Influence of Stress Assessed through Infrared Thermography and Environmental Parameters on the Performance of Fattening Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Collecting the Temperature Data
2.3. Environmental and Infrared Temperatures
2.4. Collecting the Productive Data and Calculating Fattening Performance
- DFI = Feed weight differential (g) between two data collection dates/Days elapsed between those two data collection dates.
- ADG = Live weight differential (g) between two data collection dates/Days elapsed between those two data collection dates.
- FCR = feed intake (g)/weight gain (g).
2.5. Temperature-Humidity Index
2.6. Statistical Analyses
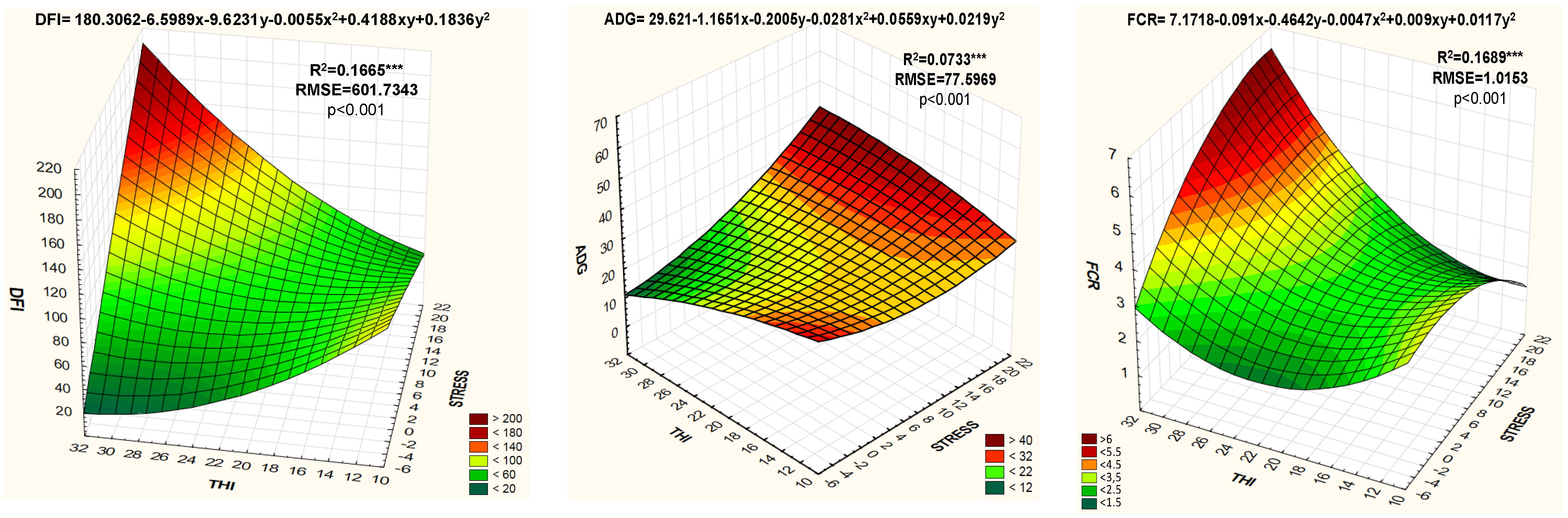
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, P. Behavioral, stress and variability. Behav. Genet. 1988, 18, 293–308. [Google Scholar] [CrossRef]
- Morton, D. Behaviour of rabbits and rodent. In The Ethology of Domestic Animal; Jensen, P., Ed.; CABI: Wallingford, UK, 2002; pp. 193–209. [Google Scholar]
- Welfare, E.P. on A.H. and Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to “The Impact of the current housing and husbandry systems on the health and welfare of farmed domestic rabbits”. EFSA J. 2005, 3, 267–271.
- Stodart, E.; Myers, K. A comparison of behaviour, reproduction and mortality of wild and domestic rabbits in confined populations. CSIRO Wildl. Res. 1964, 9, 144–159. [Google Scholar] [CrossRef]
- Price, E. Behavioral aspects of animal domestication. Q. Rev. Biol. 1984, 59, 1–32. [Google Scholar] [CrossRef]
- Csatádi, K.; Kustos, K.; Eiben, C.; Bilkó, Á.; Altbäcker, V. Even minimal human contact linked to nursing reduces fear responses toward humans in rabbits. Appl. Anim. Behav. Sci. 2005, 95, 123–128. [Google Scholar] [CrossRef]
- Andrade, O.; Orihuela, A.; Solano, J.; Galina, C. Some effects of repeated handling and the use of a mask on stress responses in zebu cattle during restraint. Appl. Anim. Behav. Sci. 2001, 71, 175–181. [Google Scholar] [CrossRef]
- Zucca, D.; Redaelli, V.; Marelli, S.P.; Bonazza, V.; Heinzl, E.; Verga, M.; Luzi, F. Effect of handling in pre-weaning rabbits. World Rabbit Sci. 2012, 20, 97–101. [Google Scholar] [CrossRef][Green Version]
- Schoen, A.; Wynn, S. Complementary and Alternative Veterinary Medicine; Editorial Mosby: Maryland Heights, MO, USA, 1998. [Google Scholar]
- Ogunjimi, L.; Ogunwande, G.; Osunade, J. Influence of building environment on rabbit weight gain, feed efficiency, rectal temperature and respiration rate in the humid tropical climate of Southwestern Nigeria. Agric. Eng. Int. CIGR J. 2008, X, 1–14. [Google Scholar]
- Finzi, A.; Daader, A.; Yamani, K.; Askar, A. Influence of high chronic relative humidity on semen quality of hot stressed bucks. In Proceedings of the 7th World Rabbit Congress of the World Rabbit Science Association (WRSA), Valencia, Spain, 4–7 July 2000; pp. 117–123. [Google Scholar]
- Marai, I.F.M.; Hareeb, A.A.M.; Gad, A. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: A review. Livest. Prod. Sci. 2002, 78, 71–90. [Google Scholar] [CrossRef]
- Fayez, I.; Marai, M.; Alnaimy, A.; Habeeb, M. Thermoregulation in rabbits. In Rabbit production in hot climates. Zaragoza CIHEAM Cah. Opitions Méditerranéennes 1994, 8, 33–41. [Google Scholar]
- Zeferino, C.; Moura, A.; Fernandes, S.; Kanayama, J.; Scapinello, C.; Sarton, J. Genetic group x ambient temperature interaction effects on physiological response and growth performance of rabbits. Livest. Sci. 2011, 140, 177–183. [Google Scholar] [CrossRef]
- Bianca, W. The significance of meteorology in animal production. Int. J. Biometeorol. 1976, 20, 139–156. [Google Scholar] [CrossRef]
- Pla, M.; Fernandez-Carmona, J.; Blas, E.; Cervera, C. Growth and some carcass traits of adult rabbits under high ambient temperature. World Rabbit Sci. 1994, 2, 147–151. [Google Scholar] [CrossRef]
- Asemota, O.; Aduba, P.; Bello-Onaghise, G.; Orheruata, A. Effect of temperature humidity index (THI) on the performance of rabbits (Oryctolagus cuniculus) in the humid tropics. Arch. Zootec. 2017, 66, 257–261. [Google Scholar]
- Wingfield, J.; Hunt, K.; Breuner, C.; Dunlap, K.; Fowler, G.; Freed, L.; Lepson, J. Environmental stress, field endocrinology, and conservation biology. In Behavioral Approaches to Conservation in the Wild; Clemmons, J.R., Buchholz, R., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 95–131. [Google Scholar]
- Cunningham, J. Fisiología veterinaria, 2nd ed.; McGraw Hill Interamericana: Mexico D.F., Mexico, 1999. [Google Scholar]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Sciencia 1984, 244, 452–459. [Google Scholar] [CrossRef]
- Burrow, H.; Corbet, N. Genetic and environmental factors affecting temperament of zebu and zebu-derived beef cattle grazed at pasture in the tropics. Aust. J. Agric. Res. 2000, 51, 155–162. [Google Scholar] [CrossRef]
- Richardson, E.; Herd, R. Biological basis for variation in residual feed intake in beef cattle. Part 2. Synthesis of results following divergent selection. Aust. J. Exp. Agric. 2004, 44, 431–440. [Google Scholar] [CrossRef]
- Monclús, R.; Rödel, H.G.; Palme, R.; von Holst, D.; De Miguel, J. Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 2006, 16, 25–29. [Google Scholar] [CrossRef]
- Cabezas, S.; Blas, J.; Marchant, T.; Moreno, S. Physiological stress levels predict survival probabilities in wild rabbits. Horm. Behav. 2007, 51, 313–320. [Google Scholar] [CrossRef]
- Broom, D. Animal welfare: Concepts, study methods and indicators. Rev. Colomb. Cienc. Pec. 2011, 24, 306–321. [Google Scholar]
- Kowalska, D.; Gugoleka, A.; Bielansky, P. Effect of stress on rabbit meat quality. Ann. Anim. Sci. 2011, 11, 465–475. [Google Scholar]
- Cafe, L.; Robinson, D.; Ferguson, D.; McIntyre, B.; Geesink, G.; Greenwood, P. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Pascua, L.; Gómez, E.; Blasco, A. Economic weights in rabbit meat production. World Rabbit Sci. 2014, 22, 165–177. [Google Scholar] [CrossRef]
- Armero, Q.; Blasco, A. Economic weights for rabbit selection indices. J. Appl. Rabbit Res. 1992, 15, 637–642. [Google Scholar]
- Gidenne, T.; Garreau, H.; Drouilhet, L.; Aubert, C.; Maertens, L. Improving feed efficiency in rabbit production, a review on nutritional, technico-economical, genetic and environmental aspects. Anim. Feed Sci. Technol. 2017, 225, 109–122. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Von der Ohe, C.; Servheen, C. Measuring stress in mammal using fecal glucocorticoids: Opportunities and challenges. Wildl. Soc. Bull. 2002, 30, 1215–1225. [Google Scholar]
- Jaén-Téllez, J.; Sánchez-Guerrero, M.; López-Campos, J.; Valera, M.; González-Redondo, P. Acute stress assessment using infrared thermography in fattening rabbits reacting to handling under winter and summer conditions. Spanish J. Agric. Res. 2020, 18, e0592. [Google Scholar] [CrossRef]
- Unruh, E.; Theurer, M.; White, B.; Larson, R.; Drouillard, J.; Scharg, N. Evaluation of infrared thermography as a diagnostic tool to predict heat stress events in feedlot cattle. Am. J. Vet. Res. 2017, 78, 771–777. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar]
- Nakayama, K.; Goto, S.; Kuraoka, K.; Nakamura, K. Decrease in nasal temperature of rhesus monkeys (Macaca mulatta) in negative emotional state. Physiol. Behav. 2005, 84, 783–790. [Google Scholar] [CrossRef]
- McCafferty, D. Applications of thermal imaging in avian science. Int. J. Avian Sci. Ibis 2013, 155, 4–15. [Google Scholar] [CrossRef]
- Weschenfelder, A.; Saucier, L.; Madague, X.; Rocha, L.; Schaefer, A.; Faucitano, L. Use of infrared ocular thermography to asses physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Bartolomé, E.; Valera, M. Genetic study of stress assessed with infrared thermography during dressage competitions in the Pura Raza Español horse. Appl. Anim. Behav. Sci. 2016, 174, 58–65. [Google Scholar] [CrossRef]
- Ludwig, N.; Gargano, M.; Luzi, F.; Carenzi, C.; Verga, M. Technical note: Applicability of infrared thermography as a non invasive measurements of stress in rabbit. World Rabbit Sci. 2007, 15, 199–206. [Google Scholar] [CrossRef]
- Emam, A.; Afonso, S.; González-Redondo, P.; Mehaisen, G.; Azoz, A.; Ahmed, N. Status and origin of Egyptian local rabbits in comparison with Spanish common rabbits using mitochondrial DNA sequence analysis. World Rabbit Sci. 2020, 28, 93–102. [Google Scholar] [CrossRef]
- González-Redondo, P. Resultados preliminares de rendimiento reproductivo y de engorde de un núcleo de cría de conejos de tipo Común Doméstico Español. In Proceedings of the XLI Symposium de Cunicultura, Hondarribia, Spain, 12–13 May 2016; pp. 180–185. [Google Scholar]
- Ministerio de Agricultura Pesca y Alimentación Resolución de la Dirección General de Producciones y Mercados Agrarios, por la que se Aprueban las Reglamentaciones Específicas de los Libros Genealógicos y los Programas de Mejora de las Razas Cunícolas Gigante de España y Antiguo Pardo Español, de Conformidad con lo Dispuesto en el Real Decreto 2129/2008, de 26 de Diciembre 2018. Available online: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia/resolucionreglamentacionesrazasgigantedeespanayantiguopardoespanolfirmada_tcm30-479539.pdf (accessed on 8 June 2021).
- Ministerio de la Presidencia Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. Boletín Oficial del Estado 2013, 34, 11370–11421.
- European Parliament and Council Directive 20102010/63/EU of 22 September 2010 on the Protection of Animals used for Scientific Purposes. Official J. European Union. 2010, L276, 33–79.
- Chapel, J.; Benedito, J.; Hernández, J.; Pereira, V.; Domínguez, R.; Castillo, C. Técnicas de manejo y sujeción del conejo doméstico. Consult. Difusión Vet. 2015, 221, 47–54. [Google Scholar]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.M.; Ayyatand, M.S.; Abd-El-Monem, U. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under egyptian conditions. Trop. Animal Health Prod. 2001, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS/Stat User´s Guide; SAS: Cary, NC, USA, 2010. [Google Scholar]
- Littell, R.; Henry, P.; Ammerman, C. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Feki, S.; Baselga, M.; Blas, E.; Cervera, C.; Gómez, E. Comparison of growth and feed efficiency among rabbit lines selected for different objectives. Livest. Prod. Sci. 1996, 45, 87–92. [Google Scholar] [CrossRef]
- Rodellar, C.; Zaragoza, P.; Osta, R. Estimación de distintos parámetros productivos en la raza de conejos Común Español. In Proceedings of the XIV Symposium de Cunicultura, Manresa, Spain, 12–14 June 1989; pp. 37–150. [Google Scholar]
- Chiericato, G.; Rizzi, C.; Rostellato, V. Effect of genotype and environmental conditions in the productive and slaughtering performance of growing meat rabbits 1. World Rabbit Sci. 1993, 1, 119–125. [Google Scholar]
- Roberts, J.; Lukefarhr, S. Evaluation of Californian, Champagne d´Argent, New Zealand White and Palomino as potential sire breeds: I. Postweaning litter performances. J. Appl. Rabbit Res. 1992, 15, 274. [Google Scholar]
- Remois, G.; Lafargue-Hauret, P.; Bourdillon, A.; Rouillere, H. Effect of weaning weight on growth performance of rabbits. In Proceedings of the 6th World Rabbit Congress of the World Rabbit Science Association (WRSA), Toulouse, France, 9–12 July 1996; pp. 237–240. [Google Scholar]
- Prayaga, K.; Eady, S. Rabbit farming for meat production in Australia: Preliminary estimates of economic values for production traits. Asian Australas. J. Anim. Sci. 2000, 13, 57–359. [Google Scholar]
- Orengo, J.; Piles, M.; Rafel, O.; Ramón, E. Gómez Crossbreeding parameters for growth and feed consumption traits from a five diallel mating scheme in rabbits. J. Anim. Sci. 2009, 87, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Garcia-Palomares, J.; Garcia-Rebollar, P.; Chamorro, S.; Carabaño, R.; De Blas, J. Effect of protein source and enzyme supplementation on ileal protein digestibility and fattening performance in rabbits. Spanish J. Agric. Res. 2006, 4, 297–303. [Google Scholar] [CrossRef]
- Gidenne, T.; De Dapper, J.; Lapanouse, A.; Aymard, P. Adaptation du lapereau á un aliment fibreuxdistribuéavantsevrage: Comportementd’ingestion, croissance et santé digestive. In Proceedings of the 2èmes Journées Recherche Cunicole, Toulouse, France, 4–5 April 1978; pp. 1–112. [Google Scholar]
- Formoso-Rafferty, N.; García-García, R.; Rodríguez, M.; Alonso, A.; Masdeu, M.; Millán, P.; Arias-Álvarez, M.; Lorenzo, L.; Rebollar, P. Reproductive and endocrine characterization of Ibicean rabbit breed. Arch. Zootec. 2016, 65, 525–534. [Google Scholar]
- Ponce de León, R.; Guzmán, G.; Pubillones, O.; García, J.; Mora, M. Comportamiento de razas de conejos importadas. Evaluación del crecimiento posdestete. Rev. Cuba. Cienc. Agrícola 2002, 36, 23–329. [Google Scholar]
- González-Redondo, P. Estado de las poblaciones y posibilidades de recuperación del conejo doméstico común español. In Proceedings of the IV Jornadas Ibéricas de Razas Autóctonas y sus Productos Tradicionales: Innovación, Seguridad y Cultura Alimentaria, Sevilla, Spain, 30 November–1 December 2007; pp. 367–371. [Google Scholar]
- Gupta, R.; Prabhakar Rao, V.; Eswara Reddy, C.; Satyanarayana, A.; Reddy, P. Feed intake and feed conversion ratio in purebred and crossbred broiler rabbits. Indian J. Anim. Res. 2000, 34, 64–67. [Google Scholar]
- Medellin, M.; Lukefahr, S. Breed and heterotic effects on postweaning traits in Altex and New Zealand White straightbred and crossbred rabbits. J. Anim. Sci. 2011, 79, 1173–1178. [Google Scholar] [CrossRef]
- Lebas, F.; Coudert, P.; de Rochambeau, H.; Thébault, R.; Rouvier, R.; de Rochambeau, H. The Rabbit—Husbandry, Health and Production; FAO: Rome, Italy, 1997. [Google Scholar]
- Sabah Abd Al-Rahman, A.; Dalal Abd Al-Sattar, A. Effect of the thermal changes on physiological, biochemical and histological traits in pregnant and embryo of New Zealand white rabbits. Int. J. Adv. Biol. Res. 2016, 6, 313–327. [Google Scholar]
- Ramon, J.; Gomez, E.; Perucho, O.; Rafel, O.; Baselga, M. Feed efficiency and postweaning growth of several Spanish selected lines. In Proceedings of the 6th World Rabbit Congress of the World Rabbit Science Association (WRSA), Tolouse, France, 9–12 July 1996; pp. 351–353. [Google Scholar]
- Ondruska, L.; Rafay, J.; Okab, A.; Ayoub, M.; Al-Haidary, A.; Samara, E.; Parkanyi, V.; Chrastinova, L.; Jurcik, R.; Massanyi, P.; et al. Influence of elevated ambient temperature upon some physiological measurements of New Zealand White rabbits. Vet. Med. 2011, 56, 180–186. [Google Scholar] [CrossRef]
- Okab, A.; El-Banna, S.; Koriem, A. Influence of environmental temperatures on some physiological and biochemical parameters of male New-Zealand rabbits. Slovak J. Anim. Sci. 2008, 41, 12–19. [Google Scholar]
- Chiericato, G.; Bailoni, L.; Rizzi, C. The effect of environmental temperature on the performance of growing rabbits. J. Appl. Rabbit Res. 1992, 15, 723–731. [Google Scholar]
- Marai, I.; Habeeb, A.; Gad, A. Biological functions in young pregnant rabbit does as affected by heat stress and lighting regime under subtropical conditions of Egypt. Trop. Subtrop. Agroecosyst. 2007, 7, 165–176. [Google Scholar]
- Kasa, I.; Thwaites, C. The effect of infrared radiation on rectal temperature and respiration rate of unacclimated female New Zealand white rabbits. J. Therm. Biol. 1992, 17, 293–296. [Google Scholar] [CrossRef]
- Odeon, M.M.; Romera, S.A. Estrés en ganado: Causas y consecuencias. Rev. Vet. 2017, 28, 69–77. [Google Scholar] [CrossRef]
- Hahn, G.; Gauchan, J.; Mader, T.; Eigenberg, R. Chapter 5: Thermal indices and their applications for livestock environments. In Livestock Energetics and Thermal Environmental Management; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009; pp. 113–130. [Google Scholar]
- Paci, G.; Preziuso, G.; D’Agata, M.; Russo, C.; Dalle-Zote, A. Effect of stocking density and group size on growth performance, carcass traits and meat quality of outdoor-reared rabbits. Meat Sci. 2013, 93, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, C.; Biagini, D.; Lussiana, C. Different rearing systems for fattening rabbits: Performance and carcass characteristics. Meat Sci. 2009, 82, 200–204. [Google Scholar] [CrossRef] [PubMed]
- McMillan, F. Stress-induced and emotional eating in animals: A review of the experimental evidence and implications for companion animal obesity. J. Vet. Behav. 2013, 8, 376–385. [Google Scholar] [CrossRef]
- Silveira, P.; Xavier, M.; Souza, F.; Manoli, L.; Rosat, R.; Ferreira, M.; Dalmaz, C. Interaction between repeated restraint stress and concomitant midazolam administration on sweet food ingestion in rats. Brazilian J. Med. Biol. Res. 2000, 33, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- López-Espinoza, A.; Moreno, A.M.; Franco, K.; Aguilera, V.; Cardenas-Villalvazo, A.; Valdes-Miramontes, E.; Magaña, C.; Macías, A.; Santoyo, F.; Rezéndis, F.D. Estrés & Comportamiento Alimentario. Modelo Bioconductual de Estrés-Alimentación; Cuevas, S., Ed.; Manual moderno: Mexico D.F., Mexico, 2012; pp. 59–70. [Google Scholar]
- Ortolani, D.; Oyama, L.; Ferrari, E.; Melo, L.; Spadari-Bratfisch, R. Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiol. Behav. 2011, 103, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, J.; Crews, D.; Basarab, J.; Price, M.; Okine, E.; Wang, Z.; Li, C.; Moore, S. Genetic and phenotypic relationships of feeding behavior and temperament with performance, feed efficiency, ultrasound, and carcass merit of beef cattle. J. Anim. Sci. 2007, 85, 2382–2390. [Google Scholar] [CrossRef]
- Llonch, P.; Somarriba, M.; Duthie, C.; Haskell, M.; Rooke, J.; Troy, S.; Roehe, S.; Turner, S. Association of temperament and acute stress responsiveness with productivity, feed efficiency, and methane emissions in beef cattle: An observational study. Front. Vet. Sci. 2016, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Halford, J. Pharmacology of appetite suppression: Implication for the treatment of obesity. Curr. Drug Targets 2001, 2, 353–370. [Google Scholar] [CrossRef]
- Takeda, E.; Terao, J.; Nakaya, Y.; Miyamoto, K.; Baba, Y.; Chuman, H.; Ryuji, K.; Ohmori, T.; Rokutan, K. Stress control and human nutrition. J. Med. Investig. 2004, 51, 139–145. [Google Scholar] [CrossRef]
- Gómez, B.; Escobar, A. Estrés y sistema inmune. Rev. Mex. Neurocienc. 2006, 7, 30–38. [Google Scholar]
- Ans, A.; Anjum, I.; Satija, V.; Inayat, A.; Asghar, Z.; Akram, I.; Shrestha, B. Neurohormonal regulation of appetite and its relationship with stress: A mini literature review. Cureus 2018, 10, e3032. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Nowson, C. Relationship between stress, eating behavior and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Rostamkhani, F.; Zardooz, H.; Zahediasl, S.; Farrokhi, B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J. Zhejiang Univ. B 2012, 13, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.; Rashwan, A. Rabbit behavioural response to climatic and managerial conditions—A review. Arch. Tierzucht 2004, 7, 469–482. [Google Scholar] [CrossRef]
- Rushen, J.; Taylor, A.; de Passilé, A. Domestic animals’ fear of humans and its effect on their welfare. Appl. Anim. Behav. Sci. 1999, 65, 285–303. [Google Scholar] [CrossRef]
- Oseni, S.; Popoola, M. Doe fertility and weaning survival rate of composite rabbits as affected by thermal environment in the humid tropical climate of Southwestern Nigeria. Int. J. Agric. Biosci. 2013, 2, 113–115. [Google Scholar]
| Variables | N | Mean ± SE | Minimum | Maximum | C.V. (%) | |
|---|---|---|---|---|---|---|
| Both seasons | TBW (g) | 39 | 1383.38 ± 40.42 | 577.30 | 1746.00 | 18.25 |
| TWG (g) | 39 | 940.18 ± 30.91 | 350.50 | 1276.10 | 30.90 | |
| TFI (g) | 39 | 2956.57 ± 94.85 | 1390.70 | 3771.80 | 19.72 | |
| ADG (g/d) | 39 | 25.80 ± 0.46 | 1.70 | 58.70 | 35.24 | |
| DFI (g/d) | 39 | 77.80 ± 1.34 | 9.40 | 163.70 | 34.32 | |
| FCR | 39 | 3.18 ± 0.06 | 0.55 | 6.66 | 34.55 | |
| THI | 39 | 19.41 ± 0.26 | 11.34 | 30.29 | 26.42 | |
| Warm season | TBW (g) | 22 | 1335.49 ± 52.66 | 577.30 | 1721.10 | 18.50 |
| TWG (g) | 22 | 924.94 ± 35.60 | 352.80 | 1106.10 | 18.05 | |
| TFI (g) | 22 | 2901.87 ± 119.90 | 1390.70 | 3754.10 | 19.38 | |
| ADG (g/d) | 22 | 24.79 ± 0.52 | 2.83 | 58.70 | 32.66 | |
| DFI (g/d) | 22 | 76.21 ± 1.42 | 9.40 | 121.70 | 29.03 | |
| FCR | 22 | 3.24 ± 0.07 | 0.55 | 6.50 | 33.95 | |
| THI | 22 | 22.95 ± 0.20 | 17.19 | 30.29 | 13.60 | |
| Cold season | TBW (g) | 17 | 1445.39 ± 61.36 | 809.30 | 1746.00 | 17.50 |
| TWG (g) | 17 | 961.72 ± 17.79 | 350.50 | 1276.10 | 23.50 | |
| TFI (g) | 17 | 3027.35 ± 50.31 | 1499.00 | 3771.80 | 21.12 | |
| ADG (g/d) | 17 | 27.39 ± 0.83 | 1.70 | 49.57 | 37.61 | |
| DFI (g/d) | 17 | 80.30 ± 2.63 | 13.13 | 163.70 | 40.56 | |
| FCR | 17 | 3.08 ± 0.09 | 1.19 | 6.66 | 35.45 | |
| THI | 17 | 13.81 ± 0.09 | 11.34 | 15.35 | 7.71 |
| Factors | Degrees of Freedom | DFI | ADG | FCR | |||
|---|---|---|---|---|---|---|---|
| F-Test | p-Value | F-Test | p-Value | F-Test | p-Value | ||
| Sex | 1 | 0.22 | 0.639 | 0.02 | 0.897 | 0.00 | 0.952 |
| Stress level | 3 | 16.38 | <0.001 | 4.69 | 0.003 | 4.05 | 0.008 |
| THI | 1 | 7.67 | 0.039 | 12.05 | 0.018 | 5.81 | 0.061 |
| Season | 1 | 7.25 | 0.007 | 0.17 | 0.676 | 15.89 | <0.001 |
| Sex*Stress level | 3 | 0.72 | 0.538 | 2.12 | 0.097 | 0.68 | 0.565 |
| Sex*Season | 1 | 0.17 | 0.678 | 0.76 | 0.385 | 3.29 | 0.071 |
| Season*Stress level | 2 | 0.33 | 0.721 | 0.49 | 0.615 | 0.00 | 0.952 |
| Season | ||||||
| Warm season | Cold season | |||||
| DFI (g/d) | 76.81 a | 80.37 b | ||||
| ADG (g/d) | 24.96 a | 27.39 a | ||||
| FCR | 3.29 b | 3.22 a | ||||
| Week of Fattening Period | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| DFI (g/d) | 52.00 a | 60.93 a | 78.68 b | 90.98 b,c | 94.09 c | 103.75 b,c |
| ADG (g/d) | 25.88 a,b | 23.89 a | 27.75 a,b | 26.96 a,b | 23.65 a | 30.77 b |
| FCR | 2.34 a | 2.88 a,b | 3.12 b,c | 3.54 c,d | 4.18 d | 3.48 b,c,d |
| Stress Level | ||||||
| 1 | 2 | 3 | 4 | |||
| DFI (g/d) | 44.71 a | 75.21 b | 81.06 b | 90.41 c | ||
| ADG (g/d) | 22.45 a | 26.22 a,b | 24.25 a | 29.14 b | ||
| FCR | 2.53 a | 3.16 a | 3.59 b | 3.24 a,b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaén-Téllez, J.A.; Sánchez-Guerrero, M.J.; Valera, M.; González-Redondo, P. Influence of Stress Assessed through Infrared Thermography and Environmental Parameters on the Performance of Fattening Rabbits. Animals 2021, 11, 1747. https://doi.org/10.3390/ani11061747
Jaén-Téllez JA, Sánchez-Guerrero MJ, Valera M, González-Redondo P. Influence of Stress Assessed through Infrared Thermography and Environmental Parameters on the Performance of Fattening Rabbits. Animals. 2021; 11(6):1747. https://doi.org/10.3390/ani11061747
Chicago/Turabian StyleJaén-Téllez, Juan Antonio, María José Sánchez-Guerrero, Mercedes Valera, and Pedro González-Redondo. 2021. "Influence of Stress Assessed through Infrared Thermography and Environmental Parameters on the Performance of Fattening Rabbits" Animals 11, no. 6: 1747. https://doi.org/10.3390/ani11061747
APA StyleJaén-Téllez, J. A., Sánchez-Guerrero, M. J., Valera, M., & González-Redondo, P. (2021). Influence of Stress Assessed through Infrared Thermography and Environmental Parameters on the Performance of Fattening Rabbits. Animals, 11(6), 1747. https://doi.org/10.3390/ani11061747







