aac(6′)aph(2″) genes (46.38% each). Of note, 79.71% of S. uberis isolates carried qac genes; among them, 55 (79.71%), 54 (78.26%), and 13 (18.84%) harbored qacED1, qacC/D, and qacA/B genes, respectively. Restriction fragment length polymorphism–polymerase chain reaction (RFLP–PCR) indicated that all analyzed isolates were S. uberis type I by their unique RFLP pattern. This study shows a significant variation in the occurrence of virulent S. uberis in dairy cows with clinical mastitis regarding the prospective hygienic concerns. Furthermore, MDR coupled with the existence of biocide resistance genes indicates the importance of S. uberis surveillance and the prudent use of antimicrobials in veterinary clinical medicine to avoid the dissemination of antimicrobial resistance.
Abstract
Mastitis remains a serious problem for dairy animals. The misappropriation of antimicrobial agents helps accelerate resistance, which poses a serious challenge in controlling environmental S. uberis infection. Here, we study the virulence attributes, antimicrobial and biocide resistance, and epidemiological typing of S. uberis recovered from bovine clinical mastitis in dairy farms of diverse hygienic interventions in Egypt. The overall S. uberis infection rate was 20.59%; all were multidrug-resistant (MDR). The sua gene was the most frequent virulence gene (42.02%), followed by pauA (40.57%), cfu (21.73%), skc (20.28%), and opp (11.59%). The erm(B) gene served as the predominant antimicrobial-resistant gene (75.36%), followed by fexA (52.63%) and tet(M), blaZ, and aac(6′)aph(2″) genes (46.38% each). Of note, 79.71%, 78.26%, and 18.84% of S. uberis isolates harbored qacED1, qacC/D, and qacA/B genes, respectively. All analyzed isolates were S. uberis type I by their unique RFLP–PCR pattern. In conclusion, the sustained presence of pauA and sua genes throughout the investigated farms contributes to a better understanding of the bacterium’s pathogenicity. Furthermore, MDR coupled with the existence of biocide resistance genes indicates the importance of S. uberis surveillance and the prudent use of antimicrobials in veterinary clinical medicine to avoid the dissemination of antimicrobial resistance.
1. Introduction
Mastitis is a significant concern affecting dairy animals worldwide, causing great losses to breeders and impacting the country’s national income [1,2]. Environmental streptococci, notably Streptococcus uberis (S. uberis), are among the main contributing agents of mastitis in many countries and have increased their significance for udder health in recent decades [3]. This pathogen is not obligatorily adapted to the udder but is ubiquitous as it is considered an environment-associated straw bedding and pasture pathogen [4]. Since S. uberis is the prime pathogen in a dairy herd, frequent antimicrobial treatments and several environmental factors favor the development of this form of mastitis [3].
Streptococcus uberis has previously been categorized into two distinct types, I and II; both were isolated from bovine mastitis cases, the latter being reclassified as Streptococcus parauberis (S. parauberis) [5]. It is impossible to differentiate between S. uberis and S. parauberis using phenotypic methods [6]. However, S. uberis isolates were verified by 16S rRNA gene restriction fragment length polymorphism (RFLP) using the HhaI restriction endonuclease for further identification of the S. uberis genetic variation [7].
Despite the economic effect of the high prevalence of environmental streptococci in dairy herds, virulence factors related to the pathogenicity of S. uberis are not well characterized; these comprise a significant existential threat to the implementation of control strategies [8]. Various potentially virulence factors were identified for S. uberis, among these, sua, cfu, opp, skc, and pauA, that play prominent roles in the adherence and early colonization of bovine mammary epithelial cells [9,10,11].
Antimicrobial resistance is one of the world’s leading threats to human and animal health [12]. It appears to have an extreme occurrence among streptococcal isolates of mastitis in Egypt [13] and S. uberis in many countries [4,14,15]. However, this susceptibility can vary from one region to another. Even within the same region, it is necessary to monitor the pathogens’ resistance to the antimicrobials used in the treatment of mastitis in various areas [16]. In Egypt, most bovine mastitis studies have focused on the inclusion of Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae, and, infrequently, S. uberis [13,17,18].
Disinfectants based on quaternary ammonium compounds (QACs) have a wide range of veterinary medicine implementations and are critical in controlling animal diseases. They are widely used worldwide, which can contribute to bacterial resistance [19].
In Egypt, there has been no exploration of the existence of S. uberis-associated virulence genes in mastitic dairy cows and the plausible allocation of virulence dynamics in the distinct hygiene measures applied in dairy farms. Moreover, there are few studies on S. uberis resistance to antimicrobials as well as biocides. Therefore, this study was designed to explore the following points: (i) ascertaining the infection rate of S. uberis in dairy cows and the hygiene correlation with its abundance, (ii) detecting the most prospective virulence-associated, antimicrobial and antiseptic resistance genes in environmental S. uberis isolates using conventional PCR, and (iii) determining the genotypic variation among the virulent S. uberis isolates using RFLP–PCR.
2. Materials and Methods
2.1. Lactating Cows and Husbandry Practices
The lactating cows under study were chosen from dairy farms of three distinct hygienic interventions in Alexandria (A) and Sharkia Governorates (B), as well as some individual smallholder cases in different villages of Sharkia Governorates (C and D), over a year, from July 2017 to August 2018. The udder of each lactating cow was screened for recurrent clinical mastitis. Hygienic interventions were based entirely on the following criteria: (i) periodic monitoring of mastitis by an indirect field check during the lactation season, such as the California mastitis test, (ii) pre-milking procedures, such as udder washing and pre- and post-milking teat dipping with antimicrobial dip; and (iii) dry period treatment after the last lactation, bedding materials, and environmental hygiene as well as balanced food. In the first farm (A; n = 75), lactating cows were milked three times daily through a computerized system using pre- and post-teat dipping. This farm followed the standard routine management, vaccination program, and control measures against infectious diseases with the implementation of all hygienic measures. On the second farm (B; n = 50), cows were milked three times daily using a machinery system with post-milking teat dipping and fair, moderate hygienic measures; the cows were placed in straw-bedding barns. The cows of the third farm (C; n = 120) and smallholder cows (D; n = 90; reared by local farmers in the villages of Sharkia Governorate) were grazed; thus, infection with S. uberis from environmental pasture reservoirs was expected. These animals lived in unhygienic environments and were fed on low nutrient rations. The cows were milked twice daily by hand, and there was no disinfection during the milking process.
2.2. Milk Sampling and Isolation of S. uberis
Three hundred and thirty-five milk samples were collected aseptically, just before treatment, from the affected mammary quarters that had clinical signs of abnormal secretions, containing clots or flakes, with udders showing inflammatory symptoms, with or without systemic reaction appearing on the cows. These samples were placed in sterile screw-capped test tubes, kept in an insulated icebox, then transported to the laboratory for further bacteriological and molecular investigations. Bacteriological analysis of milk samples was carried out following conventional protocols [20]. A milk sample loopful was plated onto Edward’s agar medium (Oxoid, Hampshire, UK) and incubated at 37 °C for 24 h. A single, well-isolated colony was subcultured onto a blood agar base (Oxoid, Hampshire, UK) enriched with 7% sterile defibrinated sheep blood and incubated aerobically at 37 °C for 24–48 h. The bacterial isolates were described based on their classic morphological and hemolytic characteristics. Suspected streptococci isolates microscopically appeared as Gram-positive cocci, either in long or short chains. Standard biochemical tests, including catalase, sodium hippurate, and esculin hydrolysis, were carried out [21]. A Christie, Atkins, and Munch-Petersen (CAMP) test was applied [22]. Growth in the presence of 6.5% NaCl at 10 or 45 °C and pH 9.6, combined with resistance to bile salt, was investigated [23].
2.3. Antimicrobial Susceptibility Testing
The Kirby-Bauer disc diffusion test was used to determine the antimicrobial susceptibilities of S. uberis isolates [24]. Commercial discs with the following antimicrobials (Oxoid, Hampshire, England, UK), commonly used in veterinary practices or for public health issues, were selected to perform the antibiogram: penicillin (10 IU), ampicillin (10 µg), amoxicillin (25 μg), amoxicillin–clavulanic acid (20/10 μg), cloxacillin (1 μg), cefoperazone (75 μg), ceftriaxone (30 μg), cephalexin (30 μg), imipenem (10 μg), tetracycline (30 μg), clindamycin (2 μg), erythromycin (15 μg), streptomycin (10 μg), neomycin (30 µg), gentamycin (10 µg), kanamycin (30 µg), novobiocin (30 µg), ciprofloxacin (5 μg), chloramphenicol (30 µg), and trimethoprim–sulphamethoxazole (23.75/1.25 μg). The interpretive criteria used for categorizing an isolate as sensitive or resistant to an antimicrobial agent are established in the Clinical and Laboratory Standards Institute guidelines [25]. Isolates showing resistance to at least three different antimicrobial classes are categorized as multidrug-resistant (MDR) [26]. MAR indices were estimated for each antimicrobial and isolate [27].
2.4. DNA Extraction and Molecular Identification of S. uberis
Streptococcus uberis isolates were cultured in tryptone soya broth (TSB, Oxoid, Hampshire, England, UK) at 37 °C for 24 h. Bacterial DNA was extracted using a QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany), as recommended by the manufacturer. PCR amplification of the tuf gene of Streptococci species [28] and the 16S rRNA gene of S. uberis [29] was performed using the oligonucleotide primer pairs listed in Table S1 to confirm the conventional bacteriological identification.
2.5. PCR Amplifications of Virulence Attributes and Antimicrobial and Biocide Resistance Genes
Virulence genes for S. uberis, cfu (encoding for CAMP factor), opp (oligopeptide binding protein), sua (S. uberis adhesion molecule), pauA (plasminogen activator), and skc (streptokinase activator), were investigated [9,11,30]. The occurrence of antimicrobial resistance genes, conferring resistance to penicillins (blaZ), phenicols (fexA), aminoglycosides (aac(6′)aph(2″)), tetracyclines (tet(M), tet(O), tet(L) and tet(K)), macrolides (erm(A), erm(B) and erm(C)), sulfonamide (sul1), and trimethoprim (dfrA) was examined [31,32,33,34,35,36,37,38,39,40]. Moreover, PCR targeting qac genes, qacA/B, qacC/D, and qacED1, conferring a high level of resistance to antiseptics, was applied [41,42]. Oligonucleotide primer sets and thermal cycling profiles are described in Table S1. The amplification reaction for each gene was conducted with a final volume of 25 μL of the following reaction mixture: 12.5 μL DreamTaq Green PCR Master Mix (2X) (Thermo Fisher Scientific, Waltham, MA, USA), 1 μL of each primer (20 pmole), 2 μL template DNA, and 8.5 μL water nuclease-free in a programmable thermal cycler PTC-100 TM (MJ Research Inc., Waltham, MA, USA). S. uberis ATCC® 27958™ was used as a reference strain. PCR products were analyzed by agarose gel electrophoresis, stained with ethidium bromide (0.5 µg/mL), and visualized using an ultraviolet transilluminator (Spectroline, Wesbury, Meadville, PA, USA).
2.6. PCR–RFLP
Epidemiological typing of recovered S. uberis isolates was then performed using HhaI restriction endonuclease (Thermo Fisher Scientific, Waltham, MA, USA), as described previously [7]. Aliquots of the amplified restriction endonuclease-digested fragments were electrophoresed on 0.5 μg/mL ethidium bromide (Sigma-Aldrich, Chemie GmbH, Schnelldorf, Germany) stained agarose gel with a 100 bp standard DNA molecular weight ladder (Fermentas, Inc., Hanover, NH, USA). The numbers of DNA fragments and their sizes in base pairs were then assessed using Pro-Score/RFLP software version 2.39 (DNA ProScan, Inc.; Nashville, TN, USA).
2.7. Bioinformatics and Statistical Analysis
The overall distribution of the antimicrobial resistance phenotypes, virulence-associated genes, and antimicrobial and biocide resistance genes in S. uberis isolates was visualized using a heatmap. The clustering pattern of the isolates and various features were determined by hierarchical clustering dendrogram [43]. These analyses were done using R software (version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria), package pheatmap. To estimate the similarity among S. uberis isolates from various farms, the binary distances were calculated among isolates based on the presence or absence of the four studied features (virulence, resistance phenotype/genes, and biocide resistance genes). This analysis was done using the functions dist and hlcust in the R environment. Correlation analyses were done on the raw data after data conversion to binary outcomes (1 = feature presence, 0 = feature absence). The correlation was estimated on a scale from +1 to −1. The significance of the correlation was assessed at a significance level of 0.05. The variables that have similar occurrences in all isolates were excluded from this analysis. The correlation analyses and visualization were done using R packages corrplot, heatmaply, hmisc, and ggpubr [44,45,46]. Fisher’s exact two-tailed test [47] was used to study the infection rates of S. uberis among farms of varying hygiene interventions and their antimicrobial resistance; p- values ˂ 0.05 were statistically significant.
3. Results
3.1. Infection Rate and Characterization of S. uberis in Clinically Mastitic Dairy Cows
The overall infection rate of S. uberis was 20.59% (69/335), which significantly (p < 0.05) differed between farms, being 8.8% (11/125) in animals living in farms with adequately applied hygiene measures and 27.61% (58/210) in animals living on low hygiene, hand machine farms and smallholders. On Edward’s media, S. uberis isolates appeared as colorless dewdrop-like, pinpoint rounded colonies. Phenotypic characteristics of the isolates denoted Gram-positive cocci, arranged mainly in chains and, sometimes, in diplococci. They showed β or γ hemolytic colonies on blood agar media. CAMP-like hemolytic activities were determined, together with beta-toxin-producing Staphylococcus aureus, on sheep blood agar in 60 out of 69 (86.9%) S. uberis isolates. Biochemically, S. uberis isolates were catalase-test-negative, whereas all isolates were positive for sodium hippurate and bile-esculin hydrolyses tests. The isolates fail to grow on MacConkey’s agar, media containing 6.5% NaCl, or at 45 °C, which is characteristic for S. uberis.
3.2. Antimicrobial Resistance Patterns of S. uberis Isolates
The antimicrobial resistance of S. uberis isolates (n = 69) was validated against 21 antimicrobials of 12 chemotherapeutic classes. As shown in Table 1 and Figure 1, S. uberis exhibited 100% resistance to cloxacillin, ceftriaxone, cephalexin, clindamycin, and novobiocin. Moreover, high levels of resistance were reported for ampicillin (89.85%), streptomycin (86.96%), penicillin (79.71%), and erythromycin (73.91%). On the other hand, kanamycin (30.43%), cefoperazone (26.04%), ciprofloxacin (21.74 %), and gentamycin (20.28%) showed the lowest resistance levels, and none of the isolates exhibited imipenem resistance. Of note, all S. uberis isolates were MDR, with MAR indices ranged from 0.38–0.81, whereas the MAR indices for tested antimicrobials were up to 0.048. Statistical analysis revealed a significant variation in the resistance levels of S. uberis isolates to various antimicrobial agents (p ˂ 0.05).

Table 1.
Antimicrobial resistance of S. uberis isolated from lactating cows with clinical mastitis.
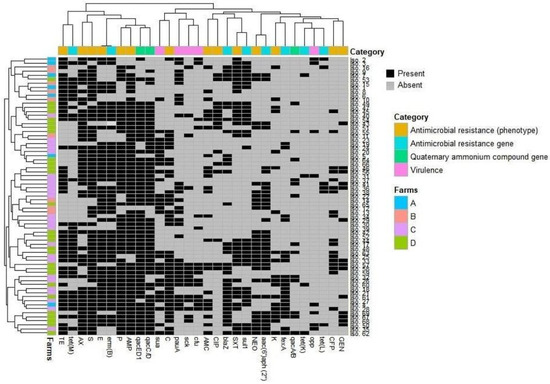
Figure 1.
Overall occurrence and clustering of S. uberis isolates (n = 69) in the investigated farms, their virulence attributes, and antimicrobial and biocide resistance patterns. The heatmap shows the occurrence of features in all isolates. The dendrogram indicates the hierarchical clustering of features and isolates. Different farms and feature categories are color-coded, as shown in the label. GEN, gentamycin; CFP, cefoperazone; K, kanamycin; NEO, neomycin; SXT, trimethoprim-sulfamethoxazole; CIP, ciprofloxacin; AMC, amoxicillin–clavulanic acid; C, chloramphenicol; AMP, ampicillin; P, penicillin; E, erythromycin; S, streptomycin; AX, amoxicillin; TE, tetracycline.
3.3. Molecular Characteristics and Virulence Gene Profiling of S. uberis
Conventional identification of S. uberis isolates (n = 69) was confirmed by PCR-based amplification of the genus-specific tuf gene (DNA fragment ~196 bp). Further, the 16S rRNA gene identified S. uberis at the species level (DNA product ~854 bp). S. uberis isolates were tested by PCR for the existence of five major genes potentially involved in virulence (Table 2). The most frequent gene was sua (42.02%), followed by pauA (40.57%), cfu (21.73%), and skc (20.28%). In contrast, the opp gene was detected with a low percentage (11.59%). The frequency of putative virulence gene patterns among S. uberis isolates is summarized in Table 3. Most of the examined isolates (58/69; 84.06%) harbored at least one virulence gene. Moreover, 11 of 69 isolates (15.94%) possessed simultaneously 3 to 4 virulence-associated genes, and 7 (10.14%) S. uberis isolates carried 2 different virulence-related genes. The most frequent virulence gene pattern was sua + pauA + skc + cfu, which was observed in 8 of 69 isolates (11.59%) from 2 different herds (C and D) of low hygiene measures.

Table 2.
Virulence traits and antimicrobial and biocide resistance profiles of S. uberis (n = 69) isolated from dairy cows of different hygiene interventions.

Table 3.
Virulence gene profiles of S. uberis isolated from lactating cattle experience clinical mastitis.
3.4. Detection of Antimicrobial Resistance Genes in S. uberis Isolates
The detection of antimicrobial resistance genes confirmed the phenotypic resistance patterns of the respective S. uberis isolates (Table 2). As presented in Figure 1, the erythromycin resistance gene erm(B) was the most prevalent among the analyzed isolates (75.36%). However, erm(C) and erm(A) genes were not amplified in either erythromycin-resistant or erythromycin-susceptible S. uberis isolates. The most frequent tetracycline resistance gene was tet(M) (46.38%), whereas tet(L) and tet(K) genes were recorded in lower frequencies (8.7 and 5.8%, respectively), and the tet(O) gene was not detected in any of the tested S. uberis isolates. The blaZ and aac(6′)aph(2″) genes, conferring resistance to penicillins and aminoglycosides, respectively, were similarly found in 32 out of 69 examined isolates (46.38% each). Furthermore, the fexA gene, conferring resistance to chloramphenicol, was detected in 20 S. uberis isolates (28.99%). The sulfonamide resistance gene, sul1, was found in 31 (44.93%) S. uberis isolates, but the trimethoprim dfrA gene was not detected in any analyzed isolate.
3.5. Biocide Resistance Genes and Biocide–Antimicrobial Cross-Resistance
Biocide resistance profiling showed that 55 out of 69 S. uberis isolates (79.71%) carried qac genes; among them, 55 (79.71%), 54 (78.26%), and 13 (18.84%) exhibited resistance to qacED1, qacC/D, and qacA/B, respectively. Biocide resistance gene combinations were detected among the isolates; 3 gene combinations were found in 11 (15.94%) isolates, and 2 combinations, either qacED1 + qacC/D (38/69, 55.07%) or qacED1 + qacA/B (2/69, 2.9%), were also reported (Table 2). The selective pressure employed by exposure to biocides may be associated with increasing antimicrobial resistance. As shown in Figure 2 and Table S2, significant (p ˂ 0.05) positive correlations (r = 0.01–0.43) between QAC tolerance and resistance to various antimicrobials indicate the pervasive occurrence of multi-drug efflux pumps. However, non-significant (p ˃ 0.05) negative correlations were observed between the existence of qac genes and resistance to certain antimicrobials such as amoxicillin–clavulanate (r = −0.01, −0.1 and −0.05), tetracycline (r = −0.06, −0.03 and −0.01), and trimethoprim-sulfamethoxazole (r = −0.02, −0.01 and −0.05) for qacED1, qacC/D, and qacA/B genes, respectively.
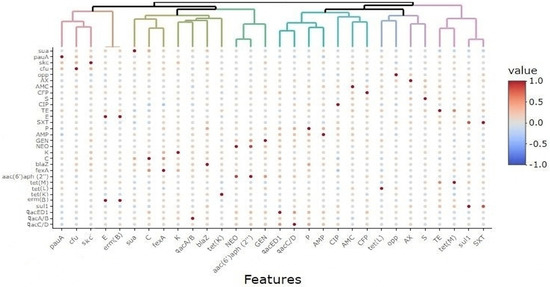
Figure 2.
Correlations among various features in S. uberis isolates (n = 69) from various farms. The color scale represents the correlation coefficient (R) on a scale from +1 to −1 (+1 is the highest positive correlation, and −1 is the highest negative correlation). AX, amoxicillin; AMC, amoxicillin–clavulanic acid; CFP, cefoperazone; S, streptomycin; CIP, ciprofloxacin; TE, tetracycline; E, erythromycin; SXT, trimethoprim-sulfamethoxazole; P, penicillin; AMP, ampicillin; GEN, gentamycin; NEO, neomycin; K, kanamycin; C, chloramphenicol.
3.6. Typing of Virulent S. uberis Isolates Using RFLP–PCR
Phenotypically, S. uberis type I and S. parauberis (S. uberis type II) isolates had similar cultural, morphological, and biochemical characteristics and could not be differentiated by conventional methods. Therefore, RFLP–PCR analysis of the 16S rRNA gene was used to characterize them, and the results indicated that all isolates (n = 69) were indeed S. uberis (S. uberis type I) by their unique RFLP pattern (Figure S1).
3.7. Association between the Existence of Virulence Traits, Antimicrobial and Biocide Resistance, and Hygienic Interventions for Dairy Cows
As shown in Table 2, the occurrence of virulence-associated and antimicrobial- and QAC resistance genes was distributed among S. uberis isolates (n = 69) over all the investigated dairy farms. However, the simultaneous existence of four virulence genes (sua + pauA + skc + cfu, 11.59%), more than four antimicrobial resistance genes (17.39%), and the three tested qac genes (qacA/B + qacED1 + qacC/D, 15.94%) was reported only in C and D dairy herds. However, nine (13.04%) S. uberis isolates recovered from the C and D dairy herds did not carry any QAC resistance genes. Overall, as presented in Figure 3, the four studied features (virulence, resistance phenotype/genes, and qac genes) were prominent in the dairy herds with moderate and low hygiene measures (C and D, respectively).
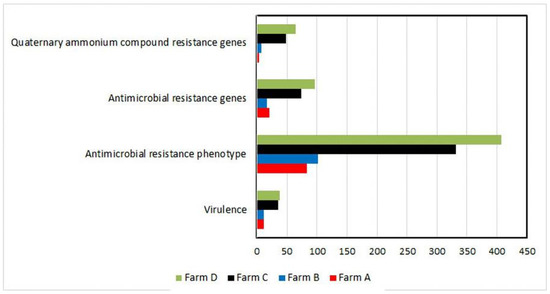
Figure 3.
Differences among farms are shown in the term of studied features. Each horizontal bar represents the overall number of isolates (x-axis) showing a certain feature (including redundancy). Farms are shown in different colors. Farm D possessed the highest number of isolates harboring the studied features compared to other farms.
Figure 4 and Table S3 demonstrate that S. uberis isolates (n= 69) had a low-to-moderate diversity (Euclidean distance = 0.11–0.73) among the investigated dairy herds. The dendrogram analysis (Figure 5) classified the isolates into four clusters (1, 2, 3, and 4). A close relatedness was noticed among certain S. uberis isolates from different dairy herds. Most S. uberis isolated from C and D dairy herds were closely related and gathered in clusters 1 and 2. In addition, S. uberis of A and B dairy herds were clustered closely in cluster 4. Few isolates of the four dairy herds clustered together in cluster 3.
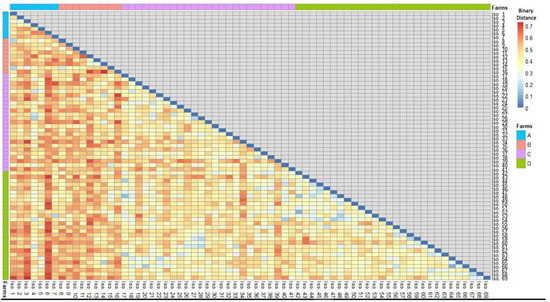
Figure 4.
A heatmap showed the binary distances among S. uberis isolates based on the presence or absence of the four studied features (Scheme 0.7).
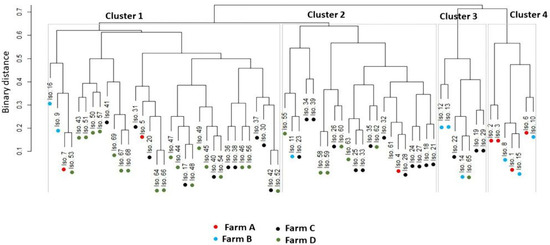
Figure 5.
Hierarchical clustering dendrogram showing the relatedness (closeness) of various isolates (shown as numbers) from different farms (shown as colored dots) based on all the feature categories. All isolates were classified into four clusters.
4. Discussion
Mastitis remains a critical problem for dairy animals, causing drastic losses during lactation seasons. Such losses are attributed mainly to decreased milk yield, lower milk quality, and higher treatment and control costs [48]. S. uberis is a well-known pathogen that causes bovine intramammary infections worldwide. Nonetheless, there are scant epidemiological data on S. uberis isolated from lactating cows in Egypt, especially in the smallholder production system, despite the fact that this extensive system type is the most common traditional livestock farming system among Egyptian farmers [49,50].
In the current study, the overall infection rate of S. uberis in dairy cows of different parity, showing gross signs of clinical mastitis associated with or without systemic reactions, was 20.59%, which is nearly similar to a previously published work (23.5%) [51]. Comparatively lower infection rates (6.3%, 9.3%, and 11.8%, respectively) of S. uberis isolated from mastitic cattle were previously reported by several studies [52,53,54]. Higher rates of S. uberis infection (55.38 and 33%, respectively) were reported in previous studies [55,56]. Our findings may represent a potential hygiene deficiency that has a significant role in the occurrence of environmental S. uberis mastitis [57].
Animals with adequate hygiene during milking (A and B dairy herds) had a lower prevalence of infection (11/125; 8.8%) than those with poor hygiene (C and D) during the milking process (58/210; 27.61%). The predominance of the microorganisms varies according to the handling practices of the animals and the hygiene conditions during milking [58]. The lower infection rate may be attributed to good management practices such as the milkers’ hygiene, sanitization of the milking machine, healthy udder environment, dry period treatment, and the control of other predisposing diseases. Meanwhile, the higher infection rate (herds C, D) may be ascribed to a group of shared breeding factors where the dairy cattle live, including bad habitats, unbalanced food, terrible drafts, and the lack of pre-milking procedures. These conditions play a role in rendering the udder more susceptible to intramammary infections [59]. Furthermore, variations in the microenvironments and management practices between the different hygiene features applied to farms can influence the existence of the disease.
Herein, 21 antimicrobials of 12 antimicrobial classes were chosen to be tested, considering their availability for the intramammary treatment of clinical mastitis. In addition, we monitored penicillin, phenicol, aminoglycoside, tetracycline, sulfonamide, and trimethoprim resistance phenotypes/genotypes among the bovine mastitis S. uberis isolates.
Penicillin is widely used in the treatment of clinical bovine mastitis. The proportions of ampicillin-, penicillin-, and amoxicillin-resistant isolates in this study were high (89.85%, 79.71%, and 69.57%, respectively). Our finding strongly supported the previous results of Haenni et al. [60], who described a shift toward penicillin resistance among a subpopulation of S. uberis isolates. Additionally, they identified the presence of resistance-associated mutations among isolates considered intermediately susceptible to penicillin. Here, the blaZ gene conferring resistance to penicillin was found in 46.38% of the examined isolates, indicating an alarming level of potential resistance in bovine mastitis. This finding conflicts with the claim that environmental streptococci are still susceptible to β-lactam-active substances [61]. Previous studies [62,63] have documented that penicillin is effective against streptococci isolates with percentages of 92% and 96%, respectively. Moreover, Minst and coauthors [64] noticed the absence of penicillin and ampicillin resistance, suggesting that β-lactam antibiotics should remain the drug of choice for treating streptococcal mastitis. In this study, a low level of gentamicin resistance (20.28%) against S. uberis isolates was observed. On the contrary, an earlier study [65] reported that up to 93% of streptococci were resistant to gentamicin. Additionally, Rato et al. [66] stated that most S. uberis isolates (80%) were resistant to gentamycin. Nevertheless, our results are comparable to a lower rate of gentamicin-resistant S. uberis in a previous German study [67]. On the other hand, the S. uberis resistance rate to streptomycin was 96%, precluding its use in the treatment of bovine mastitis, which is consistent with a previous study [66]. The aminoglycoside resistance gene, aac(6′)aph(2″), was detected here within a reasonable rate (46.38%), which provides evidence suggesting that it confers resistance to a broad spectrum of aminoglycosides in Gram-positive bacteria, including streptococci [68], whereas the aac(6′)-Ib gene confers resistance to tobramycin, kanamycin, and amikacin in Gram-negative bacteria [69].
Our results showed that S. uberis is highly resistant to erythromycin (73.91%), which is higher than previous reports from France (21%) [70], Argentina (27.6%) [71], and northwestern China (31.2%) [15]. This explains that the erm(B) gene is the most prevalent among the analyzed isolates (75.36%). Furthermore, 65.22% of S. uberis isolates displayed resistance against tetracycline, mainly due to the inclusion of the tet(M) gene (46.38%) in most resistant isolates, which is nearly similar to a previous report (60%) [66]. However, the levels in our findings were lower than that previously described in a previous research (81.3%) [15]. Another study performed on S. uberis isolates from dairy cattle with clinical mastitis found results lower than ours for tetracycline resistance (18.1%) [72]. High tetracycline resistance levels may be attributed to their widespread use in treating numerous cattle infections for several years, proposing that tetracyclines, quinolones, and aminoglycosides should be avoided for the treatment of streptococcal mastitis. Differences in the susceptibility patterns among various studies could be due to different antimicrobial use in farms or countries, which could be a consequence of antimicrobial overuse for treating clinical mastitis or for growth promotion purposes in dairy herds [14], thus resulting in the inclusion of drug-resistant bacteria even in raw milk [73].
In the present investigation, conventional PCR allowed the amplification of virulence-associated genes of S. uberis, namely, sua, skc, cfu, pauA, and opp, each represented by a single band to their respective base pairs in the corresponding region of the DNA marker. The detection of genes encoding virulence factors could explain a possible association in the pathogenesis of mammary infections. The CAMP gene (cfu) is recorded here with a percentage of 21.73%; nearly similar results have been reported (25%) [74]. However, previous studies have reported high frequencies of the cfu gene in S. uberis isolates: 76.9% [11], 55.5% [53], and 46.1% [52]. On the contrary, a lower cfu percentage (3.8%) was reported in a previous research article [7]. The results suggest that this gene might not be the only gene related to the expression of the CAMP reaction. The opp gene was found in 11.59% of the examined S. uberis isolates. Previous studies have described a higher percentage of opp in S. uberis isolates: 64.1% [11] and 22.2% [53]. In contrast, an earlier study reported that the opp gene could not be amplified from all the strains, suggesting this gene may not be the only one responsible for the growth of S. uberis in milk [75]. The pauA gene was found in 40.57% of the examined S. uberis isolates. On the contrary, Ward et al. [76] reported that expression of pauA is not essential for infection of the mammary gland, as none of the examined isolates harbored the pauA gene from mastitic cows in an experimental study. In the same way, previous reports [10,11] found the pauA gene in S. uberis isolates with a higher percentage (94.9% and 61.5%, respectively). The streptokinase gene (skc) was detected at a percentage of 20.28%. A higher result was recorded by Shome and coauthors [52], who reported the skc gene in S. uberis strains at an incidence of 100%. The sua gene was recorded in our research at 42.02%. Nearly similar results were obtained previously (38.5%) [52]. In contrast, higher rates (97.8% and 83.3%, respectively) have been previously recorded [10,11].
Quaternary ammonium compounds (QACs) are amongst the most frequently used disinfectants. They are known to hinder the activity of a broad spectrum of microorganisms. They can disrupt the microbial cell wall, resulting in the leaking of the cytoplasm out of the cells [77]. Regrettably, the prevailing usage of QAC-based antiseptics in animal husbandry may result in bacterial resistance. In this study, QAC resistance genes were examined in S. uberis isolates. The qacED1 (79.71%) and qacC/D (78.26%) genes were found more frequently than qacA/B (18.84%). A paucity of data is currently available regarding the extent of QAC resistance genes in environmental streptococcal mastitis in Egypt. In a previous study in Egypt, all examined S. uberis isolates from bovine mastitis showed 100% phenotypic resistance to QACs (TH4; concentration = 0.25%) [78]. However, there are no Egyptian reports on QAC resistance in S. uberis isolates at the genetic level. The selective pressure employed by exposure to biocides has been concomitant with increasing resistance to antimicrobial agents. It has been documented that biocides and antimicrobial agents may share joint target sites and be situated together in mobile elements, resulting in co-resistance [79]. In addition, chromosomal efflux pumps may be involved in antimicrobial and biocide resistance due to their non-specific mechanism [80]. Inconsistent with a previous investigation on biocide resistance in Staphylococcus aureus [81], we report a weak or moderate genetic correlation between the existence of QAC and antimicrobial resistance genes.
5. Conclusions
The present investigation is the first to look into the virulence attributes and genotypic resistance to antimicrobials and biocides in S. uberis isolates from bovine clinical mastitis in Egypt. It adds to our knowledge of the high diversity of S. uberis and its occurrence in relation to prospective hygienic concerns. The sustained presence of pauA and sua genes throughout the investigated farms contributes to a better understanding of the pathogenicity of the bacterium, which provides the need to use such virulence factors as potential constituents of a vaccine against S. uberis. The co-existence of MDR and biocide resistance indicates the importance of S. uberis surveillance and the prudent use of antimicrobials and antiseptics in veterinary clinical medicine to avoid the dissemination of resistance.
Supplementary Materials
The followings are available online at https://www.mdpi.com/article/10.3390/ani11071849/s1, Figure S1: RFLP–PCR analysis of the 16S rRNA gene of representative S. uberis isolates showed unique patterns, Table S1: Oligonucleotide primer sequences used for PCR assays, Table S2: Significance and correlations among pairs of the four features under study, Table S3: Calculated binary distances among isolates based on the presence or absence of the four studied features.
Author Contributions
Validation, A.M.A., E.K. and R.A.A.E.; formal analysis, N.K.A.E.-A., H.M.E.D. and E.K.; investigation, N.K.A.E.-A., H.M.E.D., A.M.A., R.A.A.E., W.E.-K. and E.K.; data curation, N.K.A.E.-A. and H.M.E.D.; writing—original draft preparation, N.K.A.E.-A. and H.M.E.D.; writing—review and editing, N.K.A.E.-A., A.M.A., W.E.-K. and E.K.; project administration, H.A.S. and W.E.-K.; funding acquisition, H.A.S. and W.E.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as the study does not involve human cases or animal interventions. In place, recruitment of dairy cows into the work was done in consultation with veterinarians, and written informed consent was obtained from dairy farm owners for the collection of milk samples from mastitic cows.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We appreciate and thank Taif University for financial support for the Taif University Researchers Supporting Project (TURSP-2020/07), Taif University, Taif, Saudi Arabia. We thank the staff members of the Faculty of Veterinary Medicine, Zagazig University, Egypt, for their technical support during this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collenburg, L.; Beyersdorf, N.; Wiese, T.; Arenz, C.; Saied, E.M.; Becker-Flegler, K.A.; Schneider-Schaulies, S.; Avota, E. The Activity of the Neutral Sphingomyelinase Is Important in T Cell Recruitment and Directional Migration. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef]
- Lone, M.A.; Hülsmeier, A.J.; Saied, E.M.; Karsai, G.; Arenz, C.; von Eckardstein, A.; Hornemann, T. Subunit Composition of the Mammalian Serine-Palmitoyltransferase Defines the Spectrum of Straight and Methyl-Branched Long-Chain Bases. Proc. Natl. Acad. Sci. USA 2020, 117, 15591–15598. [Google Scholar] [CrossRef] [PubMed]
- Käppeli, N.; Morach, M.; Zurfluh, K.; Corti, S.; Nüesch-Inderbinen, M.; Stephan, R. Sequence types and antimicrobial resistance profiles of Streptococcus uberis isolated from bovine mastitis. Front. Vet. Sci. 2019, 6, 234. [Google Scholar] [CrossRef]
- Vasiliauskaité-Brooks, I.; Healey, R.D.; Rochaix, P.; Saint-Paul, J.; Sounier, R.; Grison, C.; Waltrich-Augusto, T.; Fortier, M.; Hoh, F.; Saied, E.M.; et al. Structure of a Human Intramembrane Ceramidase Explains Enzymatic Dysfunction Found in Leukodystrophy. Nat. Commun. 2018, 9, 5437. [Google Scholar] [CrossRef] [PubMed]
- Facklam, R. What happened to the streptococci: Overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Hassan, A.A.; Abdulmawjood, A.; Lanimler, C.; Wolter, W.; Zschock, M. Identification and epidemiological characterization of Streptococcus uberis isolated from bovine mastitis using conventional methods. J. Vet. Sci. 2003, 4, 213–224. [Google Scholar] [CrossRef]
- Almeida, R.A.; Luther, D.A.; Park, H.M.; Oliver, S.P. Identification, isolation and partial characterization of a novel Streptococcus uberis adhesion molecule (SUAM). Vet. Microbiol. 2006, 115, 183–191. [Google Scholar] [CrossRef]
- Smith, A.J.; Kitt, A.J.; Ward, P.N.; Leigh, J.A. Isolation and characterization of a mutant strain of Streptococcus uberis, which fails to utilize a plasmin derived beta-casein peptide for the acquisition of methionine. J. Appl. Microbiol. 2002, 93, 631–639. [Google Scholar] [CrossRef]
- Ward, P.N.; Leigh, J.A. Genetic analysis of Streptococcus uberis plasminogen activators. Indian J. Med. Res. 2004, 119, 136–140. [Google Scholar]
- Reinoso, E.B.; Lasagno, M.C.; Dieser, S.A.; Odierno, L.M. Distribution of virulence-associated genes in Streptococcus uberis isolated from bovine mastitis. FEMS Microbiol. Lett. 2011, 318, 183–188. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 1–14. [Google Scholar] [CrossRef]
- Tomazi, T.; Freu, G.; Alves, B.G.; de Souza Filho, A.F.; Heinemann, M.B.; Veiga Dos Santos, M. Genotyping and antimicrobial resistance of Streptococcus uberis isolated from bovine clinical mastitis. PLoS ONE 2019, 14, e0223719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, F.; Li, X.-P.; Luo, J.-Y.; Wang, L.; Zhou, Y.-L.; Yan, Y.; Wang, X.-R.; Li, H.S. Detection of antimicrobial resistance and virulence-related genes in Streptococcus uberis and Streptococcus parauberis isolated from clinical bovine mastitis cases in northwestern China. J. Integr. Agric. 2020, 19, 2784–2791. [Google Scholar] [CrossRef]
- Pitkälä, A.; Koort, J.; Björkroth, J. Identification and antimicrobial resistance of Streptococcus uberis and Streptococcus parauberis isolated from bovine milk samples. J. Dairy Sci. 2008, 91, 4075–4081. [Google Scholar] [CrossRef]
- Roussel, P.; Porcherie, A.; Répérant-Ferter, M.; Cunha, P.; Gitton, C.; Rainard, P.; Germon, P. Escherichia coli mastitis strains: In Vitro phenotypes and severity of infection In Vivo. PLoS ONE 2017, 12, e0178285. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aureus recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Bjorland, J.; Steinum, T.; Kvitle, B.; Waage, S.; Sunde, M.; Heir, E. Widespread distribution of disinfectant resistance genes among staphylococci of bovine and caprine origin in Norway. J. Clin. Microbiol. 2005, 43, 4363–4368. [Google Scholar] [CrossRef]
- Quinn, P.J.; Carter, M.E.; Markey, B.; Carter, G.R. Clinical Veterinary Microbiology; Mosby: London, UK, 1999; pp. 21–66. [Google Scholar]
- Hardie, J.M. Genus Streptococcus. In Bergey’s Manual of Systematic Bacteriology; Sneath, P.H.A., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1986; Volume 2, pp. 1043–1071. [Google Scholar]
- Christie, R.; Atkins, N.E.; Munch-Petersen, E. A note on a lytic phenomenon shown by group B streptococci. Aust. J. Exp. Biol. Med. Sci. 1944, 22, 197–200. [Google Scholar] [CrossRef]
- Domig, K.J.; Mayer, H.K.; Kneifel, W. Methods used for the isolation, enumeration, characterization and identification of Enterococcus spp. 2. Pheno- and genotypic criteria. Int. J. Food Microbiol. 2003, 88, 165–188. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standard single disc method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement M100; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Tambekar, D.; Dhanorkar, D.; Gulhane, S.; Khandelwal, V.; Dudhane, M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 1562–1565. [Google Scholar]
- Picard, F.J.; Ke, D.; Boudreau, D.K.; Boissinot, M.; Huletsky, A.; Richard, D.; Ouellette, M.; Roy, P.H.; Bergeron, M.G. Use of tuf sequences for genus-specific PCR detection and phylogenetic analysis of 28 Streptococcal species. J. Clin. Microbiol. 2004, 42, 3686–3695. [Google Scholar] [CrossRef]
- Nithin Prabhu, K.; Isloor, S.; Hegde, R.; Rathnamma, D.; Veeregowda, B.M.; Narasimha Murthy, H.; Shome, R.; Suryanarayana, V.V.S. Development of polymerase chain reaction for detection of predominant streptococcal isolates causing subclinical bovine mastitis. Indian J. Biotechnol. 2013, 12, 208–212. [Google Scholar]
- Nithin Prabhu, K.; Isloor, S.K.; Hegde, R.; Suryanarayana, W. Standardization of PCR and phylogenetic analysis for predominant streptococcal species isolated from subclinical mastitis. In Proceedings of the International Symposium on “Role of Biotechnology in Conserving Biodiversity and Livestock Development for Food Security and Poverty Alleviation” and XVII Annual Convention of Indian Society of Veterinary Immunology & Biotechnology (ISVIB), Bikaner, India, 23 March 2010; Volume 50, p. 47. [Google Scholar]
- Vesterholm-Nielsen, M.; Ølholm Larsen, M.; Elmerdahl Olsen, J.; Møller Aarestrup, E. Occurrence of the blaZ Gene in penicillin resistant Staphylococcus aureus isolated from bovine mastitis in Denmark. Acta Vet. Scand. 1999, 40, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Schwarz, S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Ozer, B.; Duran, G.G.; Onlen, Y.; Demir, C. Antibiotic resistance genes & susceptibility patterns in staphylococci. Indian J. Med. Res. 2012, 135, 389–396. [Google Scholar]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell Probes. 2001, 15, 209–215. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Lammens, C.; Piessens, J.; Goossens, H. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob. Agents Chemother. 2005, 49, 4798–4800. [Google Scholar] [CrossRef]
- Lina, G.; Quaglia, A.; Reverdy, M.E.; Leclercq, R.; Vandenesch, F.; Etienne, J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999, 43, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Schlegelova, J.; Vlkova, H.; Babak, V.; Holasova, M.; Jaglic, Z.; Stosova, T.; Sauer, P. Resistance to erythromycin of Staphylococcus spp. isolates from the food chain. Vet. Med. 2008, 53, 307–314. [Google Scholar] [CrossRef]
- Jensen, L.B.; Frimodt-Møller, N.; Aarestrup, F.M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 1999, 170, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Murinda, S.E.; Graves, A.K. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS ONE 2011, 6, e20819. [Google Scholar] [CrossRef] [PubMed]
- Grape, M.; Motakefi, A.; Pavuluri, S.; Kahlmeter, G. Standard and real-time multiplex PCR methods for detection of trimethoprim resistance dfr genes in large collections of bacteria. Clin. Microbiol. Infect. 2007, 13, 1112–1118. [Google Scholar] [CrossRef]
- Noguchi, N.; Suwa, J.; Narui, K.; Sasatsu, M.; Ito, T.; Hiramatsu, K.; Song, J. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus aureus isolated in Asia during 1998 and 1999. J. Med. Microbiol. 2005, 54, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Chuanchuen, R.; Khemtong, S.; Padungtod, P. Occurrence of qacE/qacEDelta1 genes and their correlation with class 1 integrons in Salmonella enterica isolates from poultry and swine. Southeast Asian J. Trop. Med. Public Health. 2007, 38, 855–862. [Google Scholar]
- Kolde, R. Package ‘Pheatmap’: Pretty Heat Map. 2018. Available online: https://rdrr.io/cran/pheatmap/ (accessed on 12 May 2020).
- Friendly, M. Corrgrams: Exploratory displays for correlation matrices. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. Heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef]
- Harrel, F.E., Jr. Package ‘Hmisc’. Available online: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf (accessed on 4 May 2020).
- SAS. SAS Statistics User’s Guide. Statistical Analytical System, 5th rev. SAS ed.; Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Palanivel, K.M.; Suresh, R.V.; Jayakumar, R.; Ganesan, P.I.; Dhanapalan, P. Retrospective study of sub-clinical mastitis in buffaloes. Indian J. Vet. Med. 2008, 28, 34–36. [Google Scholar]
- El Damaty, H.M.; Mahmmod, Y.S.; Gouda, S.M.; Sobhy, N.M. Epidemiological and ultrasonographic investigation of bovine fascioliasis in smallholder production system in Eastern Nile Delta of Egypt. Prev. Vet. Med. 2018, 158, 35–42. [Google Scholar] [CrossRef]
- El Damaty, H.M.; Fawzi, E.M.; Neamat-Allah, A.N.F.; Elsohaby, I.; Abdallah, A.; Farag, G.K.; El-Shazly, Y.A.; Mahmmod, Y.S. Characterization of foot and mouth disease virus serotype SAT-2 in swamp water buffaloes (Bubalus bubalis) under the Egyptian smallholder production system. Animals 2021, 11, 1697. [Google Scholar] [CrossRef]
- Bradley, A.J.; Leach, K.A.; Breen, J.E.; Green, L.E.; Green, M.J. Survey of the incidence and etiology of mastitis in dairy’ farms in England and Wales. Vet. Rec. 2007, 160, 253–257. [Google Scholar] [CrossRef]
- Shome, B.R.; Bhuvana, M.; Mitra, S.D.; Krithiga, N.; Shome, R.; Velu, D.; Banerjee, A.; Barbuddhe, S.B.; Prabhudas, K.; Rahman, H. Molecular characterization of Streptococcus agalactiae and Streptococcus uberis isolates from bovine milk. Trop. Anim. Health Prod. 2012, 44, 1981–1992. [Google Scholar] [CrossRef]
- Eldesouky, I.E.; Refae, M.A.; Nada, H.S.; Elnaby, G.R.H. Molecular detection of Streptococcus species isolated from cows with mastitis. World Vet. J. 2016, 6, 193–202. [Google Scholar] [CrossRef]
- Amin, B.; Deneke, Y.; Abdela, N. Bovine mastitis: Prevalence, risk factors, and isolation of Streptococcus species from small holders dairy farms in and around Haramaya town, Eastern Ethiopia. Glob. J. Med. Res. 2017, 17, 27–38. [Google Scholar]
- Amosun, E.A.; Ajuwape, A.T.P.; Adetosoye, A.I. Bovine streptococcal mastitis in Southwest and Northern States of Nigeria. Afr. J. Biomed. Res. 2010, 13, 33–37. [Google Scholar]
- Vasiľ, M. Etiology, course, and reduction of incidence of environmental mastitis in the herd of dairy cows. Slovak J. Anim. Sci. 2009, 3, 136–144. [Google Scholar]
- Abozaid, A.A.; El Balkemy, F.A.; El Damaty, H.M. Impact of risk factors on the prevalence of mastitis in dairy cattle. Zag. Vet. J. 2013, 41, 162–168. [Google Scholar] [CrossRef]
- Jayarao, B.M.; Pillai, S.R.; Sawant, A.A.; Wolfgang, D.R.; Hegde, N.V. Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. J. Dairy Sci. 2004, 87, 3561–3573. [Google Scholar] [CrossRef]
- Kivaria, F.M.; Noordhuizen, J.P.T.M.; Msamia, H.M. Risk factors associated with the incidence rate of clinical mastitis in smallholder dairy cows in the Dar es Salaam region of Tanzania. Vet. J. 2007, 173, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Galofaro, L.; Ythier, M.; Giddey, M.; Majcherczyk, P.; Moreillon, P.; Madec, J.Y. Penicillin-bindingprotein gene alterations in Streptococcus uberis isolates presenting decreased susceptibility to penicillin. Antimicrob. Agents Chemother. 2010, 54, 1140–1145. [Google Scholar] [CrossRef]
- Cameron, M.; Saab, M.; Heider, L.; McClure, J.T.; Rodriguez-Lecompte, J.C.; Sanchez, J. Antimicrobial susceptibility patterns of environmental streptococci recovered from bovine milk samples in the Maritime Provinces of Canada. Front. Vet. Sci. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Erskine, R.J.; Walker, R.D.; Bolin, C.A.; Bartlett, P.C.; White, D.G. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 2002, 85, 1111–1118. [Google Scholar] [CrossRef]
- Gianneechini, R.E.; Concha, C.; Franklin, A. Antimicrobial susceptibility of udder pathogens isolated from dairy herds in the West Littoral region of Uruguay. Acta Vet. Scand. 2002, 43, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Minst, K.; Märtlbauer, E.; Miller, T.; Meyer, C. Short communication: Streptococcus species isolated from mastitis milk samples in Germany and their resistance to antimicrobial agents. J. Dairy Sci. 2012, 95, 6957–6962. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Hoedemacker, M.; Klein, G. Susceptibility of mastitis pathogens in northern Germany. Berl. Munch. Tierarztl. Wochenschr. 2005, 118, 393–398. [Google Scholar] [PubMed]
- Rato, M.G.; Bexiga, R.; Florindo, C.; Cavaco, L.M.; Vilela, C.L.; Santos-Sanches, I. Antimicrobial resistance and molecular epidemiology of streptococci from bovine mastitis. Vet. Microbiol. 2013, 161, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, H. Results of the antimicrobial agent susceptibility study raised in a representative, cross-sectional monitoring study on a national basis. Int. J. Med. Microbiol. 2006, 296, 69–79. [Google Scholar] [CrossRef]
- Lollai, S.A.; Ziccheddu, M.; Duprè, I.; Piras, D. Characterization of resistance to tetracyclines and aminoglycosides of sheep mastitis pathogens: Study of the effect of gene content on resistance. J. Appl. Microbiol. 2016, 121, 941–951. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Gharib, A.A. Coexistence of plasmid-mediated quinolone resistance determinants and AmpC-beta- lactamases in Escherichia coli strains in Egypt. Cell Mol. Biol. 2015, 61, 29–35. [Google Scholar] [PubMed]
- Guérin-Faublée, V.; Tardy, F.; Bouveron, C.; Carret, G. Antimicrobial susceptibility of Streptococcus species isolated from clinical mastitis in dairy cows. Int. J. Antimicrob. Agents 2002, 19, 219–226. [Google Scholar] [CrossRef]
- Denamiel, G.; Llorente, P.; Carabella, M.; Rebuelto, M.; Gen-tilini, E. Anti-microbial susceptibility of Streptococcus spp. isolated from bovine mastitis in Argentina. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.Y.; Leblond, A.; Haenni, M.; Gay, É. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; Hamdy, M.M.; Gobouri, A.A.; Azab, E.; Sewid, A.H. First report of aacC5-aadA7_4 gene cassette array and phage tail tape measure protein on class 1 integrons of Campylobacter species isolated from animal and human sources in Egypt. Animals 2020, 10, 2067. [Google Scholar] [CrossRef]
- Lasagno, M.C.; Reinoso, E.B.; Dieser, S.A.; Calvinho, L.F.; Buzzola, F.; Vissio, C.; Bogni, C.; Odierno, L.M. Phenotypic and genotypic characterization of Streptococcus uberis isolated from bovine subclinical mastitis in Argentinean dairy farms. Rev. Argent. Microbiol. 2011, 43, 212–217. [Google Scholar]
- Zadoks, R.N.; Schukken, Y.H.; Wiedmann, M. Multilocus sequence typing of Streptococcus uberis provides sensitive and epidemiologically relevant subtype information and reveals positive selection in the virulence gene pauA. J. Clin. Microbiol. 2005, 43, 2407–2417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ward, P.N.; Field, T.R.; Rapier, C.D.; Leigh, J.A. The activation of bovine plasminogen by pauA is not required for virulence of Streptococcus uberis. Infect. Immun. 2003, 71, 7193–7196. [Google Scholar] [CrossRef]
- Bragg, R.; Jansen, A.; Coetzee, M.; van der Westhuizen, W.; Boucher, C. Bacterial resistance to quaternary ammonium compounds (qac) disinfectants. In Infectious Diseases and Nanomedicine II. Advances in Experimental Medicine and Biology; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014; Volume 808. [Google Scholar]
- Haggag, Y.N.; Nossair, M.A.; Mansour, A.M.; Abd El Rahman, A.H. Streptococci in dairy farms: Isolation, antibiogram pattern and disinfectant sensitivity. Alex. J. Vet. Sci. 2018, 59, 85–92. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Cambau, E.; Guillard, T. Antimicrobials that affect the synthesis and conformation of nucleic acids. Rev. Sci. Tech. 2012, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Oggioni, M.R.; Coelho, J.R.; Furi, L.; Knight, D.R.; Viti, C.; Orefici, G.; Martinez, J.L.; Freitas, A.T.; Coque, T.M.; Morrissey, I.; et al. Significant differences characterise the correlation coefficients between biocide and antibiotic susceptibility profiles in Staphylococcus aureus. Curr. Pharm. Des. 2015, 21, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).