Recovery after General Anaesthesia in Adult Horses: A Structured Summary of the Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Narrative Reviews/Expert Opinions
4.2. Retrospective Outcome Studies
4.3. Surveys
4.4. Premedication/Sedation and Induction Drugs
4.5. Maintenance with Inhalant Agents
4.6. Maintenance with TIVA
4.7. Maintenance with PIVA
4.8. Other Drugs Used during Maintenance
4.9. Drugs before/during Recovery
4.10. Recovery Systems
4.11. Respiratory System in Recovery
4.12. Other Factors
4.13. Case Series/Reports
4.14. Systems to Score Recoveries
4.15. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, G.M.; Eastment, J.K.; Wood, J.; Taylor, P.M. The confidential enquiry into perioperative equine fatalities (CEPEF): Mortality results of Phases 1 and 2. Vet. Anaesth. Analg. 2002, 29, 159–170. [Google Scholar] [CrossRef]
- Dugdale, A.H.; Taylor, P.M. Equine anaesthesia-associated mortality: Where are we now? Vet. Anaesth. Analg. 2016, 43, 242–255. [Google Scholar] [CrossRef] [Green Version]
- Bont, M.P. Standing surgery versus general anaesthesia for resolution of acute abdomen; useful enough to become routine? Equine Vet. Educ. 2021, 33, 67–69. [Google Scholar] [CrossRef]
- Gent, T.C.; Bettschart-Wolfensberger, R. Peri-anaesthetic mortality in horses—The need for CEPEF-4. Vet. Anaesth. Analg. 2013, 40, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; Redondo, J.I.; Johnston, M.; Taylor, P.; Bettschart-Wolfensberger, R. CEPEF4: Update and plan. Vet. Anaesth. Analg. 2020, 47, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; Redondo, J.I.; Johnston, M.; Taylor, P.; Bettschart-Wolfensberger, R. A new equine anaesthetic mortality study two decades after CEPEF2: CEPEF4 is going live! Equine Vet. J. 2020, 52, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Senior, M. Post-anaesthetic pulmonary oedema in horses: A review. Vet. Anaesth. Analg. 2005, 32, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Auckburally, A.; Flaherty, D. Recovery from anaesthesia in horses 1. What can go wrong? In Practice 2009, 31, 340–347. [Google Scholar] [CrossRef]
- Auckburally, A.; Flaherty, D. Recovery from anaesthesia in horses 2. Avoiding complications. In Practice 2009, 31, 362–369. [Google Scholar] [CrossRef]
- Clark-Price, S.C. Recovery of horses from anesthesia. Vet. Clin. N. Am. Equine Pract. 2013, 29, 223–242. [Google Scholar] [CrossRef]
- Bradbury, A.G.; Eddleston, M.; Clutton, R.E. Pain management in pigs undergoing experimental surgery; a literature review (2012–2014). Br. J. Anaesth. 2016, 116, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Siddaway, A.P.; Wood, A.M.; Hedges, L.V. How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu. Rev. Psychol. 2019, 70, 747–770. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbell, J.A.E. Recovery from anaesthesia in horses. Equine Vet. Educ. 1999, 11, 160–167. [Google Scholar] [CrossRef]
- Hennig, G.E.; Court, M.H. Equine postanesthetic myopathy—An update. Compend. Contin. Educ. Vet. 1991, 13, 1709–1715. [Google Scholar]
- Stegmann, G.F. Pulmonary function in the horse during anaesthesia: A review. J. S. Afr. Vet. Assoc. 1986, 57, 49–53. [Google Scholar] [PubMed]
- Senior, J.M. Mitigating the risk of airway obstruction during recovery from anaesthesia: The way is far from clear. Equine Vet. Educ. 2015, 27, 244–246. [Google Scholar] [CrossRef]
- Wagner, A.E. Complications in equine anesthesia. Vet. Clin. N. Am. Equine Pract. 2008, 24, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Emese, B.; Zita, M. Anaesthesia-related complications in horses—Results of the last few years. Magy. Allatorvosok Lapja 2019, 141, 271–280. [Google Scholar]
- Portier, K.; Ida, K.K. Editorial: Anesthetic risk and complications in veterinary medicine. Front. Vet. Sci. 2020, 7, 397. [Google Scholar] [CrossRef]
- Deutsch, J.; Taylor, P.M. Mortality and morbidity in equine anaesthesia. Equine Vet. Educ. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Guedes, A.G.P.; Natalini, C.C. Anesthesia in horses with colic syndrome: Analysis of 48 cases and literature review. Cienc. Rural 2002, 32, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Cornick-Seahorn, J. Anesthesia of the critically ill equine patient. Vet. Clin. N. Am. Equine Pract. 2004, 20, 127–149. [Google Scholar] [CrossRef]
- Shearer, T.R.; Holcombe, S.J.; Valberg, S.J. Incisional infections associated with ventral midline celiotomy in horses. J. Vet. Emerg. Crit. Care 2020, 30, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.B. Anesthetic management and recovery of large orthopedic patients. Vet. Clin. N. Am. 1973, 3, 127–135. [Google Scholar] [CrossRef]
- Auer, J.A. Fracture fixation in horses: Recent developments in implants, management and recovery—A rewiew. Ippologia 2004, 15, 25–37. [Google Scholar]
- O’Meara, B. Bog spavin: Recognising the problem is the first step towards recovery. Vet. Rec. 2012, 170, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V. Anesthesia and sedation for late-term mares. Vet. Clin. N. Am. Equine Pract. 1994, 10, 219–236. [Google Scholar] [CrossRef]
- Kalhoro, A.B. Pre-anesthetic medication in the horse part IV. Sedative-hypnotics and drug mixtures. Pak. Vet. J. 1989, 9, 48–56. [Google Scholar]
- Short, C.E. The responses to the use of detomidine (Domosedan) in the horse. Wien. Tierarztl. Monatsschr. 1992, 79, 2–12. [Google Scholar]
- Hubbell, J.A.E.; Muir, W.W. Use of the alpha-2 agonists xylazine and detomidine in the perianaesthetic period in the horse. Equine Vet. Educ. 2004, 16, 326–332. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Gasthuys, F.; Luna, S.P.L.; Schauvliege, S. Is there a place for dexmedetomidine in equine anaesthesia and analgesia? A systematic review (2005–2017). J. Vet. Pharmacol. Ther. 2018, 41, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levionnois, O.L. Sedation in equine practice—Indications and choice of the methods. Prakt. Tierarzt. 2007, 88, 240–249. [Google Scholar]
- Southwood, L. Clinical insights: Equine anaesthesia and analgesia. Equine Vet. J. 2019, 51, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A. Neuromuscular blocking agents. Vet. Clin. N. Am. Equine Pract. 2002, 18, 181–188. [Google Scholar] [CrossRef]
- Short, C.E.; Brunson, D.B. General anesthesia in pleasure horses. Cornell Vet. 1978, 68 Suppl. 7, 276–283. [Google Scholar]
- Brunson, D.B. Use of halothane and isoflurane in the horse. Vet. Clin. N. Am. Equine Pract. 1990, 6, 529–541. [Google Scholar] [CrossRef]
- Dohoo, S.E. Isoflurane as an inhalational anesthetic agent in clinical practice. Can. Vet. J. 1990, 31, 847–850. [Google Scholar] [PubMed]
- Clarke, K.W. Desflurane and sevoflurane: New volatile anesthetic agents. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 793–810. [Google Scholar] [CrossRef]
- Stanway, G. Anaesthesia for minor surgical procedures in the horse. In Practice 2001, 23, 22–29. [Google Scholar] [CrossRef]
- Steffey, E.P. Recent advances in inhalation anesthesia. Vet. Clin. N. Am. Equine Pract. 2002, 18, 159–168. [Google Scholar] [CrossRef]
- Auckburally, A.; Flaherty, D. Use of supplemental intravenous anaesthesia/analgesia in horses. In Practice 2011, 33, 334–339. [Google Scholar] [CrossRef]
- Ratajczak, K.; Szmigielska, M.; Henklewski, R.; Biazik, A. Balanced anesthesia in horses. Med. Weter. 2011, 67, 604–608. [Google Scholar]
- Gozalo-Marcilla, M.; Gasthuys, F.; Schauvliege, S. Partial intravenous anaesthesia in the horse: A review of intravenous agents used to supplement equine inhalation anaesthesia. Part 1: Lidocaine and ketamine. Vet. Anaesth. Analg. 2014, 41, 335–345. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Gasthuys, F.; Schauvliege, S. Partial intravenous anaesthesia in the horse: A review of intravenous agents used to supplement equine inhalation anaesthesia. Part 2: Opioids and alpha-2 adrenoceptor agonists. Vet. Anaesth. Analg. 2015, 42, 1–16. [Google Scholar] [CrossRef] [PubMed]
- White, K. Total and partial intravenous anaesthesia of horses. In Practice 2015, 37, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Aubin, M.L.; Mama, K. Field anesthetic techniques for use in horses. Compend. Contin. Educ. Pract. Vet. 2002, 24, 411–417. [Google Scholar]
- Lerche, P. Total intravenous anesthesia in horses. Vet. Clin. N. Am. Equine Pract. 2013, 29, 123–129. [Google Scholar] [CrossRef] [PubMed]
- McFadzean, W.J.M.; Love, E.J. Perioperative pain management in horses. Equine Vet. Educ. 2018, 31, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Young, S.S.; Taylor, P.M. Factors influencing the outcome of equine anaesthesia: A review of 1314 cases. Equine Vet. J. 1993, 25, 147–151. [Google Scholar] [CrossRef]
- Villamandos, R.G.; Valenzuela, J.M.S.; Calatrava, I.R.; Garcia, J.R.; Jurado, I.A. Clinical problems during inhalatory anaesthesia in horses. A review of 114 cases. Vet. Mex. 1996, 27, 103–105. [Google Scholar]
- Bidwell, L.A.; Bramlage, L.R.; Rood, W.A. Equine perioperative fatalities associated with general anaesthesia at a private practice—A retrospective case series. Vet. Anaesth. Analg. 2007, 34, 23–30. [Google Scholar] [CrossRef]
- Voulgaris, D.A.; Hofmeister, E.H. Multivariate analysis of factors associated with post-anesthetic times to standing in isoflurane-anesthetized horses: 381 cases. Vet. Anaesth. Analg. 2009, 36, 414–420. [Google Scholar] [CrossRef]
- Czupalla, I.; Gerhards, H. Risk of general anesthesia in horses—A retrospective study on 1.989 cases. Pferdeheilkunde 2013, 29, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Dugdale, A.H.; Obhrai, J.; Cripps, P.J. Twenty years later: A single-centre, repeat retrospective analysis of equine perioperative mortality and investigation of recovery quality. Vet. Anaesth. Analg. 2016, 43, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Borland, K.J.; Shaw, D.J.; Clutton, R.E. Time-related changes in post-operative equine morbidity: A single-centre study. Equine Vet. Educ. 2017, 29, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Laurenza, C.; Ansart, L.; Portier, K. Risk factors of anesthesia-related mortality and morbidity in one equine hospital: A retrospective study on 1,161 cases undergoing elective or emergency surgeries. Front. Vet. Sci. 2020, 6, 514. [Google Scholar] [CrossRef] [PubMed]
- Trim, C.M.; Adams, J.G.; Cowgill, L.M.; Ward, S.L. A retrospective survey of anaesthesia in horses with colic. Equine Vet. J. Suppl. 1989, 7, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.R.; Livesey, M.A. Evaluation of exploratory laparotomy in young horses: 102 cases (1987–1992). J. Am. Vet. Med. Assoc. 1997, 211, 1158–1162. [Google Scholar]
- Stucki, F.; Spadavecchia, C.; Jaggin, N.; Schatzmann, U. Anaesthesia related problems in equine colic surgery: A retrospective study from 1995 until 1999. Tierarztl. Prax. Ausg. G. Grosstiere Nutztiere 2001, 29, 212–216. [Google Scholar]
- Driscoll, N.; Baia, P.; Fischer, A.T.; Brauer, T.; Klohnen, A. Large colon resection and anastomosis in horses: 52 cases (1996–2006). Equine Vet. J. 2008, 40, 342–347. [Google Scholar] [CrossRef]
- Rioja, E.; Cernicchiaro, N.; Costa, M.C.; Valverde, A. Perioperative risk factors for mortality and length of hospitalization in mares with dystocia undergoing general anesthesia: A retrospective study. Can. Vet. J. 2012, 53, 502–510. [Google Scholar] [PubMed]
- Frei, S.; Kummer, M.; Fürst, A.; Wehrli Eser, M. Cystoliths in the horse—A retrospective study. Pferdeheilkunde 2016, 32, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Franci, P.; Leece, E.A.; Brearley, J.C. Post anaesthetic myopathy/neuropathy in horses undergoing magnetic resonance imaging compared to horses undergoing surgery. Equine Vet. J. 2006, 38, 497–501. [Google Scholar] [CrossRef]
- Driessen, B.; Zarucco, L.; Kalir, B.; Bertolotti, L. Contemporary use of acepromazine in the anaesthetic management of male horses and ponies: A retrospective study and opinion poll. Equine Vet. J. 2011, 43, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.M.; Taylor, P.M.; Holmes, M.A.; Wood, J.L. Confidential enquiry of perioperative equine fatalities (CEPEF-1): Preliminary results. Equine Vet. J. 1995, 27, 193–200. [Google Scholar] [CrossRef]
- Kästner, S.B.R. How to manage recovery from anaesthesia in the horse—To assist or not to assist? Pferdeheilkunde 2010, 26, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Schrimpf, R.; Tichy, A.; Stanek, C. Relevance of assisting horses in recovery after general anesthesia for osteosynthesis? Pferdeheilkunde 2011, 27, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Wohlfender, F.D.; Doherr, M.G.; Driessen, B.; Hartnack, S.; Johnston, G.M.; Bettschart-Wolfensberger, R. International online survey to assess current practice in equine anaesthesia. Equine Vet. J. 2015, 47, 65–71. [Google Scholar] [CrossRef] [Green Version]
- De Miguel Garcia, C.; Campoy, L.; Parry, S.; Miller, J.E.; Martin-Flores, M.; Gleed, R.D. Questionnaire on the process of recovering horses from general anesthesia and associated personnel injury in equine practice. Vet. Anaesth. Analg. 2021, 48, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.L.; McDonell, W.N.; Young, S.S. A comparison of romifidine and xylazine when used with diazepam/ketamine for short duration anesthesia in the horse. Can. Vet. J. 1996, 37, 601–609. [Google Scholar] [PubMed]
- Marntell, S.; Nyman, G. Effects of additional premedication on romifidine and ketamine anaesthesia in horses. Acta Vet. Scand. 1996, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M.; Bennett, R.C.; Brearley, J.C.; Luna, S.P.; Johnson, C.B. Comparison of detomidine and romifidine as premedicants before ketamine and halothane anesthesia in horses undergoing elective surgery. Am. J. Vet. Res. 2001, 62, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Muir, W.W., 3rd; Tsubakishita, S.; Abrahamsen, E.; Lerch, P.; Hubbell, J.A.; Bednarski, R.M.; Skarda, R.T.; Izumisawa, Y.; Kotani, T. Clinical comparison of xylazine and medetomidine for premedication of horses. J. Am. Vet. Med. Assoc. 2002, 221, 1144–1149. [Google Scholar] [CrossRef]
- Corletto, F.; Raisis, A.A.; Brearley, J.C. Comparison of morphine and butorphanol as pre-anaesthetic agents in combination with romifidine for field castration in ponies. Vet. Anaesth. Analg. 2005, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lascurain, A.A.G.; Lopez, H.S.; Steffey, E.P.; Doherty, P.S.; Hernandez, E.N. The influence of butorphanol dose on characteristics of xylazine-butorphanol-propofol anesthesia in horses at altitude. Vet. Anaesth. Analg. 2006, 33, 104–110. [Google Scholar] [CrossRef]
- Marntell, S.; Nyman, G.; Funkquist, P. Dissociative anaesthesia during field and hospital conditions for castration of colts. Acta Vet. Scand. 2006, 47, 1–11. [Google Scholar] [CrossRef]
- Hopster, K.; Iversen, C.; Rohn, K.; Schiemann, V.; Ohnesorge, B. Influence of the combination of butorphanol and detomidine within premedication on the preoperative sedations score, the intraoperative cardiovascular situation and the early recovery period in horses. Pferdeheilkunde 2008, 24, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.G.; Sellon, D.C.; Cary, J.A.; Hines, M.T.; Farnsworth, K.D. Analgesic effects of butorphanol tartrate and phenylbutazone administered alone and in combination in young horses undergoing routine castration. J. Am. Vet. Med. Assoc. 2009, 235, 1194–1203. [Google Scholar] [CrossRef] [Green Version]
- Mane, S.; Mahajan, S.K.; Mohindroo, J.; Singh, K.; Singh, N.; Saini, N.S. Comparison of acepromazine, midazolam and xylazine as preanaesthetics to ketamine-isoflurane anaesthesia in horses. Indian J. Vet. Surg. 2014, 35, 85–88. [Google Scholar]
- Rigotti, C.; de Vries, A.; Taylor, P.M. Buprenorphine provides better anaesthetic conditions than butorphanol for field castration in ponies: Results of a randomised clinical trial. Vet. Rec. 2014, 175, 623. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.C.; Benedetti, I.C.C.; Guillebert, I.; Portier, K.G. Effect of pre- and postoperative phenylbutazone and morphine administration on the breathing response to skin incision, recovery quality, behavior, and cardiorespiratory variables in horses undergoing fetlock arthroscopy: A pilot study. Front. Vet. Sci. 2015, 2, 58. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, H.; William, B.J.; George, R.S.; Kannan, T.A. Analgesic and adjunct actions of nalbuphine hydrochloride in xylazine or xylazine and acepromazine premedicated horses. Indian J. Anim. Res. 2015, 49, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Taffarel, M.O.; Luna, S.P.L.; Cardoso, G.S.; de Oliveira, F.A.; Alonso, J.D.M.; Gozalo-Marcilla, M. Preemptive analgesia, including morphine, does not affect recovery quality and times in either pain-free horses or horses undergoing orchiectomy. J. Equine Vet. Sci. 2017, 48, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Gopinathan, A.; Singh, K.; Sarangom, S.B.; Sowbharenya, C.; John, C.; Verma, M.R. A preliminary study on effects of subanesthetic doses of preemptive ketamine given prior to premedication on total intravenous anesthesia for short- to medium-term surgical procedures in horses. Turkish J. Vet. Anim. Sci. 2019, 43, 456–468. [Google Scholar] [CrossRef]
- Carregaro, A.B.; Ueda, G.I.; Censoni, J.B.; Bisetto, S.P.; Alonso, B.B.; Reginato, G.M. Effect of methadone combined with acepromazine or detomidine on sedation and dissociative anesthesia in healthy horses. J. Equine Vet. Sci. 2020, 86, 102908. [Google Scholar] [CrossRef]
- Smith, M.C.; Bass, L.; Damone, J.; Mama, K.; Rao, S. Comparison of xylazine and detomidine in combination with midazolam/ketamine for field castration in Quarter Horses. Equine Vet. J. 2020, 52, 516–521. [Google Scholar] [CrossRef]
- Jaugstetter, H.; Jacobi, R.; Pellmann, R. Comparison of romifidine and xylazine as premedicants before general anaesthesia in horses regarding the postsurgical recovery period. Praktische Tierarzt. 2002, 83, 786–791. [Google Scholar]
- McCashin, F.B.; Gabel, A.A. Evaluation of xylazine as a sedative and preanesthetic agent in horses. Am. J. Vet. Res. 1975, 36, 1421–1429. [Google Scholar]
- Fisher, R.J. A field trial of ketamine anaesthesia in the horse. Equine Vet. J. 1984, 16, 176–179. [Google Scholar] [CrossRef]
- Diamond, M.J.; Young, L.E.; Bartram, D.H.; Gregg, A.S.; Clutton, R.E.; Long, K.J.; Jones, R.S. Clinical evaluation of romifidine/ketamine/halothane anaesthesia in horses. Vet. Rec. 1993, 132, 572–575. [Google Scholar] [CrossRef]
- Deppe, R.; Degrenade, J. Use of a propionylpromazine and meperidine combination in thiopental sodium anesthesia in horses. Zentralbl. Veterinarmed. A 1985, 32, 59–67. [Google Scholar] [CrossRef]
- Watkins, S.B.; Watney, G.C.; Hall, L.W.; Houlton, J.E. A clinical trial of three anaesthetic regimens for the castration of ponies. Vet. Rec. 1987, 120, 274–276. [Google Scholar] [CrossRef]
- Brock, N.; Hildebrand, S.V. A comparison of xylazine-diazepam-ketamine and xylazine-guaifenesin-ketamine in equine anesthesia. Vet. Surg. 1990, 19, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Mama, K.R.; Steffey, E.P.; Pascoe, P.J. Evaluation of propofol as a general anesthetic for horses. Vet. Surg. 1995, 24, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gangl, M.; Grulke, S.; Detilleux, J.; Caudron, I.; Serteyn, D. Comparison of thiopentone/guaifenesin, ketamine/guaifenesin and ketamine/midazolam for the induction of horses to be anaesthetised with isoflurane. Vet. Rec. 2001, 149, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Mama, K.R.; Steffey, E.P.; Brevard, L.F.; Hellyer, P.W. Behavioral responses following eight anesthetic induction protocols in horses. Vet. Anaesth. Analg. 2002, 29, 207–211. [Google Scholar] [CrossRef]
- Frias, A.F.; Marsico, F.; de Segura, I.A.G.; Nascimento, P.R.; Nascimento, A.; Soares, J.H.; Almosny, N.R. Evaluation of different doses of propofol in xylazine pre-medicated horses. Vet. Anaesth. Analg. 2003, 30, 193–201. [Google Scholar] [CrossRef]
- Boscan, P.; Steffey, E.P.; Farver, T.B.; Mama, K.R.; Huang, N.J.; Harris, S.B. Comparison of high (5%) and low (1%) concentrations of micellar microemulsion propofol formulations with a standard (1%) lipid emulsion in horses. Am. J. Vet. Res. 2006, 67, 1476–1483. [Google Scholar] [CrossRef]
- Keates, H.L.; van Eps, A.W.; Pearson, M.R. Alfaxalone compared with ketamine for induction of anaesthesia in horses following xylazine and guaifenesin. Vet. Anaesth. Analg. 2012, 39, 591–598. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Brosnan, R.J.; Shilo-Benjamini, Y.; Moore, S.B.; Hollingsworth, S.R. Effects of ketamine, propofol, or thiopental administration on intraocular pressure and qualities of induction of and recovery from anesthesia in horses. Am. J. Vet. Res. 2013, 74, 1070–1077. [Google Scholar] [CrossRef]
- De Vries, A.; Thomson, S.; Taylor, P.M. Comparison of midazolam and diazepam as co-induction agents with ketamine for anaesthesia in sedated ponies undergoing field castration. Vet. Anaesth. Analg. 2015, 42, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Wakuno, A.; Aoki, M.; Kushiro, A.; Mae, N.; Kodaira, K.; Maeda, T.; Yamazaki, Y.; Ohta, M. Comparison of alfaxalone, ketamine and thiopental for anaesthetic induction and recovery in Thoroughbred horses premedicated with medetomidine and midazolam. Equine Vet. J. 2017, 49, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, M.A.; Bailey, K.M.; Messenger, K.M.; Prange, T.; Gaines, B.; Posner, L.P. Recovery of horses from general anesthesia after induction with propofol and ketamine versus midazolam and ketamine. J. Am. Vet. Med. Assoc. 2018, 253, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Robinson, R.; Jolliffe, C.; Taylor, P.M. Evaluation of the use of midazolam as a co-induction agent with ketamine for anaesthesia in sedated ponies undergoing field castration. Equine Vet. J. 2018, 50, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Harðardóttir, H.; Murison, P.J.; Blissitt, K.; Olason, S.; Clutton, R.E. A comparison of two ketamine doses for field anaesthesia in horses undergoing castration. Equine Vet. J. 2019, 51, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Grono, L.R. Methohexital sodium anaesthesia in the horse. Aust. Vet. J. 1966, 42, 398–400. [Google Scholar] [CrossRef]
- Brouwer, G.J.; Hall, L.W.; Kuchel, T.R. Intravenous anaesthesia in horses after xylazine premedication. Vet. Rec. 1980, 107, 241–245. [Google Scholar] [CrossRef]
- Tokushige, H.; Araki, M.; Kusano, K.; Arima, D.; Ito, H.; Yamazaki, Y.; Urayama, S.; Kambayashi, Y.; Tateno, O.; Ohta, M. A retrospective comparison of induction with thiopental/guaifenesin and propofol/ketamine in Thoroughbred racehorses anesthetized with sevoflurane and medetomidine during arthroscopic surgery. J. Equine Sci. 2019, 30, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Eales, F.A. Effects of Saffan administered intravenously in the horse. Vet. Rec. 1976, 99, 270–272. [Google Scholar] [CrossRef]
- Kalhoro, A.B.; Rex, M.A. Observations on the use of glyceryl guaiacolate as an adjunct to general anaesthesia in horses. Aust. Vet. J. 1984, 61, 49–53. [Google Scholar] [CrossRef]
- Luna, S.P.; Taylor, P.M.; Massone, F. Midazolam and ketamine induction before halothane anaesthesia in ponies: Cardiorespiratory, endocrine and metabolic changes. J. Vet. Pharmacol. Ther. 1997, 20, 153–159. [Google Scholar] [CrossRef]
- Muir, W.W., 3rd; Gadawski, J.E.; Grosenbaugh, D.A. Cardiorespiratory effects of a tiletamine/zolazepam-ketamine-detomidine combination in horses. Am. J. Vet. Res. 1999, 60, 770–774. [Google Scholar]
- Goodwin, W.A.; Keates, H.L.; Pasloske, K.; Pearson, M.; Sauer, B.; Ranasinghe, M.G. The pharmacokinetics and pharmacodynamics of the injectable anaesthetic alfaxalone in the horse. Vet. Anaesth. Analg. 2011, 38, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, N.; Rinnovati, R.; Lambertini, C.; Spadari, A. Short-term general anesthesia with tiletamine/zolazepam in horses sedated with medetomidine for castration under field conditions. J. Equine Vet. Sci. 2018, 67, 50–54. [Google Scholar] [CrossRef]
- Longley, E.O. Thiopentone (pentothal sodium) as a general anaesthetic in the horse. Vet. Rec. 1950, 62, 17–20. [Google Scholar]
- Bishop, W.J. Glyceryl guaiacolate in equine anaesthesia. N. Z. Vet. J. 1978, 26, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Kaka, J.S.; Klavano, P.A.; Hayton, W.L. Pharmacokinetics of ketamine in the horse. Am. J. Vet. Res. 1979, 40, 978–981. [Google Scholar]
- Mama, K.R.; Steffey, E.P.; Pascoe, P.J. Evaluation of propofol for general anesthesia in premedicated horses. Am. J. Vet. Res. 1996, 57, 512–516. [Google Scholar]
- Bennett, R.C.; Taylor, P.M.; Brearley, J.C.; Johnson, C.B.; Luna, S.P. Comparison of detomidine/ketamine and guaiphenesin/thiopentone for induction of anaesthesia in horses maintained with halothane. Vet. Rec. 1998, 142, 541–545. [Google Scholar] [CrossRef]
- Matthews, N.S.; Hartsfield, S.M.; Cornick, J.L.; Williams, J.D.; Beasley, A. A comparison of injectable anesthetic combinations in horses. Vet. Surg. 1991, 20, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Posner, L.P.; Kasten, J.I.; Kata, C. Propofol with ketamine following sedation with xylazine for routine induction of general anaesthesia in horses. Vet. Rec. 2013, 173, 550. [Google Scholar] [CrossRef]
- Hall, L.W.; Taylor, P.M. Clinical trial of xylazine with ketamine in equine anaesthesia. Vet. Rec. 1981, 108, 489–493. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, R.P.; Misra, S.S. Equine castration: A practical approach. Indian Vet. Med. J. 1994, 18, 110–113. [Google Scholar]
- Oku, K.; Yamanaka, T.; Ashihara, N.; Kawasaki, K.; Mizuno, Y.; Fujinaga, T. Clinical observations during induction and recovery of xylazine-midazolam- propofol anesthesia in horses. J. Vet. Med. Sci. 2003, 65, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.P.; Sharma, S.K.; Sharma, A.; Kumar, A. Clinical evaluation of detomidine-butorphanol-guaifenesin-ketamine as short term TIVA in Spiti ponies. Pak. J. Biol. Sci. 2011, 14, 647–652. [Google Scholar] [CrossRef]
- Muir, W.W.; Skarda, R.T.; Milne, D.W. Evaluation of xylazine and ketamine hydrochloride for anesthesia in horses. Am. J. Vet. Res. 1977, 38, 195–201. [Google Scholar] [PubMed]
- Muir, W.W.; Skarda, R.T.; Sheehan, W. Evaluation of xylazine, guaifenesin, and ketamine hydrochloride for restraint in horses. Am. J. Vet. Res. 1978, 39, 1274–1278. [Google Scholar] [PubMed]
- Lin, H.C.; Branson, K.R.; Thurmon, J.C.; Benson, G.J.; Tranquilli, W.J.; Olson, W.A.; Vaha-Vahe, A.T. Ketamine, Telazol, xylazine and detomidine. A comparative anesthetic drug combinations study in ponies. Acta Vet. Scand. 1992, 33, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M.; Hall, L.W. Clinical anaesthesia in the horse: Comparison of enflurane and halothane. Equine Vet. J. 1985, 17, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Daunt, D.A.; Steffey, E.P.; Pascoe, J.R.; Willits, N.; Daels, P.F. Actions of isoflurane and halothane in pregnant mares. J. Am. Vet. Med. Assoc. 1992, 201, 1367–1374. [Google Scholar] [PubMed]
- Matthews, N.S.; Miller, S.M.; Hartsfield, S.M.; Slater, M.R. Comparison of recoveries from halothane vs isoflurane anesthesia in horses. J. Am. Vet. Med. Assoc. 1992, 201, 559–563. [Google Scholar]
- Donaldson, L.L.; Dunlop, G.S.; Holland, M.S.; Burton, B.A. The recovery of horses from inhalant anesthesia: A comparison of halothane and isoflurane. Vet. Surg. 2000, 29, 92–101. [Google Scholar] [CrossRef]
- Johnston, G.M.; Eastment, J.K.; Taylor, P.M.; Wood, J.L. Is isoflurane safer than halothane in equine anaesthesia? Results from a prospective multicentre randomised controlled trial. Equine Vet. J. 2004, 36, 64–71. [Google Scholar] [CrossRef]
- Driessen, B.; Nann, L.; Benton, R.; Boston, R. Differences in need for hemodynamic support in horses anesthetized with sevoflurane as compared to isoflurane. Vet. Anaesth. Analg. 2006, 33, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Durongphongtorn, S.; McDonell, W.N.; Kerr, C.L.; Neto, F.J.; Mirakhur, K.K. Comparison of hemodynamic, clinicopathologic, and gastrointestinal motility effects and recovery characteristics of anesthesia with isoflurane and halothane in horses undergoing arthroscopic surgery. Am. J. Vet. Res. 2006, 67, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Leece, E.A.; Corletto, F.; Brearley, J.C. A comparison of recovery times and characteristics with sevoflurane and isoflurane anaesthesia in horses undergoing magnetic resonance imaging. Vet. Anaesth. Analg. 2008, 35, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.C.; Brosnan, R.J.; Guedes, A.G. Desflurane and sevoflurane elimination kinetics and recovery quality in horses. Am. J. Vet. Res. 2015, 76, 201–207. [Google Scholar] [CrossRef]
- Auer, J.A.; Garner, H.E.; Amend, J.F.; Hutcheson, D.P.; Salem, C.A. Recovery from anaesthesia in ponies: A comparative study of the effects of isoflurane, enflurane, methoxyflurane and halothane. Equine Vet. J. 1978, 10, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.C.; Gleed, R.D.; Matthews, N.S.; Tyner, C.L.; Erb, H.N.; Short, C.E. Isoflurane anesthesia for equine colic surgery. Comparison with halothane anesthesia. Vet. Surg. 1987, 16, 184–188. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Muir, W.W. Cardiorespiratory effects of sevoflurane, isoflurane, and halothane anesthesia in horses. Am. J. Vet. Res. 1998, 59, 101–106. [Google Scholar] [PubMed]
- Arican, M.; Erol, H.; Esin, E. Clinical comparison of medetomidine with isoflurane or sevoflurane for anesthesia in horses. Pak. Vet. J. 2015, 35, 474–478. [Google Scholar]
- Titus, R.S. A study of the use of methoxyflurane general anesthesia in the horse. Southwest. Vet. 1964, 17, 279–288. [Google Scholar]
- De Moor, A.E.; Van Den Hende, C.L.; Verschooten, F.M.; Desmet, P.J.; Watte, R. Influence of a clinical anaesthesia-technique (premedication with tranquillizers and atropine, induction with chloralhydrate, maintenance with halothane in a closed circle system) on liver function tests in the horse. Zentralbl. Veterinarmed. A 1969, 16, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Seymour, C.J. Clinical experiences with isoflurane in dogs and horses. Vet. Rec. 1986, 119, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Aida, H.; Mizuno, Y.; Hobo, S.; Yoshida, K.; Fujinaga, T. Determination of the minimum alveolar concentration (MAC) and physical response to sevoflurane inhalation in horses. J. Vet. Med. Sci. 1994, 56, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Hikasa, Y.; Takase, K.; Ogasawara, S. Sevoflurane and oxygen anaesthesia following administration of atropine-xylazine-guaifenesin-thiopental in spontaneously breathing horses. Zentralbl. Veterinarmed. A 1994, 41, 700–708. [Google Scholar] [CrossRef]
- Aida, H.; Mizuno, Y.; Hobo, S.; Yoshida, K.; Fujinaga, T. Cardiovascular and pulmonary effects of sevoflurane anesthesia in horses. Vet. Surg. 1996, 25, 164–170. [Google Scholar] [CrossRef]
- Tendillo, F.J.; Mascias, A.; Santos, M.; Lopez-Sanroman, J.; de Rossi, R.; San Roman, F.; de Segura, I.A.G. Anesthetic potency of desflurane in the horse: Determination of the minimum alveolar concentration. Vet. Surg. 1997, 26, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Carroll, G.L.; Hooper, R.N.; Rains, C.B.; Martinez, E.A.; Matthews, N.S.; Hartsfield, S.M.; Beleau, M.H. Maintenance of anaesthesia with sevoflurane and oxygen in mechanically-ventilated horses subjected to exploratory laparotomy treated with intra- and post operative anaesthetic adjuncts. Equine Vet. J. 1998, 30, 402–407. [Google Scholar] [CrossRef]
- Stegmann, G.F.; Jones, R.S. Perioperative plasma cortisol concentration in the horse. J. S. Afr. Vet. Assoc. 1998, 69, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, J.M.; Gomez-Villamandos, R.J.; Santisteban, J.M.; Ruiz, I.; Redondo, J.I.; Avila, I. Romifidine-ketamine-halothane anesthesia in horses. Equine Pract. 1999, 21, 20–21. [Google Scholar]
- Ohta, M.; Oku, K.; Yamanaka, T.; Mizuno, Y. Anesthetic management with sevoflurane and oxygen for orthopedic surgeries in racehorses. J. Vet. Med. Sci. 2000, 62, 1017–1020. [Google Scholar] [CrossRef] [Green Version]
- Seddighi, M.R.; Mohri, M. Anesthesia in Caspian ponies. Vet. Anaesth. Analg. 2008, 35, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, H.; Kakizaki, M.; Ode, H.; Okano, A.; Okada, J.; Kuroda, T.; Wakuno, A.; Ohta, M. Validation of the bispectral index as an indicator of anesthetic depth in Thoroughbred horses anesthetized with sevoflurane. J. Equine Sci. 2016, 27, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangelo, B.; Robin, A.; Simpson, K.; Potier, J.; Guichardant, M.; Portier, K. The modification and performance of a large animal anesthesia machine (Tafonius®) in order to deliver xenon to a horse. Front. Vet. Sci. 2017, 4, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.C.; Thurmon, J.C.; Benson, G.J.; Tranquilli, W.J.; Olson, W.A. Guaifenesin-ketamine-xylazine anesthesia for castration in ponies: A comparative study with two different doses of ketamine. J. Equine Vet. Sci. 1993, 13, 29–32. [Google Scholar] [CrossRef]
- Muir, W.W., 3rd; Lerche, P.; Robertson, J.T.; Hubbell, J.A.; Beard, W.; Miller, T.; Badgley, B.; Bothwell, V. Comparison of four drug combinations for total intravenous anesthesia of horses undergoing surgical removal of an abdominal testis. J. Am. Vet. Med. Assoc. 2000, 217, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Mama, K.R.; Wagner, A.E.; Steffey, E.P.; Kollias-Baker, C.; Hellyer, P.W.; Golden, A.E.; Brevard, L.F. Evaluation of xylazine and ketamine for total intravenous anesthesia in horses. Am. J. Vet. Res. 2005, 66, 1002–1007. [Google Scholar] [CrossRef]
- Umar, M.A.; Yamashita, K.; Kushiro, T.; Muir, W.W. Evaluation of total intravenous anesthesia with propofol or ketamine-medetomidine-propofol combination in horses. J. Am. Vet. Med. Assoc. 2006, 228, 1221–1227. [Google Scholar] [CrossRef]
- Baetge, C.L.; Matthews, N.S.; Carroll, G.L. Comparison of 3 total intravenous anesthetic infusion combinations in adult horses. Int. J. Appl. Res. Vet. Med. 2007, 5, 1–8. [Google Scholar]
- Umar, M.A.; Yamashita, K.; Kushiro, T.; Muir, W.W., 3rd. Evaluation of cardiovascular effects of total intravenous anesthesia with propofol or a combination of ketamine-medetomidine-propofol in horses. Am. J. Vet. Res. 2007, 68, 121–127. [Google Scholar] [CrossRef]
- Rossetti, R.B.; Gaido Cortopassi, S.R.; Intelizano, T.; de Lima Machado, T.S.; da Cruz, R.S.F. Comparison of ketamine and S(+)-ketamine, with romifidine and diazepam, for total intravenous anesthesia in horses. Vet. Anaesth. Analg. 2008, 35, 30–37. [Google Scholar] [CrossRef]
- Muir, W.W.; Lerche, P.; Erichson, D. Anaesthetic and cardiorespiratory effects of propofol at 10% for induction and 1% for maintenance of anaesthesia in horses. Equine Vet. J. 2009, 41, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Valverde, A. Short-term anaesthesia with xylazine, diazepam/ketamine for castration in horses under field conditions: Use of intravenous lidocaine. Equine Vet. J. 2009, 41, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; William, B.J.; Rao, G.D.J.; Prathaban, S.; Kumar, R.S.; Leela, V. Anaesthetic evaluation of ketamine/propofol in acepromazine- xylazine premedicated horses. Indian J. Anim. Res. 2010, 44, 139–142. [Google Scholar]
- Kloppel, H.; Leece, E.A. Comparison of ketamine and alfaxalone for induction and maintenance of anaesthesia in ponies undergoing castration. Vet. Anaesth. Analg. 2011, 38, 37–43. [Google Scholar] [CrossRef]
- Ishizuka, T.; Itami, T.; Tamura, J.; Saitoh, Y.; Saitoh, M.; Umar, M.A.; Miyoshi, K.; Yamashita, K.; Muir, W.W. Anesthetic and cardiorespiratory effects of propofol, medetomidine, lidocaine and butorphanol total intravenous anesthesia in horses. J. Vet. Med. Sci. 2013, 75, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Neto, P.I.N.; Luna, S.P.; Queiroz-Williams, P.; Mama, K.R.; Steffey, E.P.; Carregaro, A.B. Cardiorespiratory and antinociceptive effects of two different doses of lidocaine administered to horses during a constant intravenous infusion of xylazine and ketamine. BMC Vet. Res. 2013, 9, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopster, K.; Muller, C.; Hopster-Iversen, C.; Stahl, J.; Rohn, K.; Kastner, S. Effects of dexmedetomidine and xylazine on cardiovascular function during total intravenous anaesthesia with midazolam and ketamine and recovery quality and duration in horses. Vet. Anaesth. Analg. 2014, 41, 25–35. [Google Scholar] [CrossRef]
- Nanda, V.S.; Kumar, A.; Kanwar, M.S.; Sharma, A.; Sharma, S.K.; Khurana, A. Continuous maintenance anaesthesia using guaifenesin or diazepam combined with xylazine and ketamine in horses. Indian J. Vet. Surg. 2014, 35, 89–92. [Google Scholar]
- Aoki, M.; Wakuno, A.; Kushiro, A.; Mae, N.; Kakizaki, M.; Nagata, S.I.; Ohta, M. Evaluation of total intravenous anesthesia with propofol-guaifenesin-medetomidine and alfaxalone-guaifenesin-medetomidine in Thoroughbred horses undergoing castration. J. Vet. Med. Sci. 2017, 79, 2011–2018. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, J.; Ekiri, A.; de Vries, A. Alfaxalone for maintenance of anaesthesia in ponies undergoing field castration: Continuous infusion compared with intravenous boluses. Vet. Anaesth. Analg. 2017, 44, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.M.; Keating, S.C.; Lascola, K.M.; Schaeffer, D.J.; Clark-Price, S.C. Cardiopulmonary effects and recovery characteristics of horses anesthetized with xylazine-ketamine with midazolam or propofol. Vet. Anaesth. Analg. 2018, 45, 772–781. [Google Scholar] [CrossRef]
- Pratt, S.; Cunneen, A.; Perkins, N.; Farry, T.; Kidd, L.; McEwen, M.; Rainger, J.; Truchetti, G.; Goodwin, W. Total intravenous anaesthesia with ketamine, medetomidine and guaifenesin compared with ketamine, medetomidine and midazolam in young horses anaesthetised for computerised tomography. Equine Vet. J. 2019, 51, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.M.; Hall, L.W. Total intravenous anaesthesia in the horse with propofol. Equine Vet. J. 1985, 17, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. The stress response to anaesthesia in ponies: Barbiturate anaesthesia. Equine Vet. J. 1990, 22, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Filzek, U.; Fischer, U.; Ferguson, J. Intravenous anaesthesia in hoses: Racemic ketamine versus S-(+)-ketamine. Pferdeheilkunde 2003, 19, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.A.; Jimenez, J.; Martin, M.F.; Uson, J.M.; Perez, E.M.; Ezquerra, L.J. Tiletamine-zolazepam anesthesia in horses: Repeated dose versus continuous infusion. Rev. Med. Vet. 2004, 155, 197–204. [Google Scholar]
- Kaegi, B. Anesthesia by injection of xylazine, ketamine and the benzodiazepine derivate climazolam and the use of the benzodiazepine antagonist Ro15-3505. Schweiz. Arch. Tierheilkd. 1990, 132, 251–257. [Google Scholar]
- McCarty, J.E.; Trim, C.M.; Ferguson, D. Prolongation of anesthesia with xylazine, ketamine, and guaifenesin in horses: 64 cases (1986–1989). J. Am. Vet. Med. Assoc. 1990, 197, 1646–1650. [Google Scholar]
- Young, L.E.; Bartram, D.H.; Diamond, M.J.; Gregg, A.S.; Jones, R.S. Clinical evaluation of an infusion of xylazine, guaifenesin and ketamine for maintenance of anaesthesia in horses. Equine Vet. J. 1993, 25, 115–119. [Google Scholar] [CrossRef]
- Lin, H.C.; Wallace, S.S.; Robbins, R.L.; Harrison, I.W.; Thurmon, J.C. A case report on the use of guaifenesin-ketamine-xylazine anesthesia for equine dystocia. Cornell Vet. 1994, 84, 61–66. [Google Scholar]
- Taylor, P.M.; Luna, S.P.L.; Sear, J.W.; Wheeler, M.J. Total intravenous anaesthesia in ponies using detomidine, ketamine and guaiphenesin: Pharmacokinetics, cardiopulmonary and endocrine effects. Res. Vet. Sci. 1995, 59, 17–23. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Taylor, P.M.; Sear, J.W.; Bloomfield, M.R.; Rentsch, K.; Dawling, S. Physiologic effects of anesthesia induced and maintained by intravenous administration of a climazolam-ketamine combination in ponies premedicated with acepromazine and xylazine. Am. J. Vet. Res. 1996, 57, 1472–1477. [Google Scholar] [PubMed]
- Davies, T.; Swan, J. Romifidine, ketamine and guaiphenesin continual infusion anaesthesia: Some experiences of its use under field conditions. Equine Vet. Educ. 1997, 9, 12–16. [Google Scholar] [CrossRef]
- Thurmon, J.C.; Ko, J.C.H.; Lin, H.C.; Olson, W.A. Guaifenesin-ketamine-detomidine anesthesia for castration of ponies. J. Equine Vet. Sci. 1997, 17, 262–266. [Google Scholar] [CrossRef]
- Matthews, N.S.; Hartsfield, S.M.; Hague, B.; Carroll, G.L.; Short, C.E. Detomidine-propofol anesthesia for abdominal surgery in horses. Vet. Surg. 1999, 28, 196–201. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Schmucker, N.; Schatzmann, U. Investigations into injection anesthesia (TIVA) of the horse with ketamine/guaifenesin/xylazin: Experiences with computerized pump infusion. Praktische Tierarzt. 1999, 80, 118–122. [Google Scholar]
- Ratajczak, K.; Stochnij, P.; Skrzypczak, P. Anaesthetic compound and its application in general anaesthesia of horses. Med. Weter. 2000, 56, 107–113. [Google Scholar]
- Bettschart-Wolfensberger, R.; Bowen, M.I.; Freeman, S.L.; Feller, R.; Bettschart, R.W.; Nolan, A.; Clarke, K.W. Cardiopulmonary effects of prolonged anesthesia via propofol-medetomidine infusion in ponies. Am. J. Vet. Res. 2001, 62, 1428–1435. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Freeman, S.L.; Jaggin-Schmucker, N.; Clarke, K.W. Infusion of a combination of propofol and medetomidine for long-term anesthesia in ponies. Am. J. Vet. Res. 2001, 62, 500–507. [Google Scholar] [CrossRef]
- Taylor, P.M.; Luna, S.P.; White, K.L.; Bloomfield, M.; Fowden, A.L. Intravenous anaesthesia using detomidine, ketamine and guaiphenesin for laparotomy in pregnant pony mares. Vet. Anaesth. Analg. 2001, 28, 119–125. [Google Scholar] [CrossRef]
- Taylor, P.M.; White, K.L.; Fowden, A.L.; Giussani, D.A.; Bloomfield, M.; Sear, J.W. Propofol anaesthesia for surgery in late gestation pony mares. Vet. Anaesth. Analg. 2001, 28, 177–187. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Freeman, S.; Bettschart, R.W.; Furst, A.; Clarke, K.W. Assessment of a medetomidine/propofol total intravenous anaesthesia (TIVA) for clinical anaesthesia in equidae. Pferdeheilkunde 2002, 18, 39–48. [Google Scholar] [CrossRef]
- Heeß, D.; Schatzmann, U. Practical experiences and clinical parameters in a xylazine-ketamine-anaesthesia of horses. Pferdeheilkunde 2003, 19, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Bettschart-Wolfensberger, R.; Bowen, I.M.; Freeman, S.L.; Weller, R.; Clarke, K.W. Medetomidine-ketamine anaesthesia induction followed by medetomidine-propofol in ponies: Infusion rates and cardiopulmonary side effects. Equine Vet. J. 2003, 35, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Oku, K.; Mukai, K.; Akiyama, K.; Mizuno, Y. Propofol-ketamine anesthesia for internal fixation of fractures in racehorses. J. Vet. Med. Sci. 2004, 66, 1433–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettschart-Wolfensberger, R.; Kalchofner, K.; Neges, K.; Kästner, S.; Fürst, A. Total intravenous anaesthesia in horses using medetomidine and propofol. Vet. Anaesth. Analg. 2005, 32, 348–354. [Google Scholar] [CrossRef]
- Yamashita, K.; Wijayathilaka, T.P.; Kushiro, T.; Umar, M.A.; Taguchi, K.; Muir, W.W. Anesthetic and cardiopulmonary effects of total intravenous anesthesia using a midazolam, ketamine and medetomidine drug combination in horses. J. Vet. Med. Sci. 2007, 69, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Leece, E.A.; Girard, N.M.; Maddern, K. Alfaxalone in cyclodextrin for induction and maintenance of anaesthesia in ponies undergoing field castration. Vet. Anaesth. Analg. 2009, 36, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Boscan, P.; Rezende, M.L.; Grimsrud, K.; Stanley, S.D.; Mama, K.R.; Steffey, E.P. Pharmacokinetic profile in relation to anaesthesia characteristics after a 5% micellar microemulsion of propofol in the horse. Br. J. Anaesth. 2010, 104, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Rezende, M.L.; Boscan, P.; Stanley, S.D.; Mama, K.R.; Steffey, E.P. Evaluation of cardiovascular, respiratory and biochemical effects, and anesthetic induction and recovery behavior in horses anesthetized with a 5% micellar microemulsion propofol formulation. Vet. Anaesth. Analg. 2010, 37, 440–450. [Google Scholar] [CrossRef]
- Oku, K.; Kakizaki, M.; Ono, K.; Ohta, M. Clinical evaluation of total intravenous anesthesia using a combination of propofol and medetomidine following anesthesia induction with medetomidine, guaifenesin and propofol for castration in Thoroughbred horses. J. Vet. Med. Sci. 2011, 73, 1639–1643. [Google Scholar] [CrossRef] [Green Version]
- Hubbell, J.A.; Aarnes, T.K.; Lerche, P.; Bednarski, R.M. Evaluation of a midazolam-ketamine-xylazine infusion for total intravenous anesthesia in horses. Am. J. Vet. Res. 2012, 73, 470–475. [Google Scholar] [CrossRef]
- Goodwin, W.A.; Keates, H.L.; Pearson, M.; Pasloske, K. Alfaxalone and medetomidine intravenous infusion to maintain anaesthesia in colts undergoing field castration. Equine Vet. J. 2013, 45, 315–319. [Google Scholar] [CrossRef]
- Casoni, D.; Spadavecchia, C.; Wampfler, B.; Thormann, W.; Levionnois, O.L. Clinical and pharmacokinetic evaluation of S-ketamine for intravenous general anaesthesia in horses undergoing field castration. Acta Vet. Scand. 2015, 57, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, M.A.; Fukui, S.; Kawase, K.; Itami, T.; Yamashita, K. Cardiovascular effects of total intravenous anesthesia using ketamine-medetomidine-propofol (KMP-TIVA) in horses undergoing surgery. J. Vet. Med. Sci. 2015, 77, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmura, H.; Okano, A.; Mukai, K.; Fukuda, K.; Takahashi, T. Cardiorespiratory and anesthetic effects of combined alfaxalone, butorphanol, and medetomidine in Thoroughbred horses. J. Equine Sci. 2016, 27, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarnes, T.K.; Lerche, P.; Bednarski, R.M.; Hubbell, J.A.E. Total intravenous anesthesia using a midazolam-ketamine-xylazine infusion in horses: 46 cases (2011–2014). Can. Vet. J. 2018, 59, 500–504. [Google Scholar]
- Goodwin, W.A.; Pasloske, K.; Keates, H.L.; Ranasinghe, M.G.; Woldeyohannes, S.; Perkins, N. Alfaxalone for total intravenous anaesthesia in horses. Vet. Anaesth. Analg. 2019, 46, 188–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.M.; Kirby, J.J.; Shrimpton, D.J.; Johnson, C.B. Cardiovascular effects of surgical castration during anaesthesia maintained with halothane or infusion of detomidine, ketamine and guaifenesin in ponies. Equine Vet. J. 1998, 30, 304–309. [Google Scholar] [CrossRef]
- Duke-Novakovski, T.; Palacios-Jimenez, C.; Wetzel, T.; Rymes, L.; Sanchez-Teran, A.F. Cardiopulmonary effects of dexmedetomidine and ketamine infusions with either propofol infusion or isoflurane for anesthesia in horses. Vet. Anaesth. Analg. 2015, 42, 39–49. [Google Scholar] [CrossRef]
- Luna, S.P.; Taylor, P.M.; Wheeler, M.J. Cardiorespiratory, endocrine and metabolic changes in ponies undergoing intravenous or inhalation anaesthesia. J. Vet. Pharmacol. Ther. 1996, 19, 251–258. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Stucki, F.; Moens, Y.; Schatzmann, U. Anaesthesia in horses using halothane and intravenous ketamine-guaiphenesin: A clinical study. Vet. Anaesth. Analg. 2002, 29, 20–28. [Google Scholar] [CrossRef]
- Yamashita, K.; Muir, W.W., 3rd; Tsubakishita, S.; Abrahamsen, E.; Lerch, P.; Izumisawa, Y.; Kotani, T. Infusion of guaifenesin, ketamine, and medetomidine in combination with inhalation of sevoflurane versus inhalation of sevoflurane alone for anesthesia of horses. J. Am. Vet. Med. Assoc. 2002, 221, 1150–1155. [Google Scholar] [CrossRef]
- Steffey, E.P.; Eisele, J.H.; Baggot, J.D. Interactions of morphine and isoflurane in horses. Am. J. Vet. Res. 2003, 64, 166–175. [Google Scholar] [CrossRef]
- Valverde, A.; Gunkelt, C.; Doherty, T.J.; Giguere, S.; Pollak, A.S. Effect of a constant rate infusion of lidocaine on the quality of recovery from sevoflurane or isoflurane general anaesthesia in horses. Equine Vet. J. 2005, 37, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Love, E.J.; Lane, J.G.; Murison, P.J. Morphine administration in horses anaesthetized for upper respiratory tract surgery. Vet. Anaesth. Analg. 2006, 33, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mosing, M.; Iff, I.; Rezabek, S. Combined anaesthesia with isoflurane and an infusion of a mixture of ketamine, midazolam and one of three α2-adrenoreceptor agonists for castration in horses. Pferdeheilkunde 2007, 23, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Ringer, S.K.; Kalchofner, K.; Boller, J.; Furst, A.; Bettschart-Wolfensberger, R. A clinical comparison of two anaesthetic protocols using lidocaine or medetomidine in horses. Vet. Anaesth. Analg. 2007, 34, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.; Clutton, R.E.; Blissitt, K.J.; Chase-Topping, M.E. The effects of morphine on the recovery of horses from halothane anaesthesia. Vet. Anaesth. Analg. 2008, 35, 22–29. [Google Scholar] [CrossRef]
- Enderle, A.K.; Levionnois, O.L.; Kuhn, M.; Schatzmann, U. Clinical evaluation of ketamine and lidocaine intravenous infusions to reduce isoflurane requirements in horses under general anaesthesia. Vet. Anaesth. Analg. 2008, 35, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kalchofner, K.S.; Picek, S.; Ringer, S.K.; Jackson, M.; Hassig, M.; Bettschart-Wolfensberger, R. A study of cardiovascular function under controlled and spontaneous ventilation in isoflurane-medetomidine anaesthetized horses. Vet. Anaesth. Analg. 2009, 36, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Knych, H.K.; Steffey, E.P.; Mama, K.R.; Stanley, S.D. Effects of high plasma fentanyl concentrations on minimum alveolar concentration of isoflurane in horses. Am. J. Vet. Res. 2009, 70, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Larenza, M.P.; Ringer, S.K.; Kutter, A.P.; Conrot, A.; Theurillat, R.; Kummer, M.; Thormann, W.; Bettschart-Wolfensberger, R. Evaluation of anesthesia recovery quality after low-dose racemic or S-ketamine infusions during anesthesia with isoflurane in horses. Am. J. Vet. Res. 2009, 70, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Devisscher, L.; Schauvliege, S.; Dewulf, J.; Gasthuys, F. Romifidine as a constant rate infusion in isoflurane anaesthetized horses: A clinical study. Vet. Anaesth. Analg. 2010, 37, 425–433. [Google Scholar] [CrossRef]
- Caure, S.; Cousty, M.; Tricaud, C. Effects of adding butorphanol to a balanced anaesthesia protocol during arthroscopic surgery in horses. Vet. Rec. 2010, 166, 324–328. [Google Scholar] [CrossRef]
- Valverde, A.; Rickey, E.; Sinclair, M.; Rioja, E.; Pedernera, J.; Hathway, A.; Cruz, A. Comparison of cardiovascular function and quality of recovery in isoflurane-anaesthetised horses administered a constant rate infusion of lidocaine or lidocaine and medetomidine during elective surgery. Equine Vet. J. 2010, 42, 192–199. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Dicht, S.; Vullo, C.; Frotzler, A.; Kuemmerle, J.M.; Ringer, S.K. A clinical study on the effect in horses during medetomidine-isoflurane anaesthesia, of butorphanol constant rate infusion on isoflurane requirements, on cardiopulmonary function and on recovery characteristics. Vet. Anaesth. Analg. 2011, 38, 186–194. [Google Scholar] [CrossRef]
- Schauvliege, S.; Marcilla, M.G.; Verryken, K.; Duchateau, L.; Devisscher, L.; Gasthuys, F. Effects of a constant rate infusion of detomidine on cardiovascular function, isoflurane requirements and recovery quality in horses. Vet. Anaesth. Analg. 2011, 38, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Santiago, I.; Gomez De Segura, I.A. Effects of constant rate infusion of lidocaine and ketamine, with or without morphine, on isoflurane MAC in horses. Equine Vet. J. 2011, 43, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Mama, K.R.; Steffey, E.P.; Ferreira, T.H.; Rezende, M.L. Comparison of the cardiovascular effects of equipotent anesthetic doses of sevoflurane alone and sevoflurane plus an intravenous infusion of lidocaine in horses. Am. J. Vet. Res. 2011, 72, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.M.; Lemke, K.A.; Lamont, L.A.; Horney, B.S.; Doyle, A.J. Comparison of the effects of xylazine bolus versus medetomidine constant rate infusion on the stress response, urine production, and anesthetic recovery characteristics in horses anesthetized with isoflurane. J. Am. Vet. Med. Assoc. 2012, 240, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Kempchen, S.; Kuhn, M.; Spadavecchia, C.; Levionnois, O.L. Medetomidine continuous rate intravenous infusion in horses in which surgical anaesthesia is maintained with isoflurane and intravenous infusions of lidocaine and ketamine. Vet. Anaesth. Analg. 2012, 39, 245–255. [Google Scholar] [CrossRef]
- Marcilla, M.G.; Schauvliege, S.; Segaert, S.; Duchateau, L.; Gasthuys, F. Influence of a constant rate infusion of dexmedetomidine on cardiopulmonary function and recovery quality in isoflurane anaesthetized horses. Vet. Anaesth. Analg. 2012, 39, 49–58. [Google Scholar] [CrossRef]
- Nannarone, S.; Spadavecchia, C. Evaluation of the clinical efficacy of two partial intravenous anesthetic protocols, compared with isoflurane alone, to maintain general anesthesia in horses. Am. J. Vet. Res. 2012, 73, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Schuhbeck, M.M.; Kuhn, M.; Spadavecchia, C.; Levionnois, O.L. Continuous intravenous lidocaine infusion during isoflurane anaesthesia in horses undergoing surgical procedures. Pferdeheilkunde 2012, 28, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Chesnel, M.A.; Clutton, R.E. A comparison of two morphine doses on the quality of recovery from general anaesthesia in horses. Res. Vet. Sci. 2013, 95, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; Hopster, K.; Gasthuys, F.; Hatz, L.; Krajewski, A.E.; Schauvliege, S. Effects of a constant-rate infusion of dexmedetomidine on the minimal alveolar concentration of sevoflurane in ponies. Equine Vet. J. 2013, 45, 204–208. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Steblaj, B.; Schauvliege, S.; Duchateau, L.; Gasthuys, F. Comparison of the influence of two different constant-rate infusions (dexmedetomidine versus morphine) on anaesthetic requirements, cardiopulmonary function and recovery quality in isoflurane anaesthetized horses. Res. Vet. Sci. 2013, 95, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, P.; Husulak, M.L.; Bracamonte, J.L.; Beazley, S.G.; Withnall, E.; Duke-Novakovski, T. Cardiopulmonary effects of an infusion of remifentanil or morphine in horses anesthetized with isoflurane and dexmedetomidine. Vet. Anaesth. Analg. 2014, 41, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.P.; Araujo, M.A.; Deschk, M.; Trein, T.A.; Pinheiro, N.C.; Perri, S.H.; Rodrigues, C.A.; Santos, P.S. Effects of a continuous rate infusion of butorphanol in isoflurane-anesthetized horses on cardiorespiratory parameters, recovery quality, gastrointestinal motility and serum cortisol concentrations. Acta Cir. Bras. 2014, 29, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Gozalo-Marcilla, M.; Hopster, K.; Gasthuys, F.; Krajewski, A.E.; Schwarz, A.; Schauvliege, S. Minimum end-tidal sevoflurane concentration necessary to prevent movement during a constant rate infusion of morphine, or morphine plus dexmedetomidine in ponies. Vet. Anaesth. Analg. 2014, 41, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Santiago, I.; de Segura, I.A.G. Effects of a constant rate infusion of medetomidine-propofol on isoflurane minimum alveolar concentrations in horses. Vet. J. 2014, 202, 329–333. [Google Scholar] [CrossRef]
- Lamuraglia, R.; Kirkby, P.; Funcia, J.P. Cardiopulmonary effects and recovery quality of remifentanil–isoflurane anesthesia in horses. J. Equine Vet. Sci. 2015, 35, 271–276. [Google Scholar] [CrossRef]
- Nannarone, S.; Cenani, A.; Gialletti, R.; Pepe, M. Clinical comparison of two regimens of lidocaine infusion in horses undergoing laparotomy for colic. Vet. Anaesth. Analg. 2015, 42, 150–156. [Google Scholar] [CrossRef]
- Tokushige, H.; Ohta, M.; Okano, A.; Kuroda, T.; Kakizaki, M.; Ode, H.; Aoki, M.; Wakuno, A.; Kawasaki, K. Effects of medetomidine constant rate infusion on sevoflurane requirement, cardiopulmonary function, and recovery quality in Thoroughbred racehorses undergoing arthroscopic urgery. J. Equine Vet. Sci. 2015, 35, 83–87. [Google Scholar] [CrossRef]
- Risberg, A.I.; Ranheim, B.; Krontveit, R.I.; Lervik, A.; Haga, H.A. The cardiovascular status of isoflurane-anaesthetized horses with and without dexmedetomidine constant rate infusion evaluated at equivalent depths of anaesthesia. Vet. Anaesth. Analg. 2016, 43, 412–423. [Google Scholar] [CrossRef]
- Menzies, M.P.; Ringer, S.K.; Conrot, A.; Theurillat, R.; Kluge, K.; Kutter, A.P.; Jackson, M.; Thormann, W.; Bettschart-Wolfensberger, R. Cardiopulmonary effects and anaesthesia recovery quality in horses anaesthetized with isoflurane and low-dose S-ketamine or medetomidine infusions. Vet. Anaesth. Analg. 2016, 43, 623–634. [Google Scholar] [CrossRef]
- Cruz Benedetti, I.C.; Nottrott, K.; Fourel, I.; le Bris, M.; Mongellas, E.; Portier, K. Comparison of the effects of an intravenous lidocaine infusion combined with 1% isoflurane versus 2% isoflurane alone on selected cardiovascular variables and recovery characteristics during equine general anaesthesia. Vet. Anaesth. Analg. 2017, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mizobe, F.; Wakuno, A.; Okada, J.; Otsuka, T.; Ishikawa, Y.; Kurimoto, S. Clinical usefulness of intravenous constant rate infusion of fentanyl and medetomidine under sevoflurane anesthesia in Thoroughbred racehorses undergoing internal fixation surgery. J. Equine Sci. 2017, 28, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Del Barrio, M.C.N.; Bennett, R.C.; Hughes, J.M.L. Effect of detomidine or romifidine constant rate infusion on plasma lactate concentration and inhalant requirements during isoflurane anaesthesia in horses. Vet. Anaesth. Analg. 2017, 44, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.; Ringer, S.K.; Bischofberger, A.S.; Berchtold, S.M.; Bettschart-Wolfensberger, R. Clinical comparison of dexmedetomidine and medetomidine for isoflurane balanced anaesthesia in horses. Vet. Anaesth. Analg. 2017, 44, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, H.; Kushiro, A.; Okano, A.; Maeda, T.; Ito, H.; Wakuno, A.; Nagata, S.I.; Ohta, M. Clinical evaluation of constant rate infusion of alfaxalone-medetomidine combined with sevoflurane anesthesia in Thoroughbred racehorses undergoing arthroscopic surgery. Acta Vet. Scand. 2018, 60, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokushige, H.; Okano, A.; Arima, D.; Ito, H.; Kambayashi, Y.; Minamijima, Y.; Ohta, M. Clinical effects of constant rate infusions of medetomidine-propofol combined with sevoflurane anesthesia in Thoroughbred racehorses undergoing arthroscopic surgery. Acta Vet. Scand. 2018, 60, 71. [Google Scholar] [CrossRef] [PubMed]
- Bettembourg, V.; Dulgheriu, D.; Haga, H.A. Plasma concentrations at two dexmedetomidine constant rate infusions in isoflurane anaesthetized horses: A clinical study. Vet. Anaesth. Analg. 2019, 46, 627–635. [Google Scholar] [CrossRef]
- Wagner, A.E.; Dunlop, C.I.; Heath, R.B.; Turner, A.S.; Trotter, G.W. Hemodynamic function during neurectomy in halothane-anesthetized horses with or without constant dose detomidine infusion. Vet. Surg. 1992, 21, 248–255. [Google Scholar] [CrossRef]
- Yamashita, K.; Satoh, M.; Umikawa, A.; Tsuda, A.; Yajima, Y.; Tsubakishita, S.; Seno, T.; Katoh, S.; Izumisawa, Y.; Kotani, T. Combination of continuous intravenous infusion using a mixture of guaifenesin-ketamine-medetomidine and sevoflurane anesthesia in horses. J. Vet. Med. Sci. 2000, 62, 229–235. [Google Scholar] [PubMed] [Green Version]
- Atasoy, N.; Mercan, N.; Atalay, C.; Bayram, E.; Tas, A. Evaluation of a mixture of thiopental-guafinesine-metedomidine and sevoflurane anesthesia in horses. Asian J. Anim. Vet. Adv. 2009, 4, 191–199. [Google Scholar] [CrossRef]
- Poppel, N.; Hopster, K.; Geburek, F.; Kastner, S. Influence of ketamine or xylazine supplementation on isoflurane anaesthetized horses—A controlled clinical trial. Vet. Anaesth. Analg. 2015, 42, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, G.P.; Dinev, D.N.; Sleiman, M.U. Comparative study on sevoflurane anesthesia alone and combined with partial intravenous anesthesia using dexmedetomidine in healthy horses. Pak. Vet. J. 2017, 37, 155–159. [Google Scholar]
- Mircica, E.; Clutton, R.E.; Kyles, K.W.; Blissitt, K.J. Problems associated with perioperative morphine in horses: A retrospective case analysis. Vet. Anaesth. Analg. 2003, 30, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Alonso, B.B.; la Rosa, L.; Carregaro, A.B.; Gasthuys, F.; Schauvliege, S. Recovery quality after romifidine versus detomidine infusion during isoflurane anesthesia in horses. J. Equine Vet. Sci. 2020, 94, 103243. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Jaggin-Schmucker, N.; Lendl, C.; Bettschart, R.W.; Clarke, K.W. Minimal alveolar concentration of desflurane in combination with an infusion of medetomidine for the anaesthesia of ponies. Vet. Rec. 2001, 148, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Feary, D.J.; Mama, K.R.; Thomasy, S.M.; Wagner, A.E.; Enns, R.M. Influence of gastrointestinal tract disease on pharmacokinetics of lidocaine after intravenous infusion in anesthetized horses. Am. J. Vet. Res. 2006, 67, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kalchofner, K.S.; Ringer, S.K.; Boller, J.; Kästner, S.B.R.; Lischer, C.J.; Bettschart-Wolfensberger, R. Clinical assessment of anesthesia with isoflurane and medetomidine in 300 equidae. Pferdeheilkunde 2006, 22, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Kruger, K.; Stegmann, G.F. Partial intravenous anaesthesia in 5 horses using ketamine, lidocaine, medetomidine and halothane. J. S. Afr. Vet. Assoc. 2009, 80, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmansour, P.; Duke-Novakovski, T. Prolonged anesthesia using sevoflurane, remifentanil and dexmedetomidine in a horse. Vet. Anaesth. Analg. 2013, 40, 521–526. [Google Scholar] [CrossRef]
- Wakuno, A.; Maeda, T.; Kodaira, K.; Kikuchi, T.; Ohta, M. Anesthetic management with sevoflurane combined with alfaxalone-medetomidine constant rate infusion in a Thoroughbred racehorse undergoing a long-time orthopedic surgery. J. Equine Sci. 2017, 28, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Doherty, T.J.; Geiser, D.R.; Rohrbach, B.W. The effect of epidural xylazine on halothane minimum alveolar concentration in ponies. J. Vet. Pharmacol. Ther. 1997, 20, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.R.; Nixon, A.J.; Fubini, S.L.; Ducharme, N.G.; Fortier, L.A.; Warnick, L.D.; Ludders, J.W. Epidural morphine and detomidine decreases postoperative hindlimb lameness in horses after bilateral stifle arthroscopy. Vet. Surg. 2002, 31, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Portier, K.G.; Jaillardon, L.; Leece, E.A.; Walsh, C.M. Castration of horses under total intravenous anaesthesia: Analgesic effects of lidocaine. Vet. Anaesth. Analg. 2009, 36, 173–179. [Google Scholar] [CrossRef]
- Abass, M.; Picek, S.; Garzon, J.F.G.; Kuhnle, C.; Zaghlou, A.; Bettschart-Wolfensberger, R. Local mepivacaine before castration of horses under medetomidine isoflurane balanced anaesthesia is effective to reduce perioperative nociception and cytokine release. Equine Vet. J. 2018, 50, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Crandall, A.; Hopster, K.; Grove, A.; Levine, D. Intratesticular mepivacaine versus lidocaine in anaesthetised horses undergoing Henderson castration. Equine Vet. J. 2020, 52, 805–810. [Google Scholar] [CrossRef]
- Gaesser, A.M.; Varner, K.M.; Douglas, H.F.; Barr, C.A.; Hopster, K.; Levine, D.G. The effect of intra-articular mepivacaine administration prior to carpal arthroscopy on anesthesia management and recovery characteristics in horses. Vet. Surg. 2020, 49, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Louro, L.F.; Milner, P.I.; Bardell, D. Epidural administration of opioid analgesics improves quality of recovery in horses anaesthetised for treatment of hindlimb synovial sepsis. Equine Vet. J. 2021. [Google Scholar] [CrossRef]
- Morris, T.B.; Lumsden, J.M.; Dunlop, C.I.; Locke, V.; Sommerauer, S.; Hurcombe, S.D.A. Clinical assessment of an ipsilateral cervical spinal nerve block for prosthetic laryngoplasty in anesthetized horses. Front. Vet. Sci. 2020, 7, 284. [Google Scholar] [CrossRef]
- Bird, A.R.; Morley, S.J.; Sherlock, C.E.; Mair, T.S. The outcomes of epidural anaesthesia in horses with perineal and tail melanomas: Complications associated with ataxia and the risks of rope recovery. Equine Vet. Educ. 2018, 31, 567–574. [Google Scholar] [CrossRef]
- Hopster, K.; Ohnesorge, B.; Von Borstel, M.; Rohn, K.; Kastner, S. Influence of atracurium on cardiovascular parameters in horses undergoing vitrectomy during general anaesthesia, and on recovery duration and quality. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2013, 41, 371–377. [Google Scholar]
- Wiese, A.J.; Brosnan, R.J.; Barter, L.S. Effects of acetylcholinesterase inhibition on quality of recovery from isoflurane-induced anesthesia in horses. Am. J. Vet. Res. 2014, 75, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, S.V.; Howitt, G.A.; Arpin, D. Neuromuscular and cardiovascular effects of atracurium in ponies anesthetized with halothane. Am. J. Vet. Res. 1986, 47, 1096–1100. [Google Scholar]
- Hildebrand, S.V.; Arpin, D. Neuromuscular and cardiovascular effects of atracurium administered to healthy horses anesthetized with halothane. Am. J. Vet. Res. 1988, 49, 1066–1071. [Google Scholar]
- Hildebrand, S.V.; Holland, M.; Copland, V.S.; Daunt, D.; Brock, N. Clinical use of the neuromuscular blocking-agents atracurium and pancuronium for equine anesthesia. J. Am. Vet. Med. Assoc. 1989, 195, 212–219. [Google Scholar] [PubMed]
- Hildebrand, S.V.; Hill, T., 3rd. Effects of atracurium administered by continuous intravenous infusion in halothane-anesthetized horses. Am. J. Vet. Res. 1989, 50, 2124–2126. [Google Scholar]
- Hildebrand, S.V.; Hill, T., 3rd. Interaction of gentamycin and atracurium in anaesthetised horses. Equine Vet. J. 1994, 26, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Aida, H.; Steffey, E.P.; Pascoe, J.R.; Yarbrough, T.B.; Takahashi, T.; Hiraga, A.; Hobo, S.; Smith, B.L.; Steffey, M.A.; Jones, J.H. Use of sevoflurane for anesthetic management of horses during thoracotomy. Am. J. Vet. Res. 2000, 61, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Mosing, M. Prolonged neuromuscular blockade in a horse following concomitant use of vecuronium and atracurium. Vet. Anaesth. Analg. 2012, 39, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Benson, G.J.; Hartsfield, S.M.; Manning, J.P.; Thurmon, J.C. Biochemical effects of succinylcholine chloride in mechanically ventilated horses anesthetized with halothane in oxygen. Am. J. Vet. Res. 1980, 41, 754–756. [Google Scholar] [PubMed]
- Lin, H.C.; Johnson, C.R.; Duran, S.H.; Waldridge, B.M. Effects of intravenous administration of dimethyl sulfoxide on cardiopulmonary and clinicopathologic variables in awake or halothane-anesthetized horses. J. Am. Vet. Med. Assoc. 2004, 225, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Neto, F.J.; McDonell, W.N.; Black, W.D.; Duronghphongtorn, S. Effects of glycopyrrolate on cardiorespiratory function in horses anesthetized with halothane and xylazine. Am. J. Vet. Res. 2004, 65, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Neto, F.J.; McDonell, W.N.; Black, W.D.; Moraes, A.N.; Duronghphongtorn, S. Effects of a muscarinic type-2 antagonist on cardiorespiratory function and intestinal transit in horses anesthetized with halothane and xylazine. Am. J. Vet. Res. 2004, 65, 464–472. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, E.C.; di Concetto, S.; Payton, M.E.; Mandsager, R.E.; Arko, M. Effect of dantrolene premedication on various cardiovascular and biochemical variables and the recovery in healthy isoflurane-anesthetized horses. Am. J. Vet. Res. 2015, 76, 293–301. [Google Scholar] [CrossRef]
- Lee, Y.H.; Clarke, K.W.; Alibhai, H.I.; Song, D. Effects of dopamine, dobutamine, dopexamine, phenylephrine, and saline solution on intramuscular blood flow and other cardiopulmonary variables in halothane-anesthetized ponies. Am. J. Vet. Res. 1998, 59, 1463–1472. [Google Scholar]
- Taylor, P.M. Doxapram infusion during halothane anaesthesia in ponies. Equine Vet. J. 1990, 22, 329–332. [Google Scholar] [CrossRef]
- Gasthuys, F.; Messeman, C.; de Moor, A. Influence of hypertonic saline solution 7.2% on different hematological parameters in awake and anaesthetized ponies. Zentralbl. Veterinarmed. A 1992, 39, 204–214. [Google Scholar] [CrossRef]
- Young, L.E.; Blissitt, K.J.; Clutton, R.E.; Molony, V. Temporal effects of an infusion of dopexamine hydrochloride in horses anesthetized with halothane. Am. J. Vet. Res. 1997, 58, 516–523. [Google Scholar]
- Kitzman, J.V.; Wilson, R.C.; Hatch, R.C.; Booth, N.H. Antagonism of xylazine and ketamine anesthesia by 4-aminopyridine and yohimbine in geldings. Am. J. Vet. Res. 1984, 45, 875–879. [Google Scholar]
- Taylor, P.M. Effect of postoperative pethidine on the anaesthetic recovery period in the horse. Equine Vet. J. 1986, 18, 70–72. [Google Scholar] [CrossRef]
- Matthews, N.S.; Hartsfield, S.M.; Mercer, D.; Beleau, M.H.; Mackenthun, A. Recovery from sevoflurane anesthesia in horses: Comparison to isoflurane and effect of postmedication with xylazine. Vet. Surg. 1998, 27, 480–485. [Google Scholar] [CrossRef]
- Glitz, F.; Lorber, K.; von Oppen, T.; Bubeck, K.; Bartmann, C.P.; Deegen, E. Recovery phase of horses after general anesthesia with inhalants with and without postanesthetic sedation with xylazine (Rompun®). Pferdeheilkunde 2001, 17, 165–172. [Google Scholar] [CrossRef]
- Santos, M.; Fuente, M.; Garcia-Iturralde, R.; Herran, R.; Lopez-Sanroman, J.; Tendillo, F.J. Effects of alpha-2 adrenoceptor agonists during recovery from isoflurane anaesthesia in horses. Equine Vet. J. 2003, 35, 170–175. [Google Scholar] [CrossRef]
- Bienert, A.; Bartmann, C.P.; von Oppen, T.; Poppe, T.; Schiemann, V.; Deegen, E. Standing behavior in horses after inhalation anesthesia with isoflurane (Isoflo) and postanesthetic sedation with romifidine (Sedivet) or xylazine (Rompun). Dtsch. Tierarztl. Wochenschr. 2003, 110, 244–248. [Google Scholar]
- Wagner, A.E.; Mama, K.R.; Steffey, E.P.; Hellyer, P.W. A comparison of equine recovery characteristics after isoflurane or isoflurane followed by a xylazine-ketamine infusion. Vet. Anaesth. Analg. 2008, 35, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Steffey, E.P.; Mama, K.R.; Brosnan, R.J.; Imai, A.; Maxwell, L.K.; Cole, C.A.; Stanley, S.D. Effect of administration of propofol and xylazine hydrochloride on recovery of horses after four hours of anesthesia with desflurane. Am. J. Vet. Res. 2009, 70, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Bauquier, S.H.; Kona-Boun, J.J. Comparison of the effects of xylazine and romifidine administered perioperatively on the recovery of anesthetized horses. Can. Vet. J. 2011, 52, 987–993. [Google Scholar]
- Wagner, A.E.; Mama, K.R.; Steffey, E.P.; Hellyer, P.W. Evaluation of infusions of xylazine with ketamine or propofol to modulate recovery following sevoflurane anesthesia in horses. Am. J. Vet. Res. 2012, 73, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, K.J.; Brosnan, R.J.; Nguyen, K.Q.; Moniz, G.W.; Galuppo, L.D. Effects of postanesthetic sedation with romifidine or xylazine on quality of recovery from isoflurane anesthesia in horses. J. Am. Vet. Med. Assoc. 2013, 242, 533–539. [Google Scholar] [CrossRef]
- Valverde, A.; Black, B.; Cribb, N.C.; Hathway, A.; Daw, A. Assessment of unassisted recovery from repeated general isoflurane anesthesia in horses following post-anesthetic administration of xylazine or acepromazine or a combination of xylazine and ketamine. Vet. Anaesth. Analg. 2013, 40, 3–12. [Google Scholar] [CrossRef]
- Ida, K.K.; Fantoni, D.T.; Ibiapina, B.T.; Souto, M.T.; Zoppa, A.L.; Silva, L.C.; Ambrosio, A.M. Effect of postoperative xylazine administration on cardiopulmonary function and recovery quality after isoflurane anesthesia in horses. Vet. Surg. 2013, 42, 877–884. [Google Scholar] [CrossRef]
- Aarnes, T.K.; Bednarski, R.M.; Bertone, A.L.; Hubbell, J.A.; Lerche, P. Recovery from desflurane anesthesia in horses with and without post-anesthetic xylazine. Can. J. Vet. Res. 2014, 78, 103–109. [Google Scholar]
- Guedes, A.G.P.; Tearney, C.C.; Cenani, A.; Aristizabal, F.; Nieto, J. Comparison between the effects of postanesthetic xylazine and dexmedetomidine on characteristics of recovery from sevoflurane anesthesia in horses. Vet. Anaesth. Analg. 2017, 44, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.; Knych, H.; Tucker, L.; Almeida, D.C.; Baldo, C.F.; Wendt-Hornickle, E.; Allweiler, S. Pharmacokinetics and clinical effects of xylazine and dexmedetomidine in horses recovering from isoflurane anesthesia. J. Vet. Pharmacol. Ther. 2020, 43, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.C.; Rezende, M.L.; Mama, K.R.; Hess, A.M. Recovery quality following a single post-anaesthetic dose of dexmedetomidine or romifidine in sevoflurane anaesthetised horses. Equine Vet. J. 2020, 52, 685–691. [Google Scholar] [CrossRef]
- Douglas, H.; Hopster, K.; Cerullo, M.; Hopster-Iversen, C.; Stefanovski, D.; Driessen, B. The effects of flumazenil on ventilatory and recovery characteristics in horses following midazolam-ketamine induction and isoflurane anaesthesia. Equine Vet. J. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Richter, M.C.; Bayly, W.M.; Keegan, R.D.; Ragle, C.A.; Weil, A.B.; Schneider, R.K. Cardiopulmonary function following anesthesia in horses experiencing hydro pool recovery versus padded stall recovery. Vet. Anaesth. Analg. 2000, 27, 107–108. [Google Scholar] [CrossRef]
- Richter, M.C.; Bayly, W.M.; Keegan, R.D.; Schneider, R.K.; Weil, A.B.; Ragle, C.A. Cardiopulmonary function in horses during anesthetic recovery in a hydropool. Am. J. Vet. Res. 2001, 62, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Ray-Miller, W.M.; Hodgson, D.S.; McMurphy, R.M.; Chapman, P.L. Comparison of recoveries from anesthesia of horses placed on a rapidly inflating-deflating air pillow or the floor of a padded stall. J. Am. Vet. Med. Assoc. 2006, 229, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Francois, I.; Laleye, F.X.; Micat, M.; Benredouane, K.; Portier, K. Arterial oxygen tension and pulmonary ventilation in horses placed in the Anderson Sling suspension system after a period of lateral recumbency and anaesthetised with constant rate infusions of romifidine and ketamine. Equine Vet. J. 2014, 46, 596–600. [Google Scholar] [CrossRef]
- Arndt, S.; Hopster, K.; Sill, V.; Rohn, K.; Kastner, S.B.R. Comparison between head-tail-rope assisted and unassisted recoveries in healthy horses undergoing general anesthesia for elective surgeries. Vet. Surg. 2020, 49, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, M.; Bettschart-Wolfensberger, R.; Hartnack, S.; Junge, H.K.; Theiss, F.; Ringer, S.K. Comparison of non-assisted versus head and tail rope-assisted recovery after emergency abdominal surgery in horses. Pferdeheilkunde 2016, 32, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Nicolaisen, A.S.K.; Nygaard, A.B.; Christophersen, M.T.; Jensen, D.B.; Lindegaard, C. Effect of head and tail rope-assisted recovery of horses after elective and emergency surgery under general anaesthesia. Equine Vet. Educ. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Sullivan, E.K.; Klein, L.V.; Richardson, D.W.; Ross, M.W.; Orsini, J.A.; Nunamaker, D.M. Use of a pool-raft system for recovery of horses from general anesthesia: 393 horses (1984–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, S.A.; Schneider, R.K.; Ragle, C.A.; Weil, A.B.; Richter, M.C. Use of a hydro-pool system to recover horses after general anesthesia: 60 cases. Vet. Surg. 2002, 31, 455–461. [Google Scholar] [CrossRef]
- Liechti, J.; Pauli, H.; Jäggin, N.; Schatzmann, U. Investigation into the assisted standing up procedure in horses during recovery phase after inhalation anaesthesia. Pferdeheilkunde 2003, 19, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.L.; Galuppo, L.D.; Steffey, E.P.; Scarlett, C.C.; Madigan, J.E. Use of the Anderson Sling suspension system for recovery of horses from general anesthesia. Vet. Surg. 2005, 34, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Elmas, C.R.; Cruz, A.M.; Kerr, C.L. Tilt table recovery of horses after orthopedic surgery: Fifty-four cases (1994–2005). Vet. Surg. 2007, 36, 252–258. [Google Scholar] [CrossRef]
- Fürst, A.E.; Keller, R.; Kummer, M.; Manera, C.; von Salis, B.; Auer, J.; Bettschart-Wolfensberger, R. Evaluation of a new full-body animal rescue and transportation sling in horses: 181 horses (1998–2006). J. Vet. Emerg. Crit. Care. 2008, 18, 619–625. [Google Scholar] [CrossRef]
- Steffey, E.P.; Brosnan, R.J.; Galuppo, L.D.; Mama, K.R.; Imai, A.; Maxwell, L.K.; Cole, C.A.; Stanley, S.D. Use of propofol-xylazine and the Anderson Sling Suspension System for recovery of horses from desflurane anesthesia. Vet. Surg. 2009, 38, 927–933. [Google Scholar] [CrossRef]
- Picek, S.; Kalchofner, K.S.; Ringer, S.K.; Kummer, M.; Fürst, A.; Bettschart-Wolfensberger, R. Anaesthetic management for hydropool recovery in 50 horses. Pferdeheilkunde 2010, 26, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Del Barrio, M.C.N.; David, F.; Hughes, J.M.L.; Clifford, D.; Wilderjans, H.; Bennett, R. A retrospective report (2003–2013) of the complications associated with the use of a one-man (head and tail) rope recovery system in horses following general anaesthesia. Ir. Vet. J. 2018, 71, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gygax, D.; Furst, A.; Picek, S.; Kummer, M. Internal fixation of a fractured axis in an adult horse. Vet. Surg. 2011, 40, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Maitland-Stuart, S.; Zeiler, G.E.; Mahne, A.T. Reduction and external coaptation as successful treatment for tarsocrural joint luxation in an Arabian mare. Equine Vet. Educ. 2018, 30, 600–604. [Google Scholar] [CrossRef]
- Lukasik, V.M.; Gleed, R.D.; Scarlett, J.M.; Ludders, J.W.; Moon, P.F.; Ballenstedt, J.L.; Sturmer, A.T. Intranasal phenylephrine reduces post anesthetic upper airway obstruction in horses. Equine Vet. J. 1997, 29, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.D.; Hildebrand, S.V. An evaluation of apnea or spontaneous ventilation in early recovery following mechanical ventilation in the anesthetized horse. Vet. Anaesth. Analg. 2001, 28, 26–33. [Google Scholar] [CrossRef]
- Duke, T.; Cruz, A.M.; Cruz, J.I.; Howden, K.J. Cardiopulmonary effects associated with head-down position in halothane-anesthetized ponies with or without capnoperitoneum. Vet. Anaesth. Analg. 2002, 29, 76–89. [Google Scholar] [CrossRef]
- Marntell, S.; Nyman, G.; Hedenstierna, G. High inspired oxygen concentrations increase intrapulmonary shunt in anaesthetized horses. Vet. Anaesth. Analg. 2005, 32, 338–347. [Google Scholar] [CrossRef]
- Hopster, K.; Kastner, S.B.; Rohn, K.; Ohnesorge, B. Intermittent positive pressure ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvre during inhalation anaesthesia in horses undergoing surgery for colic, and its influence on the early recovery period. Vet. Anaesth. Analg. 2011, 38, 169–177. [Google Scholar] [CrossRef]
- Schulte-Bahrenberg, S.; Hopster, K.; Kästner, S.B.; Rohn, K.; Ohnesorge, B. Influence on horse´s pulmonary function using a modified “Open-Lung-Concept”-Ventilation with different oxygen-concentration during general anaesthesia. Pferdeheilkunde 2011, 27, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, R.J.; Steffey, E.P.; Escobar, A. Effects of hypercapnic hyperpnea on recovery from isoflurane or sevoflurane anesthesia in horses. Vet. Anaesth. Analg. 2012, 39, 335–344. [Google Scholar] [CrossRef]
- Grubb, T.; Edner, A.; Frendin, J.H.; Funkquist, P.; Ryden, A.; Nyman, G. Oxygenation and plasma endothelin-1 concentrations in healthy horses recovering from isoflurane anaesthesia administered with or without pulse-delivered inhaled nitric oxide. Vet. Anaesth. Analg. 2013, 40, e9–e18. [Google Scholar] [CrossRef]
- Ida, K.K.; Fantoni, D.T.; Souto, M.T.; Otsuki, D.A.; Zoppa, A.L.; Silva, L.C.; Ambrosio, A.M. Effect of pressure support ventilation during weaning on ventilation and oxygenation indices in healthy horses recovering from general anesthesia. Vet. Anaesth. Analg. 2013, 40, 339–350. [Google Scholar] [CrossRef]
- Karrasch, N.M.; Hubbell, J.A.; Aarnes, T.K.; Bednarski, R.M.; Lerche, P. Comparison of cardiorespiratory variables in dorsally recumbent horses anesthetized with guaifenesin-ketamine-xylazine spontaneously breathing 50% or maximal oxygen concentrations. Can. Vet. J. 2015, 56, 387–392. [Google Scholar]
- Levionnois, O.L.; Zuehlke, N.; Kuhn, M.; Spadavecchia, C. Impact of low inspired oxygen fraction on oxygenation in clinical horses under general anesthesia. Pferdeheilkunde 2016, 32, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.R.; Bardell, D. The effect of two different intra-operative end-tidal carbon dioxide tensions on apnoeic duration in the recovery period in horses. Vet. Anaesth. Analg. 2016, 43, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hopster, K.; Rohn, K.; Ohnesorge, B.; Kastner, S.B.R. Controlled mechanical ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvres during anaesthesia in laterally or dorsally recumbent horses. Vet. Anaesth. Analg. 2017, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Uquillas, E.; Dart, C.M.; Perkins, N.R.; Dart, A.J. Effect of reducing inspired oxygen concentration on oxygenation parameters during general anaesthesia in horses in lateral or dorsal recumbency. Aust. Vet. J. 2018, 96, 46–53. [Google Scholar] [CrossRef]
- Andrade, F.S.; Faco, L.L.; Ida, K.K.; Silva, L.C.; Fantoni, D.T.; Ambrosio, A.M. Effects of 12 and 17 cm H2O positive end-expiratory pressure applied after alveolar recruitment maneuver on pulmonary gas exchange and compliance in isoflurane-anesthetized horses. Vet. Anaesth. Analg. 2019, 46, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.E.; Muir, W.W.; Wade, A. Arterial blood gas tensions in the horse during recovery from anesthesia. J. Am. Vet. Med. Assoc. 1987, 190, 989–994. [Google Scholar]
- McMurphy, R.M.; Cribb, P.H. Alleviation of postanesthetic hypoxemia in the horse. Can. Vet. J. 1989, 30, 37–41. [Google Scholar] [PubMed]
- Bardell, D.; Mosing, M.; Cripps, P.J. Restoration of arterial oxygen tension in horses recovering from general anaesthesia. Equine Vet. J. 2020, 52, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Adam, E.N.; Taylor, P.M. Evaluation of a modification of the Hudson demand valve in ventilated and spontaneously breathing horses. Vet. Rec. 1994, 135, 569–572. [Google Scholar] [PubMed]
- Tomasic, M.; Mann, L.S.; Soma, L.R. Effects of sedation, anesthesia, and endotracheal intubation on respiratory mechanics in adult horses. Am. J. Vet. Res. 1997, 58, 641–646. [Google Scholar] [PubMed]
- Ida, K.K.; Sauvage, A.; Gougnard, A.; Grauwels, M.; Serteyn, D.; Sandersen, C. Use of nasotracheal intubation during general anesthesia in two ponies with tracheal collapse. Front. Vet. Sci. 2018, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDuffee, L.A.; Stover, S.M.; Taylor, K.T. An in vitro biomechanical investigation of the mechanical properties of dynamic compression plated osteotomized adult equine tibiae. Vet. Surg. 1997, 26, 126–136. [Google Scholar] [CrossRef]
- Harriss, F.K.; Galuppo, L.D.; Decock, H.E.; McDuffee, L.A.; Macdonald, M.H. Evaluation of a technique for collection of cancellous bone graft from the proximal humerus in horses. Vet. Surg. 2004, 33, 293–300. [Google Scholar] [CrossRef]
- Richardson, D.W. Medial condylar fractures of the third metatarsal bone in horses. J. Am. Vet. Med. Assoc. 1984, 185, 761–765. [Google Scholar] [PubMed]
- Martin, F.; Richardson, D.W.; Nunamaker, D.M.; Ross, M.W.; Orsini, J.A. Use of tension band wires in horses with fractures of the ulna: 22 cases (1980–1992). J. Am. Vet. Med. Assoc. 1995, 207, 1085–1089. [Google Scholar]
- Murray, R.C.; Debowes, R.M.; Gaughan, E.M.; Bramlage, L.R. Application of a hook plate for management of equine ulnar fractures. Vet. Surg. 1996, 25, 207–212. [Google Scholar] [CrossRef]
- Wilke, M.; Nixon, A.J.; Malark, J.; Myhre, G. Fractures of the palmar aspect of the carpal bones in horses: 10 cases (1984–2000). J. Am. Vet. Med. Assoc. 2001, 219, 801–804. [Google Scholar] [CrossRef]
- Smith, L.C.; Greet, T.R.; Bathe, A.P. A lateral approach for screw repair in lag fashion of spiral third metacarpal and metatarsal medial condylar fractures in horses. Vet. Surg. 2009, 38, 681–688. [Google Scholar] [CrossRef]
- Lang, H.M.; Nixon, A.J. Arthroscopic removal of discrete palmar carpal osteochondral fragments in horses: 25 cases (1999–2013). J. Am. Vet. Med. Assoc. 2015, 246, 998–1004. [Google Scholar] [CrossRef]
- Moulin, N.; Francois, I.; Cote, N.; Alford, C.; Cleary, O.; Desjardins, M.R. Surgical repair of propagating condylar fractures of the third metacarpal/metatarsal bones with cortical screws placed in lag fashion in 26 racehorses (2007–2015). Equine Vet. J. 2018, 50, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Pollock, S.; Hull, M.L.; Stover, S.M.; Galuppo, L.D. A musculoskeletal model of the equine forelimb for determining surface stresses and strains in the humerus—Part I. Mathematical modeling. J. Biomech. Eng. 2008, 130, 041006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buehler, M.; Jackson, M.; Fuerst, A. Successful reduction and internal fixation of an open tibial fracture in an adult Icelandic horse. Pferdeheilkunde 2011, 27, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Schulze, N.; Ehrle, A.; Noguera Cender, A.C.; Lischer, C. Internal fixation of a complete ventral luxation of the dens axis in an American quarter horse yearling. Vet. Surg. 2019, 48, 1500–1506. [Google Scholar] [CrossRef]
- Smith, L.J.; Mellor, D.J.; Marr, C.M.; Reid, S.W.; Mair, T.S. Incisional complications following exploratory celiotomy: Does an abdominal bandage reduce the risk? Equine Vet. J. 2007, 39, 277–283. [Google Scholar] [CrossRef]
- Biedrzycki, A.H.; Brounts, S.H. Case series evaluating the use of absorbable staples compared with metallic staples in equine ventral midline incisions. Equine Vet. Educ. 2016, 28, 83–88. [Google Scholar] [CrossRef]
- Galuppo, L.D.; Pascoe, J.R.; Jang, S.S.; Willits, N.H.; Greenman, S.L. Evaluation of iodophor skin preparation techniques and factors influencing drainage from ventral midline incisions in horses. J. Am. Vet. Med. Assoc. 1999, 215, 963–969. [Google Scholar]
- McCoy, A.M.; Hackett, E.S.; Wagner, A.E.; Mama, K.R.; Hendrickson, D.A. Pulmonary gas exchange and plasma lactate in horses with gastrointestinal disease undergoing emergency exploratory laparotomy: A comparison with an elective surgery horse population. Vet. Surg. 2011, 40, 601–609. [Google Scholar] [CrossRef]
- Cribb, N.C.; Koenig, J.; Sorge, U. Comparison of laparoscopic versus conventional open cryptorchidectomies on intraoperative and postoperative complications and duration of surgery, anesthesia, and hospital stay in horses. J. Am. Vet. Med. Assoc. 2015, 246, 885–892. [Google Scholar] [CrossRef]
- Espinosa, P.; Le Jeune, S.S.; Cenani, A.; Kass, P.H.; Brosnan, R.J. Investigation of perioperative and anesthetic variables affecting short-term survival of horses with small intestinal strangulating lesions. Vet. Surg. 2017, 46, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Dziubinski, N.; Mahlmann, K.; Lubke-Becker, A.; Lischer, C. Retrospective identification of bacterial isolates from emergency laparotomy surgical site infections in horses. J. Equine Vet. Sci. 2020, 87, 102927. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, A.K.; Trim, C.M. Complications associated with anaesthesia for ocular surgery: A retrospective study 1989-1996. Equine Vet. J. 2000, 32, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Krein, S.R.; Lindsey, J.C.; Blaze, C.A.; Wetmore, L.A. Evaluation of risk factors, including fluconazole administration, for prolonged anesthetic recovery times in horses undergoing general anesthesia for ocular surgery: 81 cases (2006–2013). J. Am. Vet. Med. Assoc. 2014, 244, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Curto, E.M.; Griffith, E.H.; Posner, L.P.; Walsh, K.T.; Balko, J.A.; Gilger, B.C. Factors associated with postoperative complications in healthy horses after general anesthesia for ophthalmic versus non-ophthalmic procedures: 556 cases (2012–2014). J. Am. Vet. Med. Assoc. 2018, 252, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.M.; Poulin, A.E.; Doran, R.E.; Quinteros, D.D.; Abuja, G.A. Transpalpebral enucleation using a chain écraseur. Equine Vet. Educ. 2009, 21, 603–607. [Google Scholar] [CrossRef]
- Cary, J.A.; Hellyer, P.W. Anesthesia case of the month. Evaluation and treatment of suspected squamous cell carcinoma of the third eyelid. J. Am. Vet. Med. Assoc. 2002, 221, 790–792. [Google Scholar] [CrossRef]
- Kraus, B.M.; Parente, E.J.; Tulleners, E.P. Laryngoplasty with ventriculectomy or ventriculocordectomy in 104 draft horses (1992–2000). Vet. Surg. 2003, 32, 530–538. [Google Scholar] [CrossRef]
- Tomasic, M. Temporal changes in core body temperature in anesthetized adult horses. Am. J. Vet. Res. 1999, 60, 556–562. [Google Scholar]
- Tomasic, M.; Nann, L.E. Comparison of peripheral and core temperatures in anesthetized horses. Am. J. Vet. Res. 1999, 60, 648–651. [Google Scholar] [PubMed]
- Platt, J.P.; Simon, B.T.; Coleman, M.; Martinez, E.A.; Lepiz, M.A.; Watts, A.E. The effects of multiple anaesthetic episodes on equine recovery quality. Equine Vet. J. 2018, 50, 111–116. [Google Scholar] [CrossRef]
- Clark-Price, S.C.; Posner, L.P.; Gleed, R.D. Recovery of horses from general anesthesia in a darkened or illuminated recovery stall. Vet. Anaesth. Analg. 2008, 35, 473–479. [Google Scholar] [CrossRef]
- Bellei, M.H.; Kerr, C.; McGurrin, M.K.; Kenney, D.G.; Physick-Sheard, P. Management and complications of anesthesia for transvenous electrical cardioversion of atrial fibrillation in horses: 62 cases (2002–2006). J. Am. Vet. Med. Assoc. 2007, 231, 1225–1230. [Google Scholar] [CrossRef]
- Muir, W.W.; McGuirk, S.M. Hemodynamics before and after conversion of atrial fibrillation to normal sinus rhythm in horses. J. Am. Vet. Med. Assoc. 1984, 184, 965–970. [Google Scholar]
- Tzelos, T.; Blissitt, K.J.; Clutton, R.E. Electrocardiographic indicators of excitability in horses for predicting recovery quality after general anaesthesia. Vet. Anaesth. Analg. 2015, 42, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Steffey, E.P.; Mama, K.R.; Galey, F.D.; Puschner, B.; Woliner, M.J. Effects of sevoflurane dose and mode of ventilation on cardiopulmonary function and blood biochemical variables in horses. Am. J. Vet. Res. 2005, 66, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Lees, P.; Hillidge, C.J. Influence of azaperone/metomidate anaesthesia on blood biochemistry in the horse. Br. Vet. J. 1976, 132, 405–415. [Google Scholar] [CrossRef]
- Smith, R.K.; Dyson, S.J.; Head, M.J.; Butson, R.J. Ultrasonography of the equine triceps muscle before and after general anaesthesia and in post anaesthetic myopathy. Equine Vet. J. 1996, 28, 311–319. [Google Scholar] [CrossRef]
- Edner, A.H.; Essen-Gustavsson, B.; Nyman, G.C. Metabolism during anaesthesia and recovery in colic and healthy horses: A microdialysis study. Acta Vet. Scand. 2009, 51, 10. [Google Scholar] [CrossRef] [Green Version]
- Mosing, M.; Kuemmerle, J.M.; Dadak, A.; Moens, Y.P. Metabolic changes associated with anaesthesia and cherry poisoning in a pony. Vet. Anaesth. Analg. 2009, 36, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Edner, A.; Nyman, G.; Essen-Gustavsson, B. The relationship of muscle perfusion and metabolism with cardiovascular variables before and after detomidine injection during propofol-ketamine anaesthesia in horses. Vet. Anaesth. Analg. 2002, 29, 182–199. [Google Scholar] [CrossRef]
- Widmer, W.R.; Blevins, W.E.; Jakovljevic, S.; Levy, M.; Teclaw, R.F.; Han, C.M.; Hurd, C.D. A prospective clinical trial comparing metrizamide and iohexol for equine myelography. Vet. Radiol. Ultrasound 1998, 39, 106–109. [Google Scholar] [CrossRef]
- Scarabelli, S.; Timofte, D.; Malalana, F.; Bardell, D. Corneal abrasion and microbial contamination in horses following general anaesthesia for non-ocular surgery. Vet. Anaesth. Analg. 2018, 45, 278–284. [Google Scholar] [CrossRef]
- Franco, E.C.; Cassu, R.N.; Diniz, M.S.; Mattos, G.M.; Scarcelli, P.C. Eletroacupunture treatment for isoflurane induced hypotension in horses. Arq. Bras. Med. Vet. Zootec. 2014, 66, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Monticelli, P.; Adami, C. Aspiration pneumonitis (Mendelson’s syndrome) as perianaesthetic complication occurring in two horses: A case report. Equine Vet. Educ. 2017, 31, 183–187. [Google Scholar] [CrossRef]
- Littlejohn, A. The behaviour of horses recovering from anaesthesia. Br. Vet. J. 1970, 126, 617–621. [Google Scholar] [CrossRef]
- Church, S.; Wyn-Jones, G.; Parks, A.H.; Ritchie, H.E. Treatment of guttural pouch mycosis. Equine Vet. J. 1986, 18, 362–365. [Google Scholar] [CrossRef]
- Bonilla, A.G.; Scansen, B.A.; Hurcombe, S.D.; Mudge, M.C. Potential for iatrogenic coil embolization of the caudal cerebellar artery during treatment of internal carotid artery bifurcation in two horses with guttural pouch mycosis. J. Am. Vet. Med. Assoc. 2015, 247, 1427–1432. [Google Scholar] [CrossRef]
- Trim, C.M.; Mason, J. Post-anaesthetic forelimb lameness in horses. Equine Vet. J. 1973, 5, 71–76. [Google Scholar] [CrossRef]
- Dodman, N.H.; Williams, R.; Court, M.H.; Norman, W.M. Postanesthetic hind limb adductor myopathy in five horses. J. Am. Vet. Med. Assoc. 1988, 193, 83–86. [Google Scholar] [PubMed]
- Dyson, S.; Taylor, P.; Whitwell, K. Femoral nerve paralysis after general anaesthesia. Equine Vet. J. 1988, 20, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Rijkenhuizen, A.B.M.; van Dijk, P. The incidence of post-anaesthetic myopathy with the use of a static air mattress. Pferdeheilkunde 1998, 14, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Moreno, K.L.; Scallan, E.M.; Friedeck, W.O.; Simon, B.T. Transient pelvic limb neuropathy following proximal metatarsal and tarsal magnetic resonance imaging in seven horses. Equine Vet. J. 2020, 52, 359–363. [Google Scholar] [CrossRef]
- Kaartinen, M.J.; Pang, D.S.; Cuvelliez, S.G. Post-anesthetic pulmonary edema in two horses. Vet. Anaesth. Analg. 2010, 37, 136–143. [Google Scholar] [CrossRef]
- Matthews, N.S.; Hartsfield, S.M.; Sanders, E.A.; Light, G.S.; Walker, M.A. Repetitive injectable anesthesia in a 27-year-old horse. Cornell Vet. 1993, 83, 219–225. [Google Scholar]
- Clutton, R.E. Unexpected responses following intravenous pethidine injection in two horses. Equine Vet. J. 1987, 19, 72–73. [Google Scholar] [CrossRef]
- Sprick, M.; Koch, C. Successful treatment of a coxofemoral luxation in a Shetland pony by closed reduction and prolonged immobilization using a full-body Animal Rescue Sling. Case Rep. Vet. Med. 2020, 2424653. [Google Scholar] [CrossRef]
- Quinn, C.T.; Carmalt, J.L. Ruptured urinary bladder in a horse. Vet. Anaesth. Analg. 2012, 39, 557–558. [Google Scholar] [CrossRef]
- Hewes, C.A.; Johnson, A.K.; Kivett, L.E.; Stewart, A.J.; Weisman, J.L.; Caldwell, F.J. Uterine prolapse in a mare leading to metritis, systemic inflammatory response syndrome, septic shock and death. Equine Vet. Educ. 2011, 23, 273–278. [Google Scholar] [CrossRef]
- Conde Ruiz, C.; Junot, S. Successful cardiopulmonary resuscitation in a sevoflurane anaesthetized horse that suffered cardiac arrest at recovery. Front. Vet. Sci. 2018, 5, 138. [Google Scholar] [CrossRef]
- Portier, K.; Walsh, C.M. Coxofemoral luxation in a horse during recovery from general anaesthesia. Vet. Rec. 2006, 159, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, L.; Brunthaler, R.; Moens, Y.P.S. Fatal diaphragmatic rupture during recovery from general anaesthesia in a Standardbred horse. Pferdeheilkunde 2011, 27, 504–506. [Google Scholar] [CrossRef] [Green Version]
- Mosing, M.; Junot, S. Use of continuous positive airway pressure (CPAP) in a horse with diaphragmatic hernia. Pferdeheilkunde 2011, 27, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Shepard, M.K.; Lee, W.L.; Eggleston, R.B. Perianesthetic development of diaphragmatic hernia in a horse with equine pituitary pars intermedia dysfunction (PPID). Can. Vet. J. 2015, 56, 48–52. [Google Scholar] [PubMed]
- Freeman, D.E.; Staller, G.S.; Maxson, A.D.; Sweeney, C.R. Unusual internal carotid artery branching that prevented arterial occlusion with a balloon-tipped catheter in a horse. Vet. Surg. 1993, 22, 531–534. [Google Scholar] [CrossRef]
- De Fourmestraux, C.; Tessier, C.; Touzot-Jourde, G. Multimodal therapy including electroacupuncture for the treatment of facial nerve paralysis in a horse. Equine Vet. Educ. 2014, 26, 18–23. [Google Scholar] [CrossRef]
- Curtis, M.B.; Eicker, S.W.; Archer, R.M.; Lindsay, W.A. Anesthetic management of an incisional dehiscence in recovery following exploratory laparotomy in a horse. J. Am. Vet. Med. Assoc. 1992, 200, 692–695. [Google Scholar] [PubMed]
- Trim, C.M. Complications associated with the use of the cuffless endotracheal tube in the horse. J. Am. Vet. Med. Assoc. 1984, 185, 541–542. [Google Scholar] [PubMed]
- Dixon, P.M.; Railton, D.I.; McGorum, B.C. Temporary bilateral laryngeal paralysis in a horse associated with general anaesthesia and post anaesthetic myositis. Vet. Rec. 1993, 132, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, H.L.; Monticelli, P.; Smith, R.; Adami, C. Acute life-threatening laryngeal dysfunction in a draft horse recovering from general anesthesia: A case report. J. Equine Vet. Sci. 2020, 91, 103109. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.; Allen, K.; Macfarlane, P.; Holopherne-Doran, D. Broken nasotracheal tube aspiration in a horse during anaesthetic recovery. Equine Vet. Educ. 2015, 27, 240–243. [Google Scholar] [CrossRef]
- Bienert, A.; Bartmann, C.P.; Feige, K. Comparison of therapeutic techniques for the treatment of cheek teeth diseases in the horse: Extraction versus repulsion. Pferdeheilkunde 2008, 24, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Dzikiti, T.B.; Bester, L.; Cilliers, I.; Carstens, A.; Stegmann, G.F.; Hubert, J. Limb fracture during recovery from general anaesthesia: An often tragic complication of equine anaesthesia. J. S. Afr. Vet. Assoc. 2008, 79, 145–148. [Google Scholar] [CrossRef]
- Bolt, D.M.; Read, R.M.; Weller, R.; Sinclair, C.; David, F.H. Standing low-field magnetic resonance imaging of a comminuted central tarsal bone fracture in a horse. Equine Vet. Educ. 2013, 25, 618–623. [Google Scholar] [CrossRef]
- Cornick, J.L.; Seahorn, T.L.; Hartsfield, S.M. Hyperthermia during isoflurane anaesthesia in a horse with suspected hyperkalaemic periodic paralysis. Equine Vet. J. 1994, 26, 511–514. [Google Scholar] [CrossRef]
- Grint, N.; Gorvy, D.; Dugdale, A. Hyperthermia and delayed-onset myopathy after recovery from anesthesia in a horse. J. Equine Vet. Sci. 2007, 27, 221–227. [Google Scholar] [CrossRef]
- Waldronmease, E.; Klein, L.V.; Rosenberg, H.; Leitch, M. Malignant hyperthermia in a halothane-anesthetized horse. J. Am. Vet. Med. Assoc. 1981, 179, 896–898. [Google Scholar]
- Klein, L.; Ailes, N.; Fackelman, G.E.; Kellon, E.; Rosenberg, H. Postanesthetic equine myopathy suggestive of malignant hyperthermia. A case report. Vet. Surg. 1989, 18, 479–482. [Google Scholar] [CrossRef]
- Zink, M.C. Postanesthetic poliomyelomalacia in a horse. Can. Vet. J. 1985, 26, 275–277. [Google Scholar]
- Lerche, E.; Laverty, S.; Blais, D.; Sauvageau, R.; Cuvelliez, S. Hemorrhagic myelomalacia following general anesthesia in a horse. Cornell Vet. 1993, 83, 267–273. [Google Scholar]
- Wan, P.Y.; Latimer, F.G.; Silva-Krott, I.; Goble, D.O. Hematomyelia in a colt: A post anesthesia/surgery complication. J. Equine Vet. Sci. 1994, 14, 495–497. [Google Scholar] [CrossRef]
- Jouber, K.E.; Duncan, N.; Murray, S.E. Post-anaesthetic myelomalacia in a horse. J. S. Afr. Vet. Assoc. 2005, 76, 36–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, J.P.; Meertens, N.M.; van Oldruitenborgh-Oosterbaan, M.M.; van Dijk, R. Post-anaesthetic myelopathy in a 3-year-old Friesian gelding. Tijdschr. Diergeneeskd. 2010, 135, 272–277. [Google Scholar] [PubMed]
- Patschova, M.; Kabes, R.; Ludvikova, E. Post anaesthetic myelopathy in the horse: A case report. Vet. Med. 2014, 59, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Mirra, A.; Klopfenstein Bregger, M.D.; Levionnois, O.L. Suspicion of postanesthetic femoral paralysis of the non-dependent limb in a horse. Front. Vet. Sci. 2018, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Tate, L.P., Jr.; Koch, D.B.; Sembrat, R.F.; Boles, C.L. Tracheal reconstruction by resection and end-to-end anastomosis in the horse. J. Am. Vet. Med. Assoc. 1981, 178, 253–258. [Google Scholar] [PubMed]
- Rainger, J.E.; Hughes, K.J.; Kessell, A.; Dart, C.M. Pleuropneumonia as a sequela of myelography and general anaesthesia in a Thoroughbred colt. Aust. Vet. J. 2006, 84, 138–140. [Google Scholar] [CrossRef]
- Boustead, K.J.; Steyl, J.; Joubert, K. Fatal post-anaesthetic pulmonary haemorrhage in a horse suffering from chronic-active exercise-induced pulmonary haemorrhage. Equine Vet. Educ. 2021. [Google Scholar] [CrossRef]
- Robertson, S.A.; Green, S.L.; Carter, S.W.; Bolon, B.N.; Brown, M.P.; Shields, R.P. Postanesthetic recumbency associated with hyperkalemic periodic paralysis in a quarter horse. J. Am. Vet. Med. Assoc. 1992, 201, 1209–1212. [Google Scholar] [PubMed]
- Dupont, J.; Serteyn, D.; Sandersen, C. Prolonged recovery from general anesthesia possibly related to persistent hypoxemia in a draft horse. Front. Vet. Sci. 2018, 5, 235. [Google Scholar] [CrossRef]
- Tute, A.S.; Wilkins, P.A.; Gleed, R.D.; Credille, K.M.; Murphy, D.J.; Ducharme, N.G. Negative pressure pulmonary edema as a post-anesthetic complication associated with upper airway obstruction in a horse. Vet. Surg. 1996, 25, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Borer, K.E. Pulmonary oedema associated with anaesthesia for colic surgery in a horse. Vet. Anaesth. Analg. 2005, 32, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, T.C.; Dechant, J.E.; Crowson, C.L. Suspected air embolism associated with post-anesthetic pulmonary edema and neurologic sequelae in a horse. Vet. Anaesth. Analg. 2007, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Veres-Nyeki, K.O.; Graubner, C.; Aloisio, F.; Spadavecchia, C. Pulmonary edema at recovery after colic operation with in-situ nasogastric tube in a horse. Schweiz. Arch. Tierheilkd. 2011, 153, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Auckburally, A. Tracheal rupture following general anaesthesia in a horse. Equine Vet. Educ. 2018, 32, O62–O65. [Google Scholar] [CrossRef]
- Vettorato, E.; Chase-Topping, M.E.; Clutton, R.E. A comparison of four systems for scoring recovery quality after general anaesthesia in horses. Equine Vet. J. 2010, 42, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Suthers, J.M.; Christley, R.M.; Clutton, R.E. Quantitative and qualitative comparison of three scoring systems for assessing recovery quality after general anaesthesia in horses. Vet. Anaesth. Analg. 2011, 38, 352–362. [Google Scholar] [CrossRef]
- Farmer, E.; Chase-Topping, M.; Lawson, H.; Clutton, R.E. Factors affecting the perception of recovery quality in horses after anaesthesia. Equine Vet. J. 2014, 46, 328–332. [Google Scholar] [CrossRef]
- Portier, K.G.; Sena, A.; Senior, M.; Clutton, R.E. A study of the correlation between objective and subjective indices of recovery quality after inhalation anaesthesia in equids. Vet. Anaesth. Analg. 2010, 37, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Clark-Price, S.C.; Lascola, K.M.; Carter, J.E.; da Cunha, A.F.; Donaldson, L.L.; Doherty, T.J.; Martin-Flores, M.; Hofmeister, E.H.; Keating, S.C.J.; Mama, K.R.; et al. Assessment of agreement among diplomates of the American College of Veterinary Anesthesia and Analgesia for scoring the recovery of horses from anesthesia by use of subjective grading scales and development of a system for evaluation of the recovery of horses from anesthesia by use of accelerometry. Am. J. Vet. Res. 2017, 78, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, S.; Rioja, E. Retrospective evaluation of correlation and agreement between two recovery scoring systems in horses. Vet. Rec. 2018, 182, 169. [Google Scholar] [CrossRef] [Green Version]
- Toews, L.C. Compliance of systematic reviews in veterinary journals with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) literature search reporting guidelines. J. Med. Libr. Assoc. 2017, 105, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Sports Chiropr. Rehabilit. 2001, 15, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Slavin, R.E. Best evidence synthesis: An intelligent alternative to meta-analysis. J. Clin. Epidemiol. 1995, 48, 9–18. [Google Scholar] [CrossRef]
- Ballard, S.; Shults, T.; Kownacki, A.A.; Blake, J.W.; Tobin, T. The pharmacokinetics, pharmacological responses and behavioral effects of acepromazine in the horse. J. Vet. Pharmacol. Ther. 1982, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Marroum, P.J.; Webb, A.I.; Aeschbacher, G.; Curry, S.H. Pharmacokinetics and pharmacodynamics of acepromazine in horses. Am. J. Vet. Res. 1994, 55, 1428–1433. [Google Scholar]
- England, G.C.; Clarke, K.W. Alpha 2 adrenoceptor agonists in the horse—A review. Br. Vet. J. 1996, 152, 641–657. [Google Scholar] [CrossRef]
- Hall, L.W. Bromochlorotrifluoroethane (“Fluothane”): A new volatile anaesthetic agent. Vet. Rec. 1957, 69, 615. [Google Scholar]
- Hall, L.W. Equine anaesthesia: Discovery and rediscovery. Equine Vet. J. 1983, 15, 190–195. [Google Scholar] [CrossRef]
- Jones, R.S.; West, E. Environmental sustainability in veterinary anaesthesia. Vet. Anaesth. Analg. 2019, 46, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Sulbaek Andersen, M.P.; Nielsen, O.J.; Karpichev, B.; Wallington, T.J.; Sander, S.P. Atmospheric chemistry of isoflurane, desflurane, and sevoflurane: Kinetics and mechanisms of reactions with chlorine atoms and OH radicals and global warming potentials. J. Phys. Chem. A 2012, 116, 5806–5820. [Google Scholar] [CrossRef]
- McMurphy, R.M.; Young, L.E.; Marlin, D.J.; Walsh, K. Comparison of the cardiopulmonary effects of anesthesia maintained by continuous infusion of romifidine, guaifenesin, and ketamine with anesthesia maintained by inhalation of halothane in horses. Am. J. Vet. Res. 2002, 63, 1655–1661. [Google Scholar] [CrossRef]
- Nolan, A.; Reid, J.; Welsh, E.; Flaherty, D.; McCormack, R.; Monteiro, A.M. Simultaneous infusions of propofol and ketamine in ponies premedicated with detomidine: A pharmacokinetic study. Res. Vet. Sci. 1996, 60, 262–266. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Larenza, M.P. Balanced anesthesia in the equine. Clin. Tech. Equine Pract. 2007, 6, 104–110. [Google Scholar] [CrossRef]
- White, P.F.; Schuttler, J.; Shafer, A.; Stanski, D.R.; Horai, Y.; Trevor, A.J. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br. J. Anaesth. 1985, 57, 197–203. [Google Scholar] [CrossRef]
- Thomasy, S.M.; Steffey, E.P.; Mama, K.R.; Solano, A.; Stanley, S.D. The effects of i.v. fentanyl administration on the minimum alveolar concentration of isoflurane in horses. Br. J. Anaesth. 2006, 97, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoy, L.; Morris, T.B.; Ducharme, N.G.; Gleed, R.D.; Martin-Flores, M. Unilateral cervical plexus block for prosthetic laryngoplasty in the standing horse. Equine Vet. J. 2018, 50, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Kuls, N.; Trujanovic, R.; Otero, P.E.; Larenza-Menzies, M.P. Ultrasound-guided transversus abdominis plane block in Shetland ponies: A description of a three-point injection technique and evaluation of potential analgesic effects. J. Equine Vet. Sci. 2020, 90, 102994. [Google Scholar] [CrossRef] [PubMed]
- Costa-Farre, C.; Prades, M.; Ribera, T.; Valero, O.; Taura, P. Does intraoperative low arterial partial pressure of oxygen increase the risk of surgical site infection following emergency exploratory laparotomy in horses? Vet. J. 2014, 200, 175–180. [Google Scholar] [CrossRef]
- Robson, K.; Cripps, P.; Bardell, D. Lack of association between arterial oxygen tensions in horses during exploratory coeliotomy and post-operative incisional complications: A retrospective study. Vet. J. 2016, 210, 24–29. [Google Scholar] [CrossRef]
- Freeman, K.D.; Southwood, L.L.; Lane, J.; Lindborg, S.; Aceto, H.W. Post operative infection, pyrexia and perioperative antimicrobial drug use in surgical colic patients. Equine Vet. J. 2012, 44, 476–481. [Google Scholar] [CrossRef]
- Hanrath, M.; Rodgerson, D.H. Laparoscopic cryptorchidectomy using electrosurgical instrumentation in standing horses. Vet. Surg. 2002, 31, 117–124. [Google Scholar] [CrossRef]
- De Linde Henriksen, M.; Brooks, D.E. Standing ophthalmic surgeries in horses. Vet. Clin. North. Am. Equine Pract. 2014, 30, 91–110. [Google Scholar] [CrossRef]
- Diaz, M.; Becker, D.E. Thermoregulation: Pysiological and clinical considerations during sedation and general anesthesia. Anesth. Prog. 2010, 57, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, D.; van Loon, G.; Schauvliege, S.; Tavernier, R.; Baert, K.; Croubels, S.; de Backer, P.; Deprez, P. Transvenous electrical cardioversion of atrial fibrillation in six horses using custom made cardioversion catheters. Vet. J. 2008, 177, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Brakenhoff, J.E.; Holcombe, S.J.; Hauptman, J.G.; Smith, H.K.; Nickels, F.A.; Caron, J.P. The prevalence of laryngeal disease in a large population of competition draft horses. Vet. Surg. 2006, 35, 579–583. [Google Scholar] [CrossRef]
- Grandy, J.L.; Steffey, E.P.; Hodgson, D.S.; Woliner, M.J. Arterial hypotension and the development of postanesthetic myopathy in halothane-anesthetized horses. Am. J. Vet. Res. 1987, 48, 192–197. [Google Scholar] [PubMed]
- Richey, M.T.; Holland, M.S.; McGrath, C.J.; Dodman, N.H.; Marshall, D.B.; Court, M.H.; Norman, W.M.; Seeler, D.C. Equine post-anesthetic lameness. A retrospective study. Vet. Surg. 1990, 19, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Duke, T.; Filzek, U.; Read, M.R.; Read, E.K.; Ferguson, J.G. Clinical observations surrounding an increased incidence of postanesthetic myopathy in halothane-anesthetized horses. Vet. Anaesth. Analg. 2006, 33, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, M.; Hooijmans, C.R.; van Veggel, N.; Ter Riet, G.; Leeflang, M.; Hooft, L.; van der Wilt, G.J.; Tillema, A.; Ritskes-Hoitinga, M. A step-by-step guide to systematically identify all relevant animal studies. Lab. Anim. 2012, 46, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Muir, W.W., 3rd; Mason, D.E. Effects of diazepam, acepromazine, detomidine, and xylazine on thiamylal anesthesia in horses. J. Am. Vet. Med. Assoc. 1993, 203, 1031–1038. [Google Scholar] [PubMed]
- Whitehair, K.J.; Steffey, E.P.; Willits, N.H.; Woliner, M.J. Recovery of horses from inhalation anesthesia. Am. J. Vet. Res. 1993, 54, 1693–1702. [Google Scholar] [PubMed]
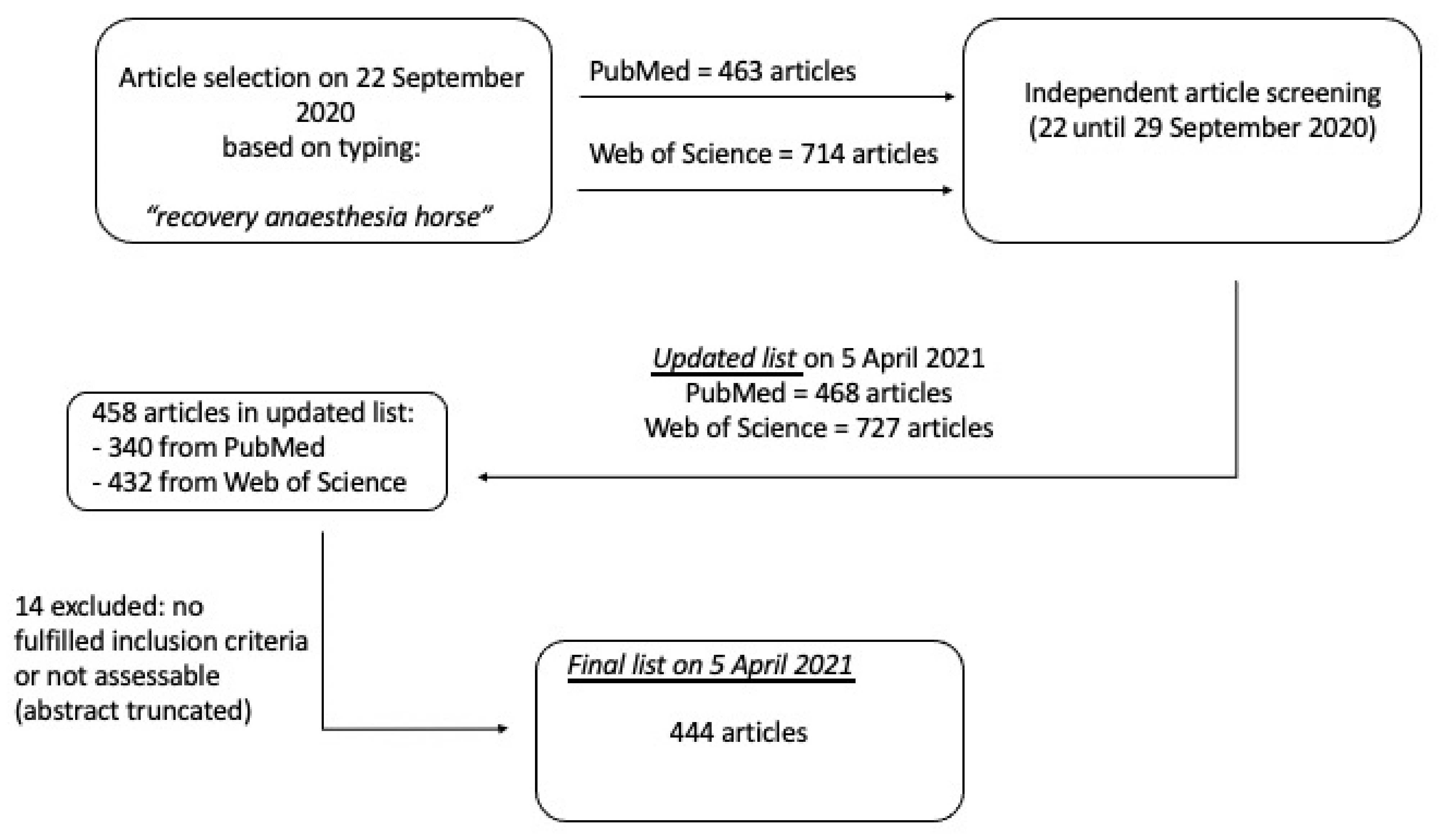
| Strength | Level | Design | Randomisation | Control |
|---|---|---|---|---|
| High | LoE 1 | Randomised control trial (RCT) | Yes | Yes |
| Meta-analysis of RCT with homogeneous results | No | |||
| LoE 2 | Prospective comparative study (therapeutic) | No | Yes | |
| Meta-analysis of Level 2 studies or Level 1 studies with inconsistent results | No | |||
| Prospective cohort study | No | Yes | ||
| LoE 3 | Retrospective cohort study | No | Yes | |
| Case-control study | No | Yes | ||
| Meta-analysis of Level 3 studies | No | |||
| LoE 4 | Case series | No | No | |
| LoE 5 | Case report | No | No | |
| Expert opinion | No | No | ||
| Low | Personal observation | No | No |
| Table | Type of Study | Number | Levels of Evidence (LoE) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ? | |||
| 3a | Narrative reviews/expert opinions | 41 | / | / | / | / | 41 | / |
| 3b | Retrospective outcome studies | 16 | / | / | 16 | / | / | / |
| 3c | Surveys | 5 | / | 5 | / | / | / | / |
| 4 | Premedication/sedation and induction drugs | 59 | 33 | 3 | 2 | 14 | / | 7 |
| 5a | Maintenance with inhalant agents | 27 | 9 | 4 | / | 13 | 1 | / |
| 5b | Maintenance with TIVA | 55 | 19 | 4 | / | 32 | / | / |
| 5c | TIVA vs. inhalants | 3 | 2 | 1 | / | / | / | / |
| 6 | Maintenance with PIVA | 56 | 43 | 5 | 2 | 4 | 2 | / |
| 7a | Loco-regional during maintenance | 9 | 6 | / | 2 | 1 | / | / |
| 7b | NMBAs during maintenance | 10 | 2 | / | / | 6 | 1 | 1 |
| 7c | Other drugs used during maintenance | 8 | 4 | 1 | 2 | 1 | / | / |
| 8 | Drugs before/during recovery | 18 | 18 | / | / | / | / | / |
| 9 | Recovery systems | 18 | 5 | / | 2 | 9 | 2 | / |
| 10 | Respiratory system in recovery | 21 | 15 | 3 | / | 3 | / | / |
| 11 | Other factors | 41 | 8 | 8 | 6 | 15 | 4 | / |
| 12 | Case series/reports | 51 | / | / | / | 12 | 39 | / |
| 13 | Systems to score recoveries | 6 | 3 | 2 | 1 | / | / | / |
| Total (5 April 2021) | 444 | 167 | 36 | 33 | 110 | 90 | 8 | |
| Type of Manuscript | References |
|---|---|
| (3a) Narrative reviews/expert opinions, LoE = 5 | |
| Recovery period per se | (1) Recovery from anaesthesia in horses [15] (2) Recovery from anaesthesia in horses 1. What can go wrong? [8] (3) Recovery from anaesthesia in horses 2. Avoiding complications [9] (4) Recovery of horses from anesthesia [10] |
| Postanaesthetic myopathy | (5) Equine postanesthetic myopathy—An update [16] |
| Pulmonary function GA | (6) Pulmonary function in the horse during anaesthesia: A review [17] |
| Upper airway during recovery | (7) Post-anaesthetic pulmonary oedema in horses: A review [7] (8) Mitigating the risk of airway obstruction during recovery from anaesthesia: The way is far from clear [18] |
| Complications during GA | (9) Complications in equine anesthesia [19] (10) Equine anaesthesia-associated mortality: where are we now? [2] (11) Anaesthesia-related complications in horses—results of the last few years [20] (12) Editorial: Anesthetic risk and complications in veterinary medicine [21] (13) Mortality and morbidity in equine anaesthesia [22] |
| Complications during GA in the clinically ill horse | (14) Anesthesia in horses with colic syndrome: Analysis of 48 cases and literature review [23] (15) Anesthesia of the critically ill equine patient [24] (16) Incisional infections associated with ventral midline celiotomy in horses [25] |
| Complications during GA in orthopaedics | (17) Anesthetic management and recovery of large orthopedic patients [26] (18) Fracture fixation in horses: Recent developments in implants, management and recovery—A rewiew [27] (19) Bog spavin: Recognising the problem is the first step towards recovery [28] |
| Complications during GA in late-term mares | (20) Anesthesia and sedation for late-term mares [29] |
| Effects of α2-agonists | (21) Pre-anesthetic medication in the horse part IV. Sedative-hypnotics and drug mixtures [30] (22) The responses to the use of detomidine (Domosedan) in the horse [31] (23) Use of the alpha-2 agonists xylazine and detomidine in the perianaesthetic period in the horse [32] (24) Is there a place for dexmedetomidine in equine anaesthesia and analgesia? A systematic review (2005–2017) [33] |
| Effects of other IV drugs | (25) Sedation in equine practice—Indications and choice of the methods [34] (26) Clinical insights: Equine anaesthesia and analgesia [35] |
| Effects of NMBAs | (27) Neuromuscular blocking agents [36] |
| Effects of inhalant agents | (28*) General anesthesia in pleasure horses [37] (29) Use of halothane and isoflurane in the horse [38] (30) Isoflurane as an inhalational anesthetic agent in clinical practice [39] (31) Desflurane and sevoflurane. New volatile anesthetic agents [40] (32*) Anaesthesia for minor surgical procedures in the horse [41] (33) Recent advances in inhalation anesthesia [42] |
| Effects of balanced anaesthesia and PIVA (or SIVA) | (34) Use of supplemental intravenous anaesthesia/analgesia in horses [43] (35) Balanced anesthesia in horses [44] (36) Partial intravenous anaesthesia in the horse: A review of intravenous agents used to supplement equine inhalation anaesthesia. Part 1: Lidocaine and ketamine [45] (37) Partial intravenous anaesthesia in the horse: A review of intravenous agents used to supplement equine inhalation anaesthesia. Part 2: Opioids and alpha-2 adrenoceptor agonists [46] (38*) Total and partial intravenous anaesthesia of horses [47] |
| Effects of TIVA | (28*) General anesthesia in pleasure horses [37] (32*) Anaesthesia for minor surgical procedures in the horse [41] (39) Field anesthetic techniques for use in horses [48] (40) Total intravenous anesthesia in horses [49] (38*) Total and partial intravenous anaesthesia of horses [47] |
| Effects of agents for pain management | (41) Perioperative pain management in horses [50] |
| Total narrative reviews/expert opinions = 41 | |
| (3b) Retrospective outcome studies, LoE = 3 | |
| Mortality, fatalities and outcome associated to GA in general | (1) Factors influencing the outcome of equine anaesthesia: A review of 1314 cases [51] (2) Clinical problems during inhalatory anaesthesia in horses. A review of 114 cases [52] (3) Equine perioperative fatalities associated with general anaesthesia at a private practice—a retrospective case series [53] (4) Multivariate analysis of factors associated with post-anesthetic times to standing in isoflurane-anesthetized horses: 381 cases [54] (5) Risk of general anesthesia in horses—A retrospective study on 1.989 cases [55] (6) Twenty years later: A single-centre, repeat retrospective analysis of equine perioperative mortality and investigation of recovery quality [56] (7) Time-related changes in post-operative equine morbidity: A single-centre study [57] (8) Risk factors of anesthesia-related mortality and morbidity in one equine hospital: A retrospective study on 1161 cases undergoing elective or emergency surgeries [58] |
| Mortality, fatalities and outcome associated to GA in colics | (9) A retrospective survey of anaesthesia in horses with colic [59] (10) Evaluation of exploratory laparotomy in young horses: 102 cases (1987–1992) [60] (11) Anaesthesia related problems in equine colic surgery: A retrospective study from 1995 until 1999 [61] (12) Large colon resection and anastomosis in horses: 52 cases (1996–2006) [62] |
| Mortality, fatalities and outcome associated to GA for dystocias | (13) Perioperative risk factors for mortality and length of hospitalization in mares with dystocia undergoing general anesthesia: A retrospective study [63] |
| Mortality, fatalities and outcome associated to GA for cystoliths removal | (14) Cystoliths in the horse—a retrospective study [64] |
| Post-anaesthetic myopathy/neuropathy in horses undergoing MRI | (15) Post anaesthetic myopathy/neuropathy in horses undergoing magnetic resonance imaging compared to horses undergoing surgery [65] |
| Use of ACP and its effects on the recovery | (16) Contemporary use of acepromazine in the anaesthetic management of male horses and ponies: A retrospective study and opinion poll [66] |
| Total retrospective outcome studies = 16 | |
| (3c) Surveys, LoE = 2 | |
| Mortality, fatalities and outcome associated to GA in general | (1) Confidential enquiry of perioperative equine fatalities (CEPEF-1): Preliminary results [67] |
| Electronic/hard copy questionnaires about assisted recovery | (2) How to manage recovery from anaesthesia in the horse—to assist or not to assist? [68] (3) Relevance of assisting horses in recovery after general anesthesia for osteosynthesis? [69] |
| Online survey evaluating practice in equine anaesthesia | (4) International online survey to assess current practice in equine anaesthesia [70] |
| Online survey evaluating current practice of recovering horses from GA | (5) Questionnaire on the process of recovering horses from general anesthesia and associated personnel injury in equine practice [71] |
| Total surveys = 5 | |
| LoE and Topic | References |
|---|---|
| (4a) LoE premedication/sedation | |
| 1 | (1) A comparison of romifidine and xylazine when used with diazepam/ketamine for short duration anesthesia in the horse [72] (2) Effects of additional premedication on romifidine and ketamine anaesthesia in horses [73] (3) Comparison of detomidine and romifidine as premedicants before ketamine and halothane anesthesia in horses undergoing elective surgery [74] (4) Clinical comparison of xylazine and medetomidine for premedication of horses [75] (5) Comparison of morphine and butorphanol as pre-anaesthetic agents in combination with romifidine for field castration in ponies [76] (6) The influence of butorphanol dose on characteristics of xylazine-butorphanol-propofol anesthesia in horses at altitude [77] (7) Dissociative anaesthesia during field and hospital conditions for castration of colts [78] (8) Influence of the combination of butorphanol and detomidine within premedication on the preoperative sedations score, the intraoperative cardiovascular situation and the early recovery period in horses [79] (9) Analgesic effects of butorphanol tartrate and phenylbutazone administered alone and in combination in young horses undergoing routine castration [80] (10) Comparison of acepromazine, midazolam and xylazine as preanaesthetics to ketamine-isoflurane anaesthesia in horses [81] (11) Buprenorphine provides better anaesthetic conditions than butorphanol for field castration in ponies: Results of a randomised clinical trial [82] (12) Effect of pre- and postoperative phenylbutazone and morphine administration on the breathing response to skin Incision, recovery quality, behavior, and cardiorespiratory variables in horses undergoing fetlock arthroscopy: A pilot study [83] (13) Analgesic and adjunct actions of nalbuphine hydrochloride in xylazine or xylazine and acepromazine premedicated horses [84] (14) Preemptive analgesia, including morphine, does not affect recovery quality and times in either pain-free horses or horses undergoing orchiectomy [85] (15) A preliminary study on effects of subanesthetic doses of preemptive ketamine given prior to premedication on total intravenous anesthesia for short- to medium-term surgical procedures in horses [86] (16) Effect of methadone combined with acepromazine or detomidine on sedation and dissociative anesthesia in healthy horses [87] (17) Comparison of xylazine and detomidine in combination with midazolam/ketamine for field castration in Quarter Horses [88] |
| 2 | (18) Comparison of romifidine and xylazine as premedicants before general anaesthesia in horses regarding the postsurgical recovery period [89] |
| 3 | / |
| 4 | (19) Evaluation of xylazine as a sedative and preanesthetic agent in horses [90] (20) A field trial of ketamine anaesthesia in the horse [91] (21) Clinical evaluation of romifidine/ketamine/halothane anaesthesia in horses [92] |
| 5 | / |
| ? | (22) Use of a propionylpromazine and meperidine combination in thiopental sodium anesthesia in horses [93] |
| Total premedication/sedation publications = 22 | |
| (4b) LoE induction drugs | |
| 1 | (1) A clinical trial of three anaesthetic regimens for the castration of ponies [94] (2) A comparison of xylazine-diazepam-ketamine and xylazine-guaifenesin-ketamine in equine anesthesia [95] (3) Evaluation of propofol as a general anesthetic for horses [96] (4) Comparison of thiopentone/guaifenesin, ketamine/guaifenesin and ketamine/midazolam for the induction of horses to be anaesthetised with isoflurane [97] (5) Behavioral responses following eight anesthetic induction protocols in horses [98] (6) Evaluation of different doses of propofol in xylazine pre-medicated horses [99] (7) Comparison of high (5%) and low (1%) concentrations of micellar microemulsion propofol formulations with a standard (1%) lipid emulsion in horses [100] (8) Alfaxalone compared with ketamine for induction of anaesthesia in horses following xylazine and guaifenesin [101] (9) Effects of ketamine, propofol, or thiopental administration on intraocular pressure and qualities of induction of and recovery from anesthesia in horses [102] (10) Comparison of midazolam and diazepam as co-induction agents with ketamine for anaesthesia in sedated ponies undergoing field castration [103] (11) Comparison of alfaxalone, ketamine and thiopental for anaesthetic induction and recovery in Thoroughbred horses premedicated with medetomidine and midazolam [104] (12) Recovery of horses from general anesthesia after induction with propofol and ketamine versus midazolam and ketamine [105] (13) Evaluation of the use of midazolam as a co-induction agent with ketamine for anaesthesia in sedated ponies undergoing field castration [106] (14) A comparison of two ketamine doses for field anaesthesia in horses undergoing castration [107] |
| 2 | (15*) Methohexital sodium anesthesia in the horse (Trial 1) [108] (16) Intravenous anaesthesia in horses after xylazine premedication [109] |
| 3 | (17) A retrospective comparison of induction with thiopental/guaifenesin and propofol/ketamine in Thoroughbred racehorses anesthetized with sevoflurane and medetomidine during arthroscopic surgery [110] |
| 4 | (15*) Methohexital sodium anesthesia in the horse (Trials 2 and 3) [108] (18) Effects of Saffan administered intravenously in the horse [111] (19) Observations on the use of glyceryl guaiacolate as an adjunct to general anaesthesia in horses [112] (20) Midazolam and ketamine induction before halothane anaesthesia in ponies: Cardiorespiratory, endocrine and metabolic changes [113] (21) Cardiorespiratory effects of a tiletamine/zolazepam-ketamine-detomidine combination in horses [114] (22) The pharmacokinetics and pharmacodynamics of the injectable anaesthetic alfaxalone in the horse [115] (23) Short-term general anesthesia with tiletamine/zolazepam in horses sedated with medetomidine for castration under field conditions [116] |
| 5 | / |
| ? | (24) Thiopentone (pentothal sodium) as a general anaesthetic in the horse [117] (25) Glyceryl guaiacolate in equine anesthesia [118] (26) Pharmacokinetics of ketamine in the horse [119] |
| Total induction drugs publications = 26 | |
| (4c) LoE sedation and induction drugs | |
| 1 | (1) Evaluation of propofol for general anesthesia in premedicated horses [120] (2) Comparison of detomidine/ketamine and guaiphenesin/thiopentone for induction of anaesthesia in horses maintained with halothane [121] |
| 2 | (3) A comparison of injectable anesthetic combinations in horses [122] |
| 3 | (4) Propofol with ketamine following sedation with xylazine for routine induction of general anaesthesia in horses [123] |
| 4 | (5) Clinical trial of xylazine with ketamine in equine anaesthesia [124] (6) Equine castration: A practical approach [125] (7) Clinical observations during induction and recovery of xylazine-midazolam- propofol anesthesia in horses [126] (8) Clinical evaluation of detomidine-butorphanol-guaifenesin-ketamine as short term TIVA in Spiti ponies [127] |
| 5 | / |
| ? | (9) Evaluation of xylazine and ketamine hydrochloride for anesthesia in horses [128] (10) Evaluation of xylazine, guaifenesin, and ketamine hydrochloride for restraint in horses [129] (11) Ketamine, Telazol, xylazine and detomidine. A comparative anesthetic drug combinations study in ponies [130] |
| Total sedation and induction drugs publications = 11 | |
| LoE and Topic | References |
|---|---|
| (5a) LoE inhalant agents | |
| 1 | (1) Clinical anaesthesia in the horse: Comparison of enflurane and halothane [131] (2) Actions of isoflurane and halothane in pregnant mares [132] (3) Comparison of recoveries from halothane vs isoflurane anesthesia in horses [133] (4) The recovery of horses from inhalant anesthesia: A comparison of halothane and isoflurane [134] (5) Is isoflurane safer than halothane in equine anaesthesia? Results from a prospective multicentre randomised controlled trial [135] (6) Differences in need for hemodynamic support in horses anesthetized with sevoflurane as compared to isoflurane [136] (7) Comparison of hemodynamic, clinicopathologic, and gastrointestinal motility effects and recovery characteristics of anesthesia with isoflurane and halothane in horses undergoing arthroscopic surgery [137] (8) A comparison of recovery times and characteristics with sevoflurane and isoflurane anaesthesia in horses undergoing magnetic resonance imaging [138] (9) Desflurane and sevoflurane elimination kinetics and recovery quality in horses [139] |
| 2 | (10) Recovery from anaesthesia in ponies: A comparative study of the effects of isoflurane, enflurane, methoxyflurane and halothane [140] (11) Isoflurane anesthesia for equine colic surgery. Comparison with halothane anesthesia [141] (12) Cardiorespiratory effects of sevoflurane, isoflurane, and halothane anesthesia in horses [142] (13) Clinical comparison of medetomidine with isoflurane or sevoflurane for anesthesia in horses [143] |
| 3 | / |
| 4 | (14) A study of the use of methoxyflurane general anesthesia in the horse [144] (15) Influence of a clinical anaesthesia-technique (premedication with tranquillizers and atropine, induction with chloralhydrate, maintenance with halothane in a closed circle system) on liver function tests in the horse [145] (16) Clinical experiences with isoflurane in dogs and horses [146] (17) Determination of the minimum alveolar concentration (MAC) and physical response to sevoflurane inhalation in horses [147] (18) Sevoflurane and oxygen anaesthesia following administration of atropine-xylazine-guaifenesin-thiopental in spontaneously breathing horses [148] (19) Cardiovascular and pulmonary effects of sevoflurane anesthesia in horses [149] (20) Anesthetic potency of desflurane in the horse: Determination of the minimum alveolar concentration [150] (21) Maintenance of anaesthesia with sevoflurane and oxygen in mechanically-ventilated horses subjected to exploratory laparotomy treated with intra- and post operative anaesthetic adjuncts [151] (22) Perioperative plasma cortisol concentration in the horse [152] (23) Romifidine-ketamine-halothane anesthesia in horses [153] (24) Anesthetic management with sevoflurane and oxygen for orthopedic surgeries in racehorses [154] (25) Anesthesia in Caspian ponies [155] (26) Validation of the bispectral index as an indicator of anesthetic depth in Thoroughbred horses anesthetized with sevoflurane [156] |
| 5 | (27) The modification and performance of a large animal anesthesia machine (Tafonius®) in order to deliver Xenon to a horse [157] |
| Total inhalant agents publications = 27 | |
| (5b) LoE TIVA | |
| 1 | (1) Guaifenesin-ketamine-xylazine anesthesia for castration in ponies—a comparative study with two different doses of ketamine [158] (2) Comparison of four drug combinations for total intravenous anesthesia of horses undergoing surgical removal of an abdominal testis [159] (3) Evaluation of xylazine and ketamine for total intravenous anesthesia in horses [160] (4) Evaluation of total intravenous anesthesia with propofol or ketamine-medetomidine-propofol combination in horses [161] (5) Comparison of 3 total intravenous anesthetic infusion combinations in adult horses [162] (6) Evaluation of cardiovascular effects of total intravenous anesthesia with propofol or a combination of ketamine-medetomidine-propofol in horses [163] (7) Comparison of ketamine and S(+)-ketamine, with romifidine and diazepam, for total intravenous anesthesia in horses [164] (8) Anaesthetic and cardiorespiratory effects of propofol at 10% for induction and 1% for maintenance of anaesthesia in horses [165] (9) Short-term anaesthesia with xylazine, diazepam/ketamine for castration in horses under field conditions: Use of intravenous lidocaine [166] (10) Anaesthetic evaluation of ketamine/propofol in acepromazine- xylazine premedicated horses [167] (11) Comparison of ketamine and alfaxalone for induction and maintenance of anaesthesia in ponies undergoing castration [168] (12) Anesthetic and cardiorespiratory effects of propofol, medetomidine, lidocaine and butorphanol total intravenous anesthesia in horses [169] (13) Cardiorespiratory and antinociceptive effects of two different doses of lidocaine administered to horses during a constant intravenous infusion of xylazine and ketamine [170] (14) Effects of dexmedetomidine and xylazine on cardiovascular function during total intravenous anaesthesia with midazolam and ketamine and recovery quality and duration in horses [171] (15) Continuous maintenance anaesthesia using guaifenesin or diazepam combined with xylazine and ketamine in horses [172] (16) Evaluation of total intravenous anesthesia with propofol-guaifenesin-medetomidine and alfaxalone-guaifenesin-medetomidine in Thoroughbred horses undergoing castration [173] (17) Alfaxalone for maintenance of anaesthesia in ponies undergoing field castration: Continuous infusion compared with intravenous boluses [174] (18) Cardiopulmonary effects and recovery characteristics of horses anesthetized with xylazine-ketamine with midazolam or propofol [175] (19) Total intravenous anaesthesia with ketamine, medetomidine and guaifenesin compared with ketamine, medetomidine and midazolam in young horses anaesthetised for computerised tomography [176] |
| 2 | (20) Total intravenous anaesthesia in the horse with propofol [177] (21) The stress response to anaesthesia in ponies: Barbiturate anaesthesia [178] (22) Intravenous anaesthesia in hoses: Racemic ketamine versus S-(+)-ketamine [179] (23) Tiletamine-zolazepam anesthesia in horses: Repeated dose versus continuous infusion [180] |
| 3 | / |
| 4 | (24) Anesthesia by injection of xylazine, ketamine and the benzodiazepine derivative climazolam and the use of the benzodiazepine antagonist Ro 15–3505 [181] (25) Prolongation of anesthesia with xylazine, ketamine, and guaifenesin in horses: 64 cases (1986–1989) [182] (26) Clinical evaluation of an infusion of xylazine, guaifenesin and ketamine for maintenance of anaesthesia in horses [183] (27) A case report on the use of guaifenesin-ketamine-xylazine anesthesia for equine dystocia [184] (28) Total intravenous anaesthesia in ponies using detomidine, ketamine and guaiphenesin: Pharmacokinetics, cardiopulmonary and endocrine effects [185] (29) Physiologic effects of anesthesia induced and maintained by intravenous administration of a climazolam-ketamine combination in ponies premedicated with acepromazine and xylazine [186] (30) Romifidine, ketamine and guaiphenesin continual infusion anaesthesia: Some experiences of its use under field conditions [187] (31) Guaifenesin-ketamine-detomidine anesthesia for castration of ponies [188] (32) Detomidine-propofol anesthesia for abdominal surgery in horses [189] (33) Investigations into injection anesthesia (TIVA) of the horse with ketamine/guaifenesin/xylazin: Experiences with computerized pump infusion [190] (34) Anaesthetic compound and its application in general anaesthesia of horses [191] (35) Cardiopulmonary effects of prolonged anesthesia via propofol-medetomidine infusion in ponies [192] (36) Infusion of a combination of propofol and medetomidine for long-term anesthesia in ponies [193] (37) Intravenous anaesthesia using detomidine, ketamine and guaiphenesin for laparotomy in pregnant pony mares [194] (38) Propofol anaesthesia for surgery in late gestation pony mares [195] (39) Assessment of a medetomidine/propofol total intravenous anaesthesia (TIVA) for clinical anaesthesia in equidae [196] (40) Practical experiences and clinical parameters in a xylazine-ketamine-anaesthesia of horses [197] (41) Medetomidine-ketamine anaesthesia induction followed by medetomidine-propofol in ponies: Infusion rates and cardiopulmonary side effects [198] (42) Propofol-ketamine anesthesia for internal fixation of fractures in racehorses [199] (43) Total intravenous anaesthesia in horses using medetomidine and propofol [200] (44) Anesthetic and cardiopulmonary effects of total intravenous anesthesia using a midazolam, ketamine and medetomidine drug combination in horses [201] (45) Alfaxalone in cyclodextrin for induction and maintenance of anaesthesia in ponies undergoing field castration [202] (46) Pharmacokinetic profile in relation to anaesthesia characteristics after a 5% micellar microemulsion of propofol in the horse [203] (47) Evaluation of cardiovascular, respiratory and biochemical effects, and anesthetic induction and recovery behavior in horses anesthetized with a 5% micellar microemulsion propofol formulation [204] (48) Clinical evaluation of total intravenous anesthesia using a combination of propofol and medetomidine following anesthesia induction with medetomidine, guaifenesin and propofol for castration in Thoroughbred horses [205] (49) Evaluation of a midazolam-ketamine-xylazine infusion for total intravenous anesthesia in horses [206] (50) Alfaxalone and medetomidine intravenous infusion to maintain anaesthesia in colts undergoing field castration [207] (51) Clinical and pharmacokinetic evaluation of S-ketamine for intravenous general anaesthesia in horses undergoing field castration [208] (52) Cardiovascular effects of total intravenous anesthesia using ketamine-medetomidine-propofol (KMP-TIVA) in horses undergoing surgery [209] (53) Cardiorespiratory and anesthetic effects of combined alfaxalone, butorphanol, and medetomidine in Thoroughbred horses [210] (54) Total intravenous anesthesia using a midazolam-ketamine-xylazine infusion in horses: 46 cases (2011–2014) [211] (55) Alfaxalone for total intravenous anaesthesia in horses [212] |
| 5 | / |
| Total TIVA publications = 55 | |
| (5c) LoE TIVA vs. inhalant agents | |
| 1 | (1) Cardiovascular effects of surgical castration during anaesthesia maintained with halothane or infusion of detomidine, ketamine and guaifenesin in ponies [213] (2) Cardiopulmonary effects of dexmedetomidine and ketamine infusions with either propofol infusion or isoflurane for anesthesia in horses [214] |
| 2 | (3) Cardiorespiratory, endocrine and metabolic changes in ponies undergoing intravenous or inhalation anaesthesia [215] |
| Total TIVA versus inhalants publications = 3 | |
| LoE | References |
|---|---|
| 1 | (1) Anaesthesia in horses using halothane and intravenous ketamine-guaiphenesin: A clinical study [216] (2) Infusion of guaifenesin, ketamine, and medetomidine in combination with inhalation of sevoflurane versus inhalation of sevoflurane alone for anesthesia of horses [217] (3) Interactions of morphine and isoflurane in horses [218] (4) Effect of a constant rate infusion of lidocaine on the quality of recovery from sevoflurane or isoflurane general anaesthesia in horses [219] (5) Morphine administration in horses anaesthetized for upper respiratory tract surgery [220] (6) Combined anaesthesia with isoflurane and an infusion of a mixture of ketamine, midazolam and one of three α2-adrenoreceptor agonists for castration in horses [221] (7) A clinical comparison of two anaesthetic protocols using lidocaine or medetomidine in horses [222] (8) The effects of morphine on the recovery of horses from halothane anaesthesia [223] (9) Clinical evaluation of ketamine and lidocaine intravenous infusions to reduce isoflurane requirements in horses under general anaesthesia [224] (10) A study of cardiovascular function under controlled and spontaneous ventilation in isoflurane-medetomidine anaesthetized horses [225] (11) Effects of high plasma fentanyl concentrations on minimum alveolar concentration of isoflurane in horses [226] (12) Evaluation of anesthesia recovery quality after low-dose racemic or S-ketamine infusions during anesthesia with isoflurane in horses [227] (13) Romifidine as a constant rate infusion in isoflurane anaesthetized horses: A clinical study [228] (14) Effects of adding butorphanol to a balanced anaesthesia protocol during arthroscopic surgery in horses [229] (15) Comparison of cardiovascular function and quality of recovery in isoflurane-anaesthetised horses administered a constant rate infusion of lidocaine or lidocaine and medetomidine during elective surgery [230] (16) A clinical study on the effect in horses during medetomidine-isoflurane anaesthesia, of butorphanol constant rate infusion on isoflurane requirements, on cardiopulmonary function and on recovery characteristics [231] (17) Effects of a constant rate infusion of detomidine on cardiovascular function, isoflurane requirements and recovery quality in horses [232] (18) Effects of constant rate infusion of lidocaine and ketamine, with or without morphine, on isoflurane MAC in horses [233] (19) Comparison of the cardiovascular effects of equipotent anesthetic doses of sevoflurane alone and sevoflurane plus an intravenous infusion of lidocaine in horses [234] (20) Comparison of the effects of xylazine bolus versus medetomidine constant rate infusion on the stress response, urine production, and anesthetic recovery characteristics in horses anesthetized with isoflurane [235] (21) Medetomidine continuous rate intravenous infusion in horses in which surgical anaesthesia is maintained with isoflurane and intravenous infusions of lidocaine and ketamine [236] (22) Influence of a constant rate infusion of dexmedetomidine on cardiopulmonary function and recovery quality in isoflurane anaesthetized horses [237] (23) Evaluation of the clinical efficacy of two partial intravenous anesthetic protocols, compared with isoflurane alone, to maintain general anesthesia in horses [238] (24) Continuous intravenous lidocaine infusion during isoflurane anaesthesia in horses undergoing surgical procedures [239] (25) A comparison of two morphine doses on the quality of recovery from general anaesthesia in horses [240] (26) Effects of a constant-rate infusion of dexmedetomidine on the minimal alveolar concentration of sevoflurane in ponies [241] (27) Comparison of the influence of two different constant-rate infusions (dexmedetomidine versus morphine) on anaesthetic requirements, cardiopulmonary function and recovery quality in isoflurane anaesthetized horses [242] (28) Cardiopulmonary effects of an infusion of remifentanil or morphine in horses anesthetized with isoflurane and dexmedetomidine [243] (29) Effects of a continuous rate infusion of butorphanol in isoflurane-anesthetized horses on cardiorespiratory parameters, recovery quality, gastrointestinal motility and serum cortisol concentrations [244] (30) Minimum end-tidal sevoflurane concentration necessary to prevent movement during a constant rate infusion of morphine, or morphine plus dexmedetomidine in ponies [245] (31) Effects of a constant rate infusion of medetomidine-propofol on isoflurane minimum alveolar concentrations in horses [246] (32) Cardiopulmonary effects and recovery quality of remifentanil–isoflurane anesthesia in horses [247] (33) Clinical comparison of two regimens of lidocaine infusion in horses undergoing laparotomy for colic [248] (34) Effects of medetomidine constant rate infusion on sevoflurane requirement, cardiopulmonary function, and recovery quality in Thoroughbred racehorses undergoing arthroscopic surgery [249] (35) The cardiovascular status of isoflurane-anaesthetized horses with and without dexmedetomidine constant rate infusion evaluated at equivalent depths of anaesthesia [250] (36) Cardiopulmonary effects and anaesthesia recovery quality in horses anaesthetized with isoflurane and low-dose S-ketamine or medetomidine infusions [251] (37) Comparison of the effects of an intravenous lidocaine infusion combined with 1% isoflurane versus 2% isoflurane alone on selected cardiovascular variables and recovery characteristics during equine general anaesthesia [252] (38) Clinical usefulness of intravenous constant rate infusion of fentanyl and medetomidine under sevoflurane anesthesia in Thoroughbred racehorses undergoing internal fixation surgery [253] (39) Effect of detomidine or romifidine constant rate infusion on plasma lactate concentration and inhalant requirements during isoflurane anaesthesia in horses [254] (40) Clinical comparison of dexmedetomidine and medetomidine for isoflurane balanced anaesthesia in horses [255] (41) Clinical evaluation of constant rate infusion of alfaxalone-medetomidine combined with sevoflurane anesthesia in Thoroughbred racehorses undergoing arthroscopic surgery [256] (42) Clinical effects of constant rate infusions of medetomidine-propofol combined with sevoflurane anesthesia in Thoroughbred racehorses undergoing arthroscopic surgery [257] (43) Plasma concentrations at two dexmedetomidine constant rate infusions in isoflurane anaesthetized horses: A clinical study [258] |
| 2 | (44) Hemodynamic function during neurectomy in halothane-anesthetized horses with or without constant dose detomidine infusion [259] (45) Combination of continuous intravenous infusion using a mixture of guaifenesin-ketamine-medetomidine and sevoflurane anesthesia in horses [260] (46) Evaluation of a mixture of thiopental-guafinesine-medetomidine and sevoflurane anesthesia in horses [261] (47) Influence of ketamine or xylazine supplementation on isoflurane anaesthetized horses--a controlled clinical trial [262] (48) Comparative study on sevoflurane anesthesia alone and combined with partial intravenous anesthesia using dexmedetomidine in healthy horses [263] |
| 3 | (49) Problems associated with perioperative morphine in horses: A retrospective case analysis [264] (50) Recovery quality after romifidine versus detomidine infusion during isoflurane anesthesia in horses [265] |
| 4 | (51) Minimal alveolar concentration of desflurane in combination with an infusion of medetomidine for the anaesthesia of ponies [266] (52) Influence of gastrointestinal tract disease on pharmacokinetics of lidocaine after intravenous infusion in anesthetized horses [267] (53) Clinical assessment of anesthesia with isoflurane and medetomidine in 300 equidae [268] (54) Partial intravenous anaesthesia in 5 horses using ketamine, lidocaine, medetomidine and halothane [269] |
| 5 | (55) Prolonged anesthesia using sevoflurane, remifentanil and dexmedetomidine in a horse [270] (56) Anesthetic management with sevoflurane combined with alfaxalone-medetomidine constant rate infusion in a Thoroughbred racehorse undergoing a long-time orthopedic surgery [271] |
| Total PIVA publications = 56 | |
| LoE and Topic | References |
|---|---|
| (7a) Loco-regional | |
| 1 | (1) The effect of epidural xylazine on halothane minimum alveolar concentration in ponies [272] (2) Epidural morphine and detomidine decreases postoperative hindlimb lameness in horses after bilateral stifle arthroscopy [273] (3) Castration of horses under total intravenous anaesthesia: Analgesic effects of lidocaine [274] (4) Local mepivacaine before castration of horses under medetomidine isoflurane balanced anaesthesia is effective to reduce perioperative nociception and cytokine release [275] (5) Intratesticular mepivacaine versus lidocaine in anaesthetised horses undergoing Henderson castration [276] (6) The effect of intra-articular mepivacaine administration prior to carpal arthroscopy on anesthesia management and recovery characteristics in horses [277] |
| 2 | / |
| 3 | (7) Epidural administration of opioid analgesics improves quality of recovery in horses anaesthetised for treatment of hindlimb synovial sepsis [278] (8) Clinical assessment of an ipsilateral cervical spinal nerve block for prosthetic laryngoplasty in anesthetized horses [279] |
| 4 | (9) The outcome of epidural anaesthesia in horses with perineal and tail melanomas: Complications associated with ataxia and the risks of rope recovery [280] |
| 5 | / |
| Total loco-regional publications = 9 | |
| (7b) NMBAs | |
| 1 | (1) Influence of atracurium on cardiovascular parameters in horses undergoing vitrectomy during general anaesthesia, and on recovery duration and quality [281] (2) Effects of acetylcholinesterase inhibition on quality of recovery from isoflurane-induced anesthesia in horses [282] |
| 2 | / |
| 3 | / |
| 4 | (3) Neuromuscular and cardiovascular effects of atracurium in ponies anesthetized with halothane [283] (4) Neuromuscular and cardiovascular effects of atracurium administered to healthy horses anesthetized with halothane [284] (5) Clinical use of the neuromuscular blocking agents atracurium and pancuronium for equine anesthesia [285] (6) Effects of atracurium administered by continuous intravenous infusion in halothane-anesthetized horses [286] (7) Interaction of gentamycin and atracurium in anaesthetised horses [287] (8) Use of sevoflurane for anesthetic management of horses during thoracotomy [288] |
| 5 | (9) Prolonged neuromuscular blockade in a horse following concomitant use of vecuronium and atracurium [289] |
| ? | (10) Biochemical effects of succinylcholine chloride in mechanically ventilated horses anesthetized with halothane in oxygen [290] |
| Total NMBAs publications = 10 | |
| (7c) Other drugs | |
| 1 | (1) Effects of intravenous administration of dimethyl sulfoxide on cardiopulmonary and clinicopathologic variables in awake or halothane-anesthetized horses [291] (2) Effects of glycopyrrolate on cardiorespiratory function in horses anesthetized with halothane and xylazine [292] (3) Effects of a muscarinic type-2 antagonist on cardiorespiratory function and intestinal transit in horses anesthetized with halothane and xylazine [293] (4) Effect of dantrolene premedication on various cardiovascular and biochemical variables and the recovery in healthy isoflurane-anesthetized horses [294] |
| 2 | (5) Effects of dopamine, dobutamine, dopexamine, phenylephrine, and saline solution on intramuscular blood flow and other cardiopulmonary variables in halothane-anesthetized ponies [295] |
| 3 | (6) Doxapram infusion during halothane anaesthesia in ponies [296] (7) Influence of hypertonic saline solution 7.2% on different hematological parameters in awake and anaesthetized ponies [297] |
| 4 | (8) Temporal effects of an infusion of dopexamine hydrochloride in horses anesthetized with halothane [298] |
| 5 | / |
| Total other drugs publications = 8 | |
| LoE | References |
|---|---|
| 1 | (1) Antagonism of xylazine and ketamine anesthesia by 4-aminopyridine and yohimbine in geldings [299] (2) Effect of postoperative pethidine on the anaesthetic recovery period in the horse [300] (3) Recovery from sevoflurane anesthesia in horses: Comparison to isoflurane and effect of postmedication with xylazine [301] (4) Recovery phase of horses after general anesthesia with inhalants with and without postanesthetic sedation with xylazine (Rompun®) [302] (5) Effects of alpha-2 adrenoceptor agonists during recovery from isoflurane anaesthesia in horses [303] (6) Standing behavior in horses after inhalation anesthesia with isoflurane (Isoflo) and postanesthetic sedation with romifidine (Sedivet) or xylazine (Rompun) [304] (7) A comparison of equine recovery characteristics after isoflurane or isoflurane followed by a xylazine-ketamine infusion [305] (8) Effect of administration of propofol and xylazine hydrochloride on recovery of horses after four hours of anesthesia with desflurane [306] (9) Comparison of the effects of xylazine and romifidine administered perioperatively on the recovery of anesthetized horses [307] (10) Evaluation of infusions of xylazine with ketamine or propofol to modulate recovery following sevoflurane anesthesia in horses [308] (11) Effects of postanesthetic sedation with romifidine or xylazine on quality of recovery from isoflurane anesthesia in horses [309] (12) Assessment of unassisted recovery from repeated general isoflurane anesthesia in horses following post-anesthetic administration of xylazine or acepromazine or a combination of xylazine and ketamine [310] (13) Effect of postoperative xylazine administration on cardiopulmonary function and recovery quality after isoflurane anesthesia in horses [311] (14) Recovery from desflurane anesthesia in horses with and without post-anesthetic xylazine [312] (15) Comparison between the effects of postanesthetic xylazine and dexmedetomidine on characteristics of recovery from sevoflurane anesthesia in horses [313] (16) Pharmacokinetics and clinical effects of xylazine and dexmedetomidine in horses recovering from isoflurane anesthesia [314] (17) Recovery quality following a single post-anaesthetic dose of dexmedetomidine or romifidine in sevoflurane anaesthetised horses [315] (18) The effects of flumazenil on ventilatory and recovery characteristics in horses following midazolam-ketamine induction and isoflurane anaesthesia [316] |
| 2 | / |
| 3 | / |
| 4 | / |
| 5 | / |
| Total drugs before/during recovery publications = 18 | |
| LoE | References |
|---|---|
| 1 | (1) Cardiopulmonary function following anesthesia in horses experiencing hydro pool recovery versus padded stall recovery [317] (2) Cardiopulmonary function in horses during anesthetic recovery in a hydropool [318] (3) Comparison of recoveries from anesthesia of horses placed on a rapidly inflating-deflating air pillow or the floor of a padded stall [319] (4) Arterial oxygen tension and pulmonary ventilation in horses placed in the Anderson Sling suspension system after a period of lateral recumbency and anaesthetised with constant rate infusions of romifidine and ketamine [320] (5) Comparison between head-tail-rope assisted and unassisted recoveries in healthy horses undergoing general anesthesia for elective surgeries [321] |
| 2 | / |
| 3 | (6) Comparison of non-assisted versus head and tail rope-assisted recovery after emergency abdominal surgery in horses [322] (7) Effect of head and tail rope-assisted recovery of horses after elective and emergency surgery under general anaesthesia [323] |
| 4 | (8) Use of a pool-raft system for recovery of horses from general anesthesia: 393 horses (1984–2000) [324] (9) Use of a hydro-pool system to recover horses after general anesthesia: 60 cases [325] (10) Investigation into the assisted standing up procedure in horses during recovery phase after inhalation anaesthesia [326] (11) Use of the Anderson Sling suspension system for recovery of horses from general anesthesia [327] (12) Tilt table recovery of horses after orthopedic surgery: Fifty-four cases (1994–2005) [328] (13) Evaluation of a new full-body animal rescue and transportation sling in horses: 181 horses (1998–2006) [329] (14) Use of propofol-xylazine and the Anderson Sling Suspension System for recovery of horses from desflurane anesthesia [330] (15) Anaesthetic management for hydropool recovery in 50 horses [331] (16) A retrospective report (2003–2013) of the complications associated with the use of a one-man (head and tail) rope recovery system in horses following general anaesthesia [332] |
| 5 | (17) Internal fixation of a fractured axis in an adult horse [333] (18) Reduction and external coaptation as successful treatment for tarsocrural joint luxation in an Arabian mare [334] |
| Total recovery systems publications = 18 | |
| LoE | References |
|---|---|
| 1 | (1) Intranasal phenylephrine reduces post anesthetic upper airway obstruction in horses [335] (2) An evaluation of apnea or spontaneous ventilation in early recovery following mechanical ventilation in the anesthetized horse [336] (3) Cardiopulmonary effects associated with head-down position in halothane-anesthetized ponies with or without capnoperitoneum [337] (4) High inspired oxygen concentrations increase intrapulmonary shunt in anaesthetized horses [338] (5) Intermittent positive pressure ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvre during inhalation anaesthesia in horses undergoing surgery for colic, and its influence on the early recovery period [339] (6) Influence on horse’s pulmonary function using a modified “Open-Lung-Concept”-Ventilation with different oxygen-concentration during general anaesthesia [340] (7) Effects of hypercapnic hyperpnea on recovery from isoflurane or sevoflurane anesthesia in horses [341] (8) Oxygenation and plasma endothelin-1 concentrations in healthy horses recovering from isoflurane anaesthesia administered with or without pulse-delivered inhaled nitric oxide [342] (9) Effect of pressure support ventilation during weaning on ventilation and oxygenation indices in healthy horses recovering from general anesthesia [343] (10) Comparison of cardiorespiratory variables in dorsally recumbent horses anesthetized with guaifenesin-ketamine-xylazine spontaneously breathing 50% or maximal oxygen concentrations [344] (11) Impact of low inspired oxygen fraction on oxygenation in clinical horses under general anesthesia [345] (12) The effect of two different intra-operative end-tidal carbon dioxide tensions on apnoeic duration in the recovery period in horses [346] (13) Controlled mechanical ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvres during anaesthesia in laterally or dorsally recumbent horses [347] (14) Effect of reducing inspired oxygen concentration on oxygenation parameters during general anaesthesia in horses in lateral or dorsal recumbency [348] (15) Effects of 12 and 17 cm H2O positive end-expiratory pressure applied after alveolar recruitment maneuver on pulmonary gas exchange and compliance in isoflurane-anesthetized horses [349] |
| 2 | (16) Arterial blood gas tensions in the horse during recovery from anesthesia [350] (17) Alleviation of postanesthetic hypoxemia in the horse [351] (18) Restoration of arterial oxygen tension in horses recovering from general anaesthesia [352] |
| 3 | / |
| 4 | (19) Evaluation of a modification of the Hudson demand valve in ventilated and spontaneously breathing horses [353] (20) Effects of sedation, anesthesia, and endotracheal intubation on respiratory mechanics in adult horses [354] (21) Use of nasotracheal intubation during general anesthesia in two ponies with tracheal collapse [355] |
| 5 | / |
| Total respiratory system in recovery publications = 21 | |
| Other Factors | LoE | References |
|---|---|---|
| Orthopaedic | 1 | (1) An in vitro biomechanical investigation of the mechanical properties of dynamic compression plated osteotomized adult equine tibiae [356] (2) Evaluation of a technique for collection of cancellous bone graft from the proximal humerus in horses [357] |
| 2 | / | |
| 3 | / | |
| 4 | (3) Medial condylar fractures of the third metatarsal bone in horses [358] (4) Use of tension band wires in horses with fractures of the ulna: 22 cases (1980–1992) [359] (5) Application of a hook plate for management of equine ulnar fractures [360] (6) Fractures of the palmar aspect of the carpal bones in horses: 10 cases (1984–2000) [361] (7) A lateral approach for screw repair in lag fashion of spiral third metacarpal and metatarsal medial condylar fractures in horses [362] (8) Arthroscopic removal of discrete palmar carpal osteochondral fragments in horses: 25 cases (1999–2013) [363] (9) Surgical repair of propagating condylar fractures of the third metacarpal/metatarsal bones with cortical screws placed in lag fashion in 26 racehorses (2007–2015) [364] | |
| 5 | (10) A musculoskeletal model of the equine forelimb for determining surface stresses and strains in the humerus--part I. Mathematical modeling [365] (11) Successful reduction and internal fixation of an open tibial fracture in an adult Icelandic horse [366] (12) Internal fixation of a complete ventral luxation of the dens axis in an American quarter horse yearling [367] | |
| Abdominal | 1 | (13) Incisional complications following exploratory celiotomy: Does an abdominal bandage reduce the risk? [368] (14) Case series evaluating the use of absorbable staples compared with metallic staples in equine ventral midline incisions [369] |
| 2 | (15) Evaluation of iodophor skin preparation techniques and factors influencing drainage from ventral midline incisions in horses [370] (16) Pulmonary gas exchange and plasma lactate in horses with gastrointestinal disease undergoing emergency exploratory laparotomy: A comparison with an elective surgery horse population [371] | |
| 3 | (17) Comparison of laparoscopic versus conventional open cryptorchidectomies on intraoperative and postoperative complications and duration of surgery, anesthesia, and hospital stay in horses [372] | |
| 4 | (18) Investigation of perioperative and anesthetic variables affecting short-term survival of horses with small intestinal strangulating lesions [373] (19) Retrospective identification of bacterial isolates from emergency laparotomy surgical site infections in horses [374] | |
| 5 | / | |
| Ocular surgery | 1 | / |
| 2 | / | |
| 3 | (20) Complications associated with anaesthesia for ocular surgery: A retrospective study 1989–1996 [375] (21) Evaluation of risk factors, including fluconazole administration, for prolonged anesthetic recovery times in horses undergoing general anesthesia for ocular surgery: 81 cases (2006–2013) [376] (22) Factors associated with postoperative complications in healthy horses after general anesthesia for ophthalmic versus non-ophthalmic procedures: 556 cases (2012–2014) [377] | |
| 4 | (23) Transpalpebral enucleation using a chain écraseur [378] | |
| 5 | (24) Anesthesia case of the month. Evaluation and treatment of suspected squamous cell carcinoma of the third eyelid [379] | |
| Others | ||
| Airway | 3 | (25) Laryngoplasty with ventriculectomy or ventriculocordectomy in 104 draft horses (1992–2000) [380] |
| Body temperature | 1 | (26) Temporal changes in core body temperature in anesthetized adult horses [381] |
| 2 | (27) Comparison of peripheral and core temperatures in anesthetized horses [382] | |
| Repeated GA | 2 | (28) The effects of multiple anaesthetic episodes on equine recovery quality [383] |
| Environmental light | 1 | (29) Recovery of horses from general anesthesia in a darkened or illuminated recovery stall [384] |
| Cardiac | 3 | (30) Management and complications of anesthesia for transvenous electrical cardioversion of atrial fibrillation in horses: 62 cases (2002–2006) [385] |
| 4 | (31) Hemodynamics before and after conversion of atrial-fibrillation to normal sinus rhythm in horses [386] (32) Electrocardiographic indicators of excitability in horses for predicting recovery quality after general anaesthesia [387] | |
| Metabolic | 1 | (33) Effects of sevoflurane dose and mode of ventilation on cardiopulmonary function and blood biochemical variables in horses [388] |
| 2 | (34) Influence of azaperone/metomidate in anesthesia on blood biochemistry in horse [389] (35) Ultrasonography of the equine triceps muscle before and after general anaesthesia and in post anaesthetic myopathy [390] (36) Metabolism during anaesthesia and recovery in colic and healthy horses: A microdialysis study [391] | |
| 4 | (37) Metabolic changes associated with anaesthesia and cherry poisoning in a pony [392] (38) The relationship of muscle perfusion and metabolism with cardiovascular variables before and after detomidine injection during propofol-ketamine anaesthesia in horses [393] | |
| Miscellaneous | 1 | (39) A prospective clinical trial comparing metrizamide and iohexol for equine myelography [394] |
| 2 | (40) Corneal abrasion and microbial contamination in horses following general anaesthesia for non-ocular surgery [395] | |
| 4 | (41) Electroacupuncture treatment for isoflurane induced hypotension in horses [396] | |
| Total other factors publications = 41 | ||
| (12a) Case series, LoE = 4 | References |
| Aspiration pneumonitis | (1) Aspiration pneumonitis (Mendelson’s syndrome) as perianaesthetic complication occurring in two horses: A case report [397] |
| Behaviour during recovery from GA | (2) The behaviour of horses recovering from anaesthesia [398] |
| Guttural pouch mycosis | (3) Treatment of guttural pouch mycosis [399] (4) Potential for iatrogenic coil embolization of the caudal cerebellar artery during treatment of internal carotid artery bifurcation in two horses with guttural pouch mycosis [400] |
| Post-anaesthetic myopathy/neuropathy | (5) Post-anaesthetic forelimb lameness in horses [401] (6) Postanesthetic hind limb adductor myopathy in five horses [402] (7) Femoral nerve paralysis after general anaesthesia [403] (8) The incidence of post-anaesthetic myopathy with the use of a static air mattress [404] (9) Transient pelvic limb neuropathy following proximal metatarsal and tarsal magnetic resonance imaging in seven horses [405] |
| Pulmonary oedema | (10) Post-anesthetic pulmonary edema in two horses [406] |
| Repetitive injectable anaesthesia | (11) Repetitive injectable anesthesia in a 27-year-old horse [407] |
| Sweating | (12) Unexpected responses following intravenous pethidine injection in two horses [408] |
| Total case series = 12 | |
| (12b) Case reports, LoE = 5 | References |
| Assisted recovery with an Animal Rescue and Transport Sling (ARTS) | (1) Successful treatment of a coxofemoral luxation in a Shetland pony by closed reduction and prolonged immobilization using a full-body Animal Rescue Sling [409] |
| Bladder rupture | (2) Ruptured urinary bladder in a horse [410] |
| Cardiac arrest in recovery | (3) Uterine prolapse in a mare leading to metritis, systemic inflammatory response syndrome, septic shock and death [411] (4) Successful cardiopulmonary resuscitation in a sevoflurane anaesthetized horse that suffered cardiac arrest at recovery [412] |
| Coxofemoral luxation | (5) Coxofemoral luxation in a horse during recovery from general anaesthesia [413] |
| Diaphragmatic hernia/rupture | (6) Fatal diaphragmatic rupture during recovery from general anaesthesia in a Standardbred horse [414] (7) Use of continuous positive airway pressure (CPAP) in a horse with diaphragmatic hernia [415] (8) Perianesthetic development of diaphragmatic hernia in a horse with equine pituitary pars intermedia dysfunction (PPID) [416] |
| Epistaxis/Guttural pouch mycosis | (9) Unusual internal carotid artery branching that prevented arterial occlusion with a balloon-tipped catheter in a horse [417] |
| Facial paralysis | (10) Multimodal therapy including electroacupuncture for the treatment of facial nerve paralysis in a horse [418] |
| Incisional dehiscence | (11) Anesthetic management of an incisional dehiscence in recovery following exploratory laparotomy in a horse [419] |
| Laryngeal oedema | (12) Complications associated with the use of the cuffless endotracheal tube in the horse [420] |
| Laryngeal paralysis/dysfunction | (13) Temporary bilateral laryngeal paralysis in a horse associated with general anaesthesia and post anaesthetic myositis [421] (14) Acute life-threatening laryngeal dysfunction in a draft horse recovering from general anesthesia: A case report. [422] |
| Nasotracheal | (15) Broken nasotracheal tube aspiration in a horse during anaesthetic recovery [423] |
| Fractures | (16) Comparison of therapeutic techniques for the treatment of cheek teeth diseases in the horse: Extraction versus repulsion [424] (17) Limb fracture during recovery from general anaesthesia: An often tragic complication of equine anaesthesia [425] (18) Standing low-field magnetic resonance imaging of a comminuted central tarsal bone fracture in a horse [426] |
| Hyperthermia | (19*) Hyperthermia during isoflurane anaesthesia in a horse with suspected hyperkalemic periodic paralysis [427] (20*) Hyperthermia and delayed-onset myopathy after recovery from anesthesia in a horse [428] |
| Malignant hyperthermia | (21) Malignant hyperthermia in a halothane-anesthetized horse [429] (22*) Postanesthetic equine myopathy suggestive of malignant hyperthermia. A case report [430] |
| Post-anaesthetic myelomalacia | (23) Postanesthetic poliomyelomalacia in a horse [431] (24) Hemorrhagic myelomalacia following general anesthesia in a horse [432] (25) Hematomyelia in a colt: A post anesthesia surgery complication [433] (26) Post-anaesthetic myelomalacia in a horse [434] |
| Post-anaesthetic myelopathy | (27) Post-anaesthetic myelopathy in a 3-year-old Friesian gelding [435] (28) Post anaesthetic myelopathy in the horse: A case report [436] |
| Post-anaesthetic myopathy/neuropathy | (22*) Postanesthetic equine myopathy suggestive of malignant hyperthermia. A case report [430] (20*) Hyperthermia and delayed-onset myopathy after recovery from anesthesia in a horse [428] (29) Suspicion of postanesthetic femoral paralysis of the non-dependent limb in a horse [437] |
| Post-anaesthetic myositis | (30) Tracheal reconstruction by resection and end-to-end anastomosis in the horse [438] |
| Post-anaesthetic pleuropneumonia | (31) Pleuropneumonia as a sequela of myelography and general anaesthesia in a Thoroughbred colt [439] |
| Post-anaesthetic pulmonary haemorrhage | (32) Fatal post-anaesthetic pulmonary haemorrhage in a horse suffering from chronic-active exercise-induced pulmonary haemorrhage [440] |
| Prolonged recovery by HYPP | (33) Postanesthetic recumbency associated with hyperkalemic periodic paralysis in a quarter horse [441] (19*) Hyperthermia during isoflurane anesthesia in a horse with suspected hyperkalemic periodic paralysis [427] |
| Prolonged recovery by persistent hypoxaemia | (34) Prolonged recovery from general anesthesia possibly related to persistent hypoxemia in a draft horse [442] |
| Pulmonary oedema | (35) Negative pressure pulmonary edema as a post-anesthetic complication associated with upper airway obstruction in a horse [443] (36) Pulmonary oedema associated with anaesthesia for colic surgery in a horse [444] (37) Suspected air embolism associated with post-anesthetic pulmonary edema and neurologic sequelae in a horse [445] (38) Pulmonary edema at recovery after colic operation with in-situ nasogastric tube in a horse [446] |
| Tracheal rupture | (39) Tracheal rupture following general anaesthesia in a horse [447] |
| Total case reports = 39 |
| LoE | References |
|---|---|
| 1 | (1) A comparison of four systems for scoring recovery quality after general anaesthesia in horses [448] (2) Quantitative and qualitative comparison of three scoring systems for assessing recovery quality after general anaesthesia in horses [449] (3) Factors affecting the perception of recovery quality in horses after anaesthesia [450] |
| 2 | (4) A study of the correlation between objective and subjective indices of recovery quality after inhalation anaesthesia in equids [451] (5) Assessment of agreement among diplomates of the American College of Veterinary Anesthesia and Analgesia for scoring the recovery of horses from anesthesia by use of subjective grading scales and development of a system for evaluation of the recovery of horses from anesthesia by use of accelerometry [452] |
| 3 | (6) Retrospective evaluation of correlation and agreement between two recovery scoring systems in horses [453] |
| 4 | / |
| 5 | / |
| Total systems to score recoveries publications = 6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozalo-Marcilla, M.; Ringer, S.K. Recovery after General Anaesthesia in Adult Horses: A Structured Summary of the Literature. Animals 2021, 11, 1777. https://doi.org/10.3390/ani11061777
Gozalo-Marcilla M, Ringer SK. Recovery after General Anaesthesia in Adult Horses: A Structured Summary of the Literature. Animals. 2021; 11(6):1777. https://doi.org/10.3390/ani11061777
Chicago/Turabian StyleGozalo-Marcilla, Miguel, and Simone Katja Ringer. 2021. "Recovery after General Anaesthesia in Adult Horses: A Structured Summary of the Literature" Animals 11, no. 6: 1777. https://doi.org/10.3390/ani11061777
APA StyleGozalo-Marcilla, M., & Ringer, S. K. (2021). Recovery after General Anaesthesia in Adult Horses: A Structured Summary of the Literature. Animals, 11(6), 1777. https://doi.org/10.3390/ani11061777






