An Investigation into the Influence of Different Types of Nesting Materials upon the Welfare of Captive Chimpanzees (Pan troglodytes)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Sample
2.3. Study Materials
2.4. Data Collection
2.5. Sampling Method
2.6. Schedule
2.7. Measures of Welfare
2.8. Statistical Analysis
3. Results
3.1. Summary
3.2. Affiliative Behaviours
3.3. Abnormal Behaviours
3.4. Agonistic Behaviours
3.5. Social Competence
3.6. Behavioural Competence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humle, T.; Boesch, C.; Campbell, G.; Junker, J.; Koops, K.; Kuehl, H.; Sop, T. Pan troglodytes ssp. verus (errata version published in 2016). IUCN Red List Threat. Species 2016, 2016. [Google Scholar] [CrossRef]
- Hockings, K.J.; McLennan, M.; Carvalho, S.; Ancrenaz, M.; Bobe, R.; Byrne, R.; Dunbar, R.I.; Matsuzawa, T.; McGrew, W.C.; Williamson, E.; et al. Apes in the Anthropocene: Flexibility and survival. Trends Ecol. Evol. 2015, 30, 215–222. [Google Scholar] [CrossRef] [Green Version]
- McLennan, M.R. Beleaguered chimpanzees in the Agricultural District of Hoima, Western Uganda. Primate Conserv. 2008, 23, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Garriga, R.M.; Marco, I.; Casas-Díaz, E.; Acevedo, P.; Amarasekaran, B.; Cuadrado, L.; Humle, T. Factors influencing wild chimpanzee (Pan troglodytes verus) relative abundance in an agriculture-swamp matrix outside protected areas. PLoS ONE 2019, 14, e0215545. [Google Scholar] [CrossRef]
- Gronqvist, G.; Kingston-Jones, M.; May, A.; Lehmann, J. The effects of three types of environmental enrichment on the behaviour of captive Javan gibbons (Hylobates moloch). Appl. Anim. Behav. Sci. 2013, 147, 214–223. [Google Scholar] [CrossRef]
- Young, R.J. 1966—Environmental Enrichment for Captive Animals; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Shapiro, M.E.; Shapiro, H.G.; Ehmke, E.E. Behavioral responses of three lemur species to different food enrichment devices. Zoo Biol. 2018, 37, 146–155. [Google Scholar] [CrossRef]
- Acklin, C.J.; Gault, R.A. Effects of natural enrichment materials on stress, memory and exploratory behavior in Mice|Lab Animal. Lab. Anim. 2015, 44, 262–267. [Google Scholar] [CrossRef]
- Coleman, K.; Novak, M. Environmental Enrichment in the 21st Century. ILAR J. 2017, 58, 295–307. [Google Scholar] [CrossRef]
- Hopper, L.M.; Shender, M.A.; Ross, S.R. Behavioral research as physical enrichment for captive chimpanzees. Zoo Biol. 2016, 35, 293–297. [Google Scholar] [CrossRef]
- Dolins, F.L.; Schweller, K.; Milne, S. Technology advancing the study of animal cognition: Using virtual reality to present virtually simulated environments to investigate nonhuman primate spatial cognition. Curr. Zoöl. 2017, 63, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Clark, F. Cognitive enrichment and welfare: Current approaches and future directions. Anim. Behav. Cogn. 2017, 4, 52–71. [Google Scholar] [CrossRef] [Green Version]
- Carter, R.L.; Lipman, N.S. Feed and bedding. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Doane, C.J.; Andrews, K.; Schaefer, L.J.; Morelli, N.; McAllister, S.; Coleman, K. Dry bedding provides cost-effective enrichment for group-housed rhesus macaques (Macaca Mulatta). J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 247–252. [Google Scholar]
- Chamove, A.S.; Anderson, J.R.; Morgan-Jones, S.C.; Jones, S.P. Deep woodchip litter: Hygiene, feeding, and behavioral enhancement in eight primate species. Int. J. Study Anim. Probl. 1982, 3, 308–318. [Google Scholar]
- Brent, L. Woodchip bedding as enrichment for captive chimpanzees in an outdoor enclosure. Anim. Welfare 1992, 1, 161–170. [Google Scholar]
- Baker, K.C. Straw and Forage Material Ameliorate Abnormal Behaviors in Adult Chimpanzees. Zoo Biol. 1997, 16, 225–236. [Google Scholar] [CrossRef]
- Fruth, B.; McGrew, W.C.; Marchant, L.F.; Nishida, T. Nest building behavior in the great apes: The great leap forward? In Great Ape Societies; Cambridge University Press: Cambridge, UK, 1996; pp. 225–240. [Google Scholar]
- Videan, E.N. Bed-building in captive chimpanzees (Pan troglodytes): The importance of early rearing. Am. J. Primatol. 2006, 68, 745–751. [Google Scholar] [CrossRef]
- Samson, D.R.; Shumaker, R. Pre-sleep and sleeping platform construction behavior in captive orangutans (Pongo spp.): Implications for ape health and welfare. Folia Primatol. 2015, 86, 187–202. [Google Scholar] [CrossRef]
- Anderson, J.R.; Ang, M.Y.L.; Lock, L.C.; Weiche, I. Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 2019, 60, 321–332. [Google Scholar] [CrossRef]
- Koops, K.; Humle, T.; Sterck, E.H.M.; Matsuzawa, T. Ground-nesting by the chimpanzees of the Nimba Mountains, Guinea: Environmentally or socially determined? Am. J. Primatol. 2007, 69, 407–419. [Google Scholar] [CrossRef]
- Ndiaye, P.I.; Badji, L.; Lindshield, S.M.; Pruetz, J.D. Nest-building behaviour by chimpanzees (Pan troglodytes verus) in the non-protected area of Diaguiri (Kedougou, Senegal): Implications for conservation. Folia Primatol. 2018, 89, 316–326. [Google Scholar] [CrossRef]
- Brownlow, A.; Plumptre, A.; Reynolds, V.; Ward, R. Sources of variation in the nesting behavior of chimpanzees (Pan troglodytes schweinfurthii) in the Budongo forest, Uganda. Am. J. Primatol. 2001, 55, 49–55. [Google Scholar] [CrossRef]
- Plumptre, A.J.; Reynolds, V. Nesting behavior of chimpanzees: Implications for censuses. Int. J. Primatol. 1997, 18, 475–485. [Google Scholar] [CrossRef]
- Goodall, J.M. Nest building behavior in the free ranging chimpanzee. Ann. N. Y. Acad. Sci. 1962, 102, 455–467. [Google Scholar] [CrossRef]
- Dugard, P.; Todman, J. Analysis of pre-test-post-test control group designs in educational research. Educ. Psychol. 1995, 15, 181–198. [Google Scholar] [CrossRef]
- Palagi, E. Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behav. Ecol. Sociobiol. 2018, 72, 90. [Google Scholar] [CrossRef]
- Llorente, M.; Riba, D.; Ballesta, S.; Feliu, O.; Rostan, C. Rehabilitation and socialization of chimpanzees (Pan troglodytes) used for entertainment and as pets: An 8-Year Study at Fundació Mona. Int. J. Primatol. 2015, 36, 605–624. [Google Scholar] [CrossRef]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Forstmeier, W.; Schielzeth, H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 2010, 65, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Lavery, M.R.; Acharya, P.; Sivo, S.A.; Xu, L. Number of predictors and multicollinearity: What are their effects on error and bias in regression? Commun. Stat. Simul. Comput. 2017, 48, 27–38. [Google Scholar] [CrossRef]
- Lewis, F.; Butler, A.J.; Gilbert, L. A unified approach to model selection using the likelihood ratio test. Methods Ecol. Evol. 2010, 2, 155–162. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org (accessed on 4 March 2020).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). 2020. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 4 March 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2020. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 4 March 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models Usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. 2020. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 4 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009; p. 213. [Google Scholar]
- Videan, E.N.; Fritz, J.; Schwandt, M.L.; Smith, H.F.; Howell, S. Controllability in Environmental Enrichment for Captive Chimpanzees (Pan troglodytes). J. Appl. Anim. Welf. Sci. 2005, 8, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Samuni, L.; Wittig, R.; Crockford, C.; Boesch, C.; Vigilant, L.; Deschner, T.; Leendertz, F. Adoption in the Taï chimpanzees: Costs, benefits and strong social relationships. In The Chimpanzees of the Taï Forest; Cambridge University Press: Cambridge, UK, 2019; pp. 141–158. [Google Scholar]
- Reddy, R.B.; Mitani, J.C. Social relationships and caregiving behavior between recently orphaned chimpanzee siblings. Primates 2019, 60, 389–400. [Google Scholar] [CrossRef]
- Boesch, C.; Bolé, C.; Eckhardt, N.; Boesch, H. Altruism in forest chimpanzees: The case of adoption. PLoS ONE 2010, 5, e8901. [Google Scholar] [CrossRef] [Green Version]



| Subject | Age | Sex | Group |
|---|---|---|---|
| Mac | 13 | Male | G1 |
| Nico | 15 | Male | G1 |
| Monko | 8 | Female | G2 |
| Hashi | 8 | Female | G2 |
| Winnie | 8 | Female | G2 |
| Lola | 9 | Female | G2 |
| Kortu | 8 | Female | G2 |
| Perry | 9 | Male | G2 |
| Mortes | 9 | Male | G2 |
| Tetina | 8 | Male | G2 |
| Molly | 9 | Female | G2 |
| Mus | 9 | Female | G2 |
| Michael | 7 | Male | G2 |
| Morlai | 8 | Male | G2 |
| Jean | 5 | Female | G3 |
| Joko | 4 | Male | G3 |
| Bousy | 4 | Male | G3 |
| Sophie | 5 | Female | G4 |
| Matilda | 5 | Female | G4 |
| Woron | 4 | Male | G4 |
| Treatment | Type of Bedding |
|---|---|
| 1 | Leaves and branches |
| 2 | Long grass |
| 3 | Cotton sheets |
| 4 | Shredded newspaper |
| 5 | Leaves and branches + Long grass |
| 6 | Cotton sheets + Shredded newspaper |
| Response Variable | Condition | Mean | Median | St. Deviation | Maximum | Minimum |
|---|---|---|---|---|---|---|
| Rel. Freq. of Affiliative | Control | 0.055 | 0.025 | 0.070 | 0.335 | 0 |
| During Treatment | 0.083 | 0.030 | 0.112 | 0.634 | 0 | |
| Post-Treatment | 0.064 | 0.030 | 0.084 | 0.404 | 0 | |
| Rel. Freq. of Abnormal | Control | 0.042 | 0.016 | 0.064 | 0.365 | 0 |
| During Treatment | 0.019 | 0.005 | 0.038 | 0.249 | 0 | |
| Post-Treatment | 0.043 | 0.015 | 0.070 | 0.410 | 0 | |
| Rel. Freq. of Agonistic | Control | 0.008 | 0 | 0.015 | 0.099 | 0 |
| During Treatment | 0.007 | 0 | 0.012 | 0.083 | 0 | |
| Post-Treatment | 0.008 | 0 | 0.015 | 0.107 | 0 | |
| SCI | Control | −0.870 | −0.923 | 0.140 | −0.311 | −1 |
| During Treatment | −0.815 | −0.905 | 0.227 | 0.268 | −1 | |
| Post-Treatment | −0.856 | −0.927 | 0.168 | −0.220 | −1 | |
| BCI | Control | −0.241 | −0.342 | 0.561 | 0.950 | −1 |
| During Treatment | 0.272 | 0.359 | 0.495 | 1 | −0.936 | |
| Post-Treatment | −0.245 | −0.345 | 0.554 | 0.924 | −1 |
| Response Variable | Independent Variable | F | df | p | V (%) |
|---|---|---|---|---|---|
| Rel. Freq. of Affiliative | Condition | 6.566 | 2 | 0.002 ** | 30.7 |
| Treatment | 1.807 | 5 | 0.1095 | - | |
| Time of Day | 13.559 | 1 | 0.000 *** | - | |
| Sex | 0.808 | 1 | 0.379 | - | |
| Group | 15.202 | 3 | 0.000 *** | - | |
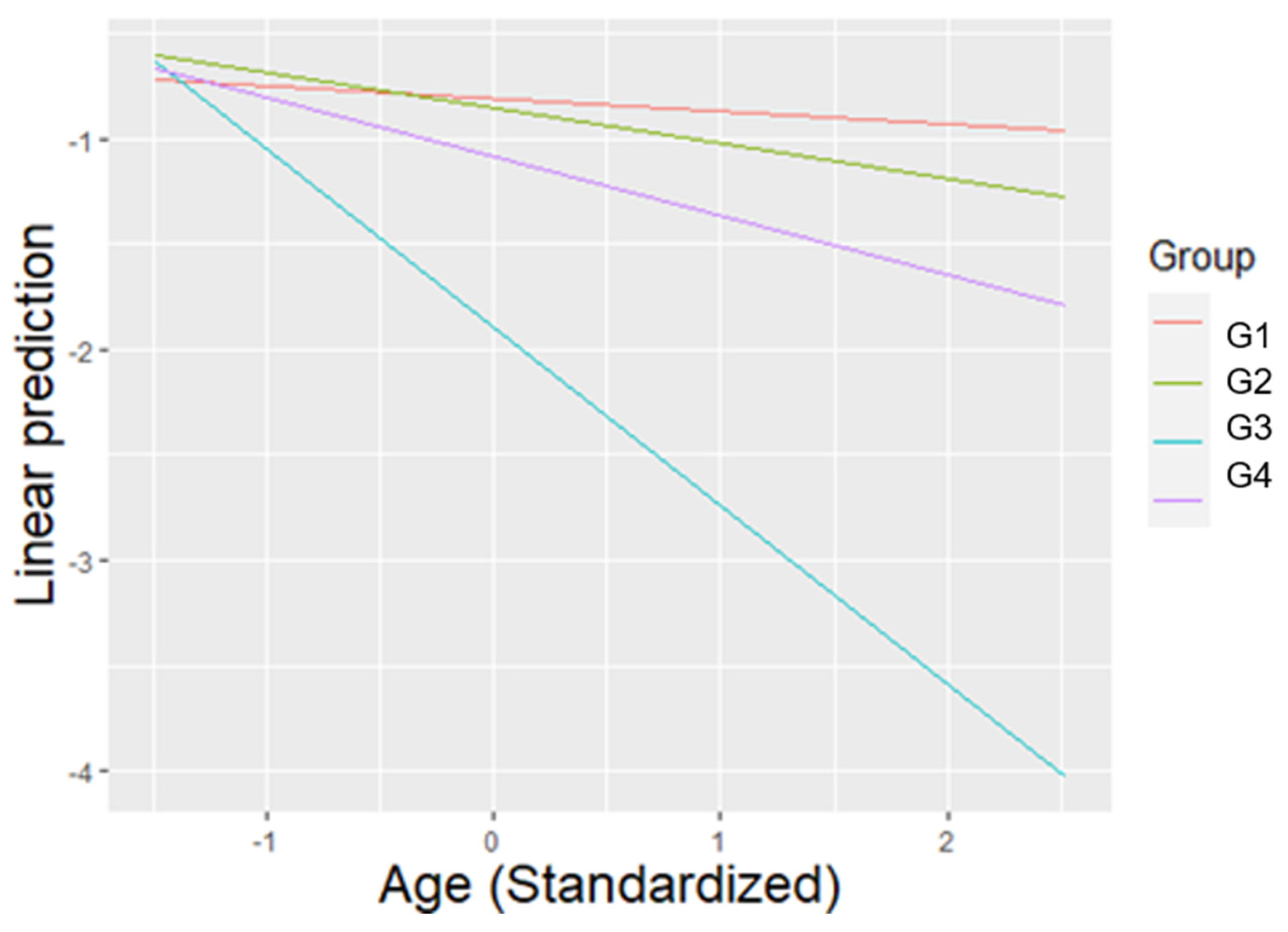
| Group × Age | 17.762 | 4 | 0.000 *** | - | |
| Rel. Freq. of Abnormal | Condition | 15.498 | 2 | 0.000 *** | 33.0 |
| Rel. Freq. of Agonistic | Condition | 0.404 | 2 | 0.668 | 17.2 |
| Treatment | 6.219 | 5 | 0.000 *** | - | |
| Time of Day | 36.483 | 1 | 0.000 *** | - | |
| Sex | 12.194 | 1 | 0.001 *** | - | |
| Group | 4.069 | 3 | 0.007 ** | - | |
| Group × Age | 3.233 | 4 | 0.012 * | - | |
| Social Competence Index | Condition | 6.353 | 2 | 0.002 ** | 28.1 |
| Treatment | 0.894 | 5 | 0.485 | - | |
| Time of Day | 19.859 | 1 | 0.000 *** | - | |
| Sex | 0.353 | 1 | 0.559 | - | |
| Group | 15.419 | 3 | 0.000 *** | - | |
| Group × Age | 18.544 | 4 | 0.000 *** | - | |
| Behavioural Competence Index | Condition | 151.544 | 2 | 0.000 *** | 67.4 |
| Treatment | 12.909 | 5 | 0.000 *** | - | |
| Time of Day | 130.421 | 1 | 0.000 *** | - | |
| Sex | 13.996 | 1 | 0.000 *** | - | |
| Group | 28.550 | 3 | 0.000 *** | - | |
| Group × Age | 23.645 | 4 | 0.000 *** | - |
| Model | Model Structure | AICc |
|---|---|---|
| Full Model | Condition, treatment, time of day, sex, group, group × age, random effect for subject identity | −1333.05 |
| Model 1 | Condition, treatment, time of day, sex, group × age, random effect for subject identity | −1310.08 |
| Model 2 | Condition, treatment, time of day, sex, random effect for subject identity | −1298.67 |
| Model 3 | Condition, treatment, time of day, random effect for subject identity | −1300.37 |
| Model 4 | Condition, treatment, random effect for subject identity | −1289.37 |
| Model 5 | Condition, random effect for subject identity | −1293.12 |
| Null Model | random effect for subject identity | −1284.43 |
| Model | Model Structure | AICc |
|---|---|---|
| Full Model | Condition, treatment, time of day, sex, group, group × age, random effect for subject identity | −1810.52 |
| Model 1 | Condition, treatment, time of day, sex, group × age, random effect for subject identity | −1807.56 |
| Model 2 | Condition, treatment, time of day, sex, random effect for subject identity | −1805.61 |
| Model 3 | Condition, treatment, time of day, random effect for subject identity | −1807.57 |
| Model 4 | Condition, treatment, random effect for subject identity | −1807.61 |
| Model 5 | Condition, random effect for subject identity | −1813.68 |
| Null Model | random effect for subject identity | −1787.57 |
| Model | Model Structure | AICc |
|---|---|---|
| Full Model | Condition, treatment, time of day, sex, group, group × age, random effect for subject identity | −3460.27 |
| Model 1 | Condition, treatment, time of day, sex, group × age, random effect for subject identity | −3456.21 |
| Model 2 | Condition, treatment, time of day, sex, random effect for subject identity | −3451.75 |
| Model 3 | Condition, treatment, time of day, random effect for subject identity | −3445.16 |
| Model 4 | Condition, treatment, random effect for subject identity | −3412.33 |
| Model 5 | Condition, random effect for subject identity | −3394.79 |
| Null Model | random effect for subject identity | −3397.95 |
| Model | Model Structure | AICc |
|---|---|---|
| Full Model | Condition, treatment, time of day, sex, group, group × age, random effect for subject identity | −485.05 |
| Model 1 | Condition, treatment, time of day, sex, Group × age, random effect for subject identity | −461.36 |
| Model 2 | Condition, treatment, time of day, sex, random effect for subject identity | −451.27 |
| Model 3 | Condition, treatment, time of day, random effect for subject identity | −452.43 |
| Model 4 | Condition, treatment, random effect for subject identity | −435.40 |
| Model 5 | Condition, random effect for subject identity | −442.59 |
| Null Model | random effect for subject identity | −434.46 |
| Model | Model Structure | AICc |
|---|---|---|
| Full Model | Condition, treatment, time of day, sex, group, group × age, random effect for subject identity | 443.47 |
| Model 1 | Condition, treatment, time of day, sex, group × age, random effect for subject identity | 471.41 |
| Model 2 | Condition, treatment, time of day, sex, random effect for subject identity | 490.05 |
| Model 3 | Condition, treatment, time of day, random effect for subject identity | 489.05 |
| Model 4 | Condition, treatment, random effect for subject identity | 603.74 |
| Model 5 | Condition, random effect for subject identity | 638.75 |
| Null Model | random effect for subject identity | 827.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, N.; Amarasekaran, B.; Riba, D. An Investigation into the Influence of Different Types of Nesting Materials upon the Welfare of Captive Chimpanzees (Pan troglodytes). Animals 2021, 11, 1835. https://doi.org/10.3390/ani11061835
Anderson N, Amarasekaran B, Riba D. An Investigation into the Influence of Different Types of Nesting Materials upon the Welfare of Captive Chimpanzees (Pan troglodytes). Animals. 2021; 11(6):1835. https://doi.org/10.3390/ani11061835
Chicago/Turabian StyleAnderson, Naomi, Bala Amarasekaran, and David Riba. 2021. "An Investigation into the Influence of Different Types of Nesting Materials upon the Welfare of Captive Chimpanzees (Pan troglodytes)" Animals 11, no. 6: 1835. https://doi.org/10.3390/ani11061835
APA StyleAnderson, N., Amarasekaran, B., & Riba, D. (2021). An Investigation into the Influence of Different Types of Nesting Materials upon the Welfare of Captive Chimpanzees (Pan troglodytes). Animals, 11(6), 1835. https://doi.org/10.3390/ani11061835






