Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Determination of Plasma Glucose, Triacylglycerol, NEFA, Insulin, and IL-1β Concentrations
2.3. Isolation of Peripheral Leukocytes
2.4. Cell Cultures
2.5. Respiratory Burst and Phagocytosis Analysis
2.6. Cell Chemotaxis Analysis
2.7. Bacterial Killing Analysis
2.8. Cell Viability and Livability Analysis
2.9. Western Blot Analysis
2.10. Statistics
3. Results
3.1. Body Weight, Feed Intake, Plasma Glucose, Triglyceride, NEFA, Insulin, and IL-1β Levels
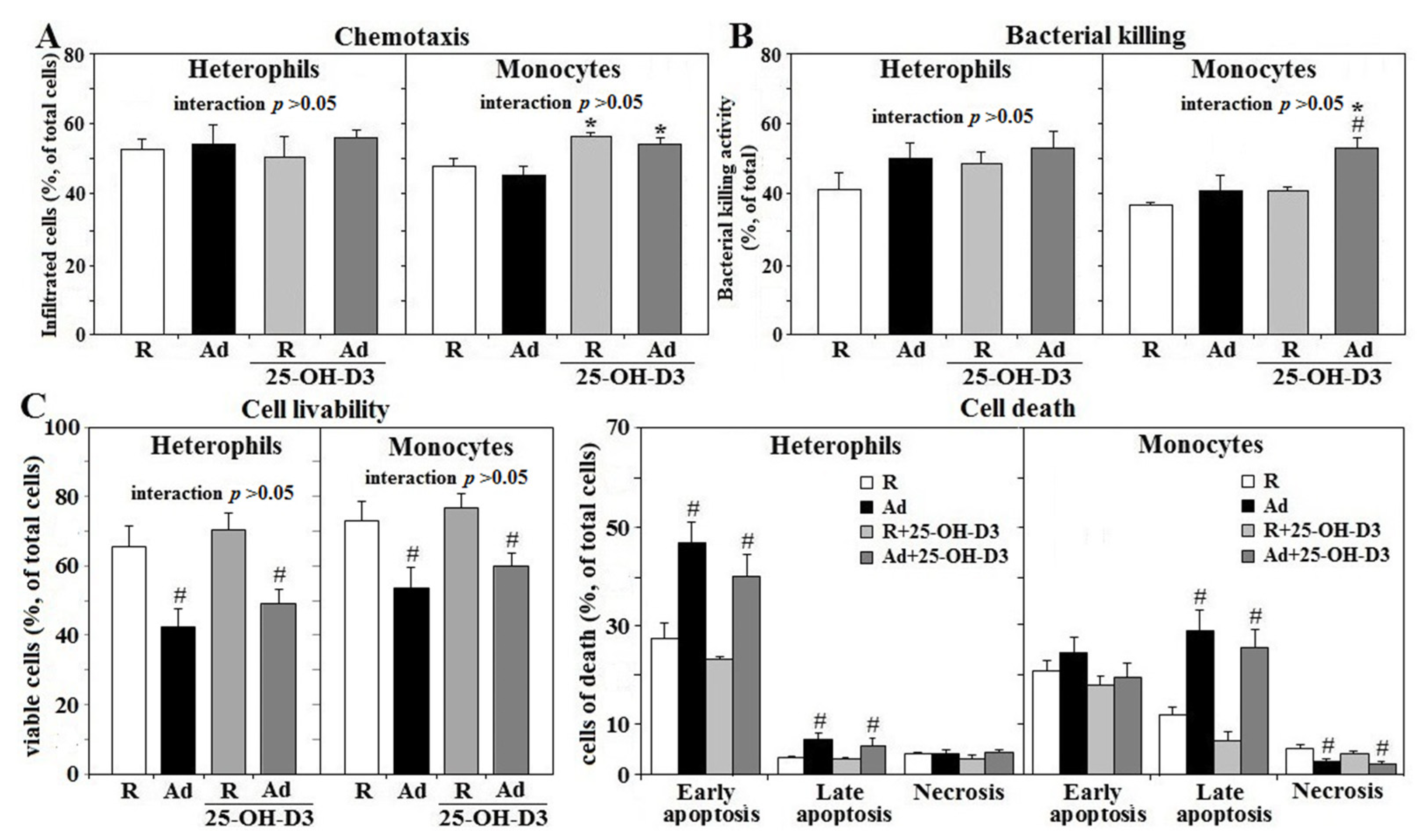
3.2. IL-1β Secretion, Phagocytosis, and Respiratory Burst of Fresh Leukocytes
3.3. Chemotaxis and Bacterial Killing of Fresh Leukocytes
3.4. VDR Protein Amounts and NFκB Activation of Fresh Leukocytes
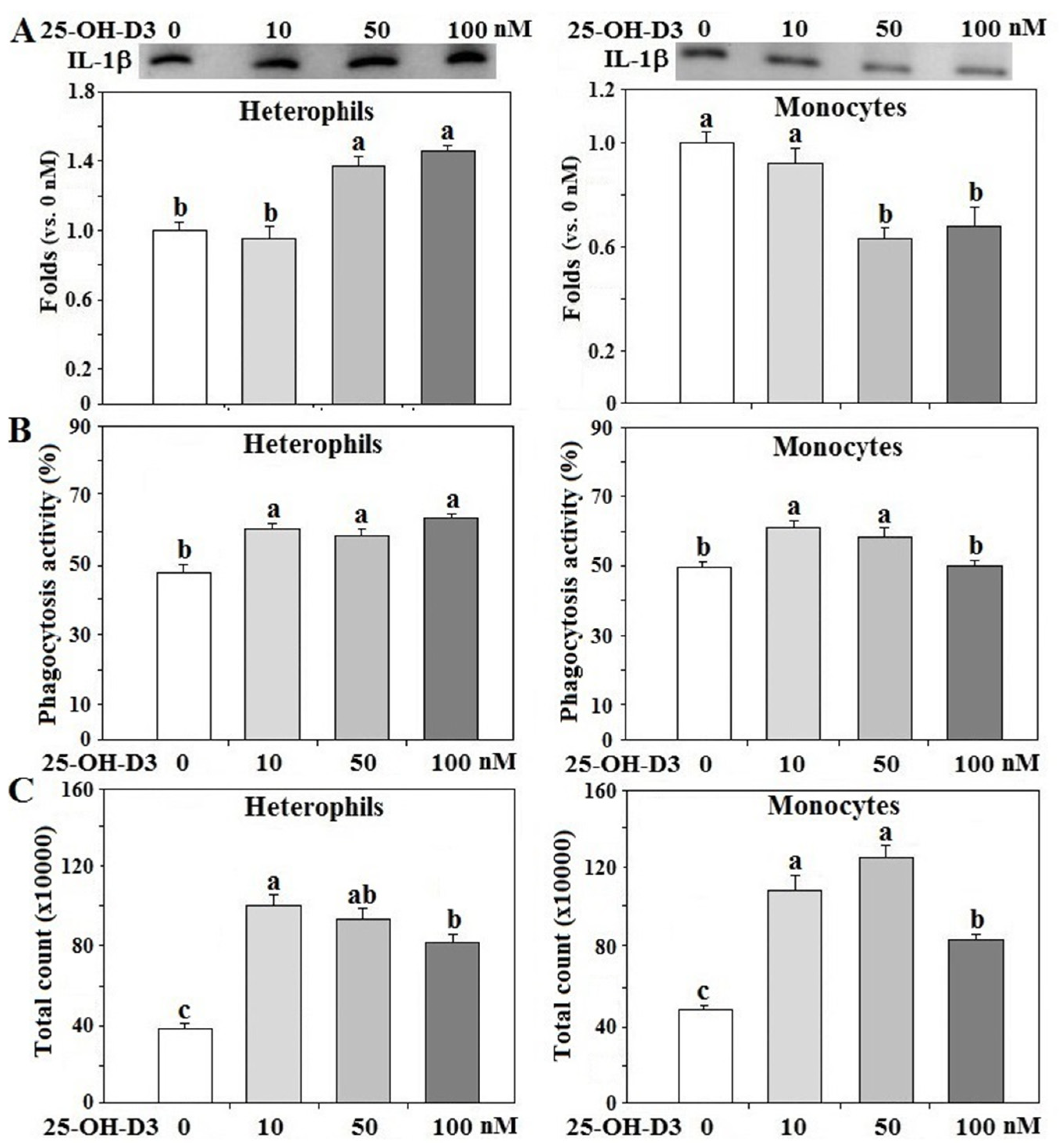
3.5. Effects of 25-OH-D3 on IL-1β Secretion of Leukocytes
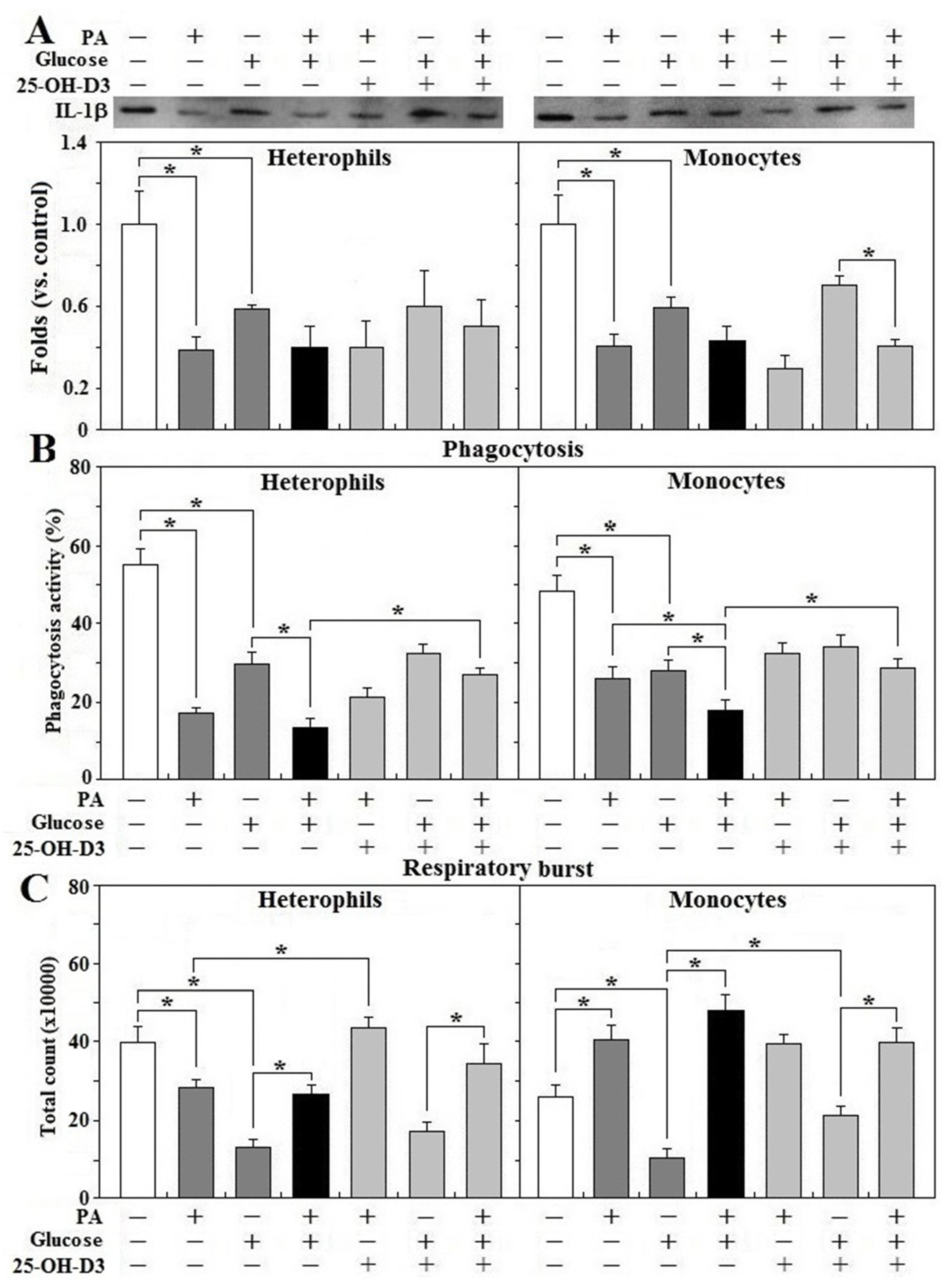
3.6. Effects of Glucose and Fatty Acid on Leukocyte Functions
3.7. Effects of 25-OH-D3 on Leukocyte Functions during Glucolipotoxicity
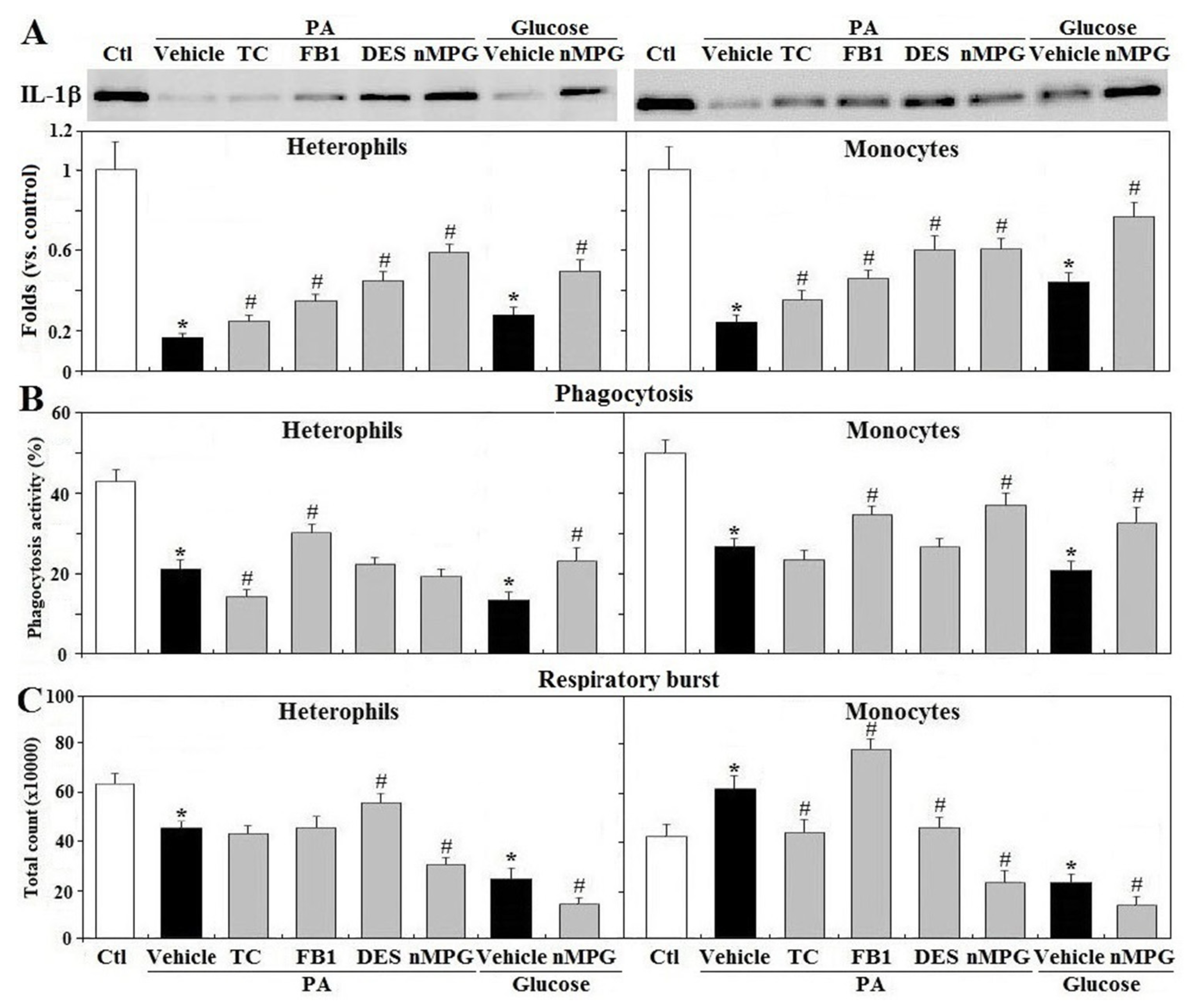
3.8. Mechanisms of Gluco/Lipotoxicity on Leukocyte Functions
3.9. Effects of 25-OH-D3 on Leukocyte Viability Following Glucose or Palmitic Acid Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, H.D.; Goddard, C. Rapidly growing broiler (meat-type) chickens: Their origin and use for comparative studies of the regulation of growth. Int. J. Biochem. 1994, 26, 19–28. [Google Scholar] [CrossRef]
- Yu, M.W.; Robinson, F.E.; Etches, R.J. Effect of feed allowance during rearing and breeding on female broiler breeders. 3. Ovarian steroidogenesis. Poult. Sci. 1992, 71, 1762–1767. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, H.Y.; Chen, Y.W.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Pathological adaption of cardiac hypertrophy. Poult. Sci. 2017, 96, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Development of metabolic cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Nadeau, K.; Wang, P.W.; Lee, J.Y.; Guttman, D.S.; Sharif, S.; Korver, D.R.; Brumell, J.H.; Parkinson, J. Accumulation of genetic variants associated with immunity in the selective breeding of broilers. BMC Genet. 2020, 21, 5. [Google Scholar] [CrossRef]
- Willson, N.L.; Forder, R.E.A.; Tearle, R.G.; Nattrass, G.S.; Hughes, R.J.; Hynd, P.I. Evaluation of fatty acid metabolism and innate immunity interactions between commercial broiler, F1 layer × broiler cross and commercial layer strains selected for different growth potentials. J. Anim. Sci. Biotechnol. 2017, 8, 70. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Pevzner, I.Y.; Kaiser, P.; Kogut, M.H. Profiling pro-inflammatory cytokine and chemokine mRNA expression levels as a novel method for selection of increased innate immune responsiveness. Vet. Immunol. Immunopathol. 2008, 126, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.A.; Qureshi, M.A.; Havenstein, G.B. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1519–1529. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of adaptive and innate immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lam, K.S. Obesity-induced insulin resistance and macrophage infiltration of the adipose tissue: A vicious cycle. J. Diabetes Investig. 2019, 10, 29–31. [Google Scholar] [CrossRef]
- Richardson, V.R.; Smith, K.A.; Carter, A.M. Adipose tissue inflammation: Feeding the development of type 2 diabetes mellitus. Immunobiology 2013, 218, 1497–1504. [Google Scholar] [CrossRef]
- Liu, Z.C.; Xie, Y.L.; Chang, C.J.; Su, C.M.; Chen, Y.H.; Huang, S.Y.; Walzem, R.L.; Chen, S.E. Feed intake alters immune cell functions and ovarian infiltration in broiler hens: Implications for reproductive performance. Biol. Reprod. 2014, 90, 1–8. [Google Scholar] [CrossRef]
- Xie, Y.L.; Pan, Y.E.; Chang, C.J.; Tang, P.C.; Huang, Y.F.; Walzem, R.L.; Chen, S.E. Palmitic acid in chicken granulosa cell death-lipotoxic mechanisms mediate reproductive inefficacy of broiler breeder hens. Theriogenology 2012, 78, 1917–1928. [Google Scholar] [CrossRef]
- Pan, Y.E.; Liu, Z.C.; Chang, C.J.; Xie, Y.L.; Chen, C.Y.; Chen, C.F.; Walzem, R.L.; Chen, S.E. Ceramide accumulation and up-regulation of proinflammatory interleukin-1β exemplify lipotoxicity to mediate declines of reproductive efficacy of broiler hens. Domest. Anim. Endocrinol. 2012, 42, 183–194. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chang, L.C.; Chen, C.Y.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Unrestricted Feed Intake Induces β-Cell Death and Impairs Insulin Secretion in Broiler Breeder Hens. Animals 2020, 10, 1969. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Eapen, M.S.; Zosky, G.R. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci. Rep. 2017, 7, 45172. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.T. The Osler slide, a demonstration of phagocytosis from 1876 Reports of phagocytosis before Metchnikoff’s 1880 paper. Cell. Immunol. 2006, 240, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vanlint, S. Vitamin D and obesity. Nutrients 2013, 5, 949–956. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; Marz, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010, 321, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Geng, Y.; Ma, Q.; Wang, Z.; Guo, Y. Dietary vitamin D3 supplementation protects laying hens against lipopolysaccharide-induced immunological stress. Nutr. Metab. 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.C.; Chen, Y.H.; Chung, T.K.; Walzem, R.L.; Lai, L.S.; Chen, S.E. Supplemental 25-hydroxycholecalciferol Alleviates Inflammation and Cardiac Fibrosis in Hens. Int. J. Mol. Sci. 2020, 21, 8379. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chung, T.K.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol improves livability in broiler breeder hens. Poult. Sci. 2019, 98, 6108–6116. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chou, P.C.; Chen, Y.H.; Lai, L.S.; Chung, T.K.; Walzem, R.L.; Huang, S.Y.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol Improves livability in broiler breeder hens-amelioration of cardiac pathogenesis and hepatopathology. Animals 2019, 9, 770. [Google Scholar] [CrossRef]
- Yeh, Y.L.; Chou, P.C.; Chen, Y.H.; Lai, L.S.; Chung, T.K.; Walzem, R.L.; Huang, S.Y.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol improves cardiac function and livability in broiler breeder hens–amelioration of blood pressure and vascular remodeling. Poult. Sci. 2020, 99, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Franssens, L.; Lesuisse, J.; Wang, Y.; De Ketelaere, B.; Willems, E.; Koppenol, A.; Guo, X.; Buyse, J.; Decuypere, E.; Everaert, N. Prenatal tolbutamide treatment alters plasma glucose and insulin concentrations and negatively affects the postnatal performance of chickens. Domest. Anim. Endocrinol. 2015, 52, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sevimli, A.; Misirlioglu, D.; Yagci, A.; Bulbul, A.; Yilmaztepe, A.; Altunbas, K. The role of chicken IL-1β, IL-6 and TNF-α in the occurrence of amyloid arthropathy. Vet. Res. Commun. 2008, 32, 499–508. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; Lowry, V.K. Differential activation of signal transduction pathways mediating phagocytosis, oxidative burst, and degranulation by chicken heterophils in response to stimulation with opsonized Salmonella enteritidis. Inflammation 2001, 25, 7–15. [Google Scholar] [CrossRef]
- Liu, Z.C.; Su, C.M.; Xie, Y.L.; Chang, C.J.; Chen, J.Y.; Wu, S.W.; Chen, Y.H.; Walzem, R.L.; Huang, S.Y.; Chen, S.E. Intracellular lipid dysregulation interferes with leukocyte function in the ovaries of meat-type hens under unrestricted feed intake. Anim. Reprod. Sci. 2016, 167, 40–50. [Google Scholar] [CrossRef]
- Chen, S.E.; McMurtry, J.P.; Walzem, R.L. Overfeeding-Induced ovarian dysfunction in broiler breeder hens Is associated with lipotoxicity. Poult. Sci. 2006, 85, 70–81. [Google Scholar] [CrossRef]
- Osawa, Y.; Uchinami, H.; Bielawski, J.; Schwabe, R.F.; Hannun, Y.A.; Brenner, D.A. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J. Biol. Chem. 2005, 280, 27879–27887. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Structure and lipid binding properties of serum albumin. Meth. Enzymol. 1986, 128, 320–339. [Google Scholar] [CrossRef]
- Hala, K.; Moore, C.; Plachy, J.; Kaspers, B.; Bock, G.; Hofmann, A. Genes of chicken MHC regulate the adherence activity of blood monocytes in Rous sarcomas progressing and regressing lines. Vet. Immunol. Immunopathol. 1998, 66, 143–157. [Google Scholar] [CrossRef]
- Chuammitri, P.; Redmond, S.B.; Kimura, K.; Andreasen, C.B.; Lamont, S.J.; Palic, D. Heterophil functional responses to dietary immunomodulators vary in genetically distinct chicken lines. Vet. Immunol. Immunopathol. 2011, 142, 219–227. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, K.A.; Morris, J.L.; Feterl, M.L.; Govan, B.L.; Ketheesan, N. Altered macrophage function is associated with severe Burkholderia pseudomallei infection in a murine model of type 2 diabetes. Microbes Infect. 2011, 13, 1177–1184. [Google Scholar] [CrossRef]
- Naundrup Thøfner, I.C.; Poulsen, L.L.; Bisgaard, M.; Christensen, H.; Olsen, R.H.; Christensen, J.P. Longitudinal Study on Causes of Mortality in Danish Broiler Breeders. Avian Dis. 2019, 63, 400–410. [Google Scholar] [CrossRef]
- Lenin, R.; Maria, M.S.; Agrawal, M.; Balasubramanyam, J.; Mohan, V.; Balasubramanyam, M. Amelioration of glucolipotoxicity-induced endoplasmic reticulum stress by a “chemical chaperone” in human THP-1 monocytes. Exp. Diabetes Res. 2012, 2012, 356487. [Google Scholar] [CrossRef]
- Restaino, R.M.; Deo, S.H.; Parrish, A.R.; Fadel, P.J.; Padilla, J. Increased monocyte-derived reactive oxygen species in type 2 diabetes: Role of endoplasmic reticulum stress. Exp. Physiol. 2017, 102, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Roszer, T.; Ricote, M. Lipotoxicity in macrophages: Evidence from diseases associated with the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E145–E154. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.D.; Machkovech, H.M.; He, L.; Sidhu, R.; Fujiwara, H.; Weber, K.; Ory, D.S.; Schaffer, J.E. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 2013, 288, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Sitrin, R.G.; Sassanella, T.M.; Petty, H.R. An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils. Am. J. Respir. Cell Mol. Biol. 2011, 44, 205–212. [Google Scholar] [CrossRef]
- Niwa, M.; Kozawa, O.; Matsuno, H.; Kanamori, Y.; Hara, A.; Uematsu, T. Tumor necrosis factor-α-mediated signal transduction in human neutrophils: Involvement of sphingomyelin metabolites in the priming effect of TNF-α on the fMLP-stimulated superoxide production. Life Sci. 2000, 66, 245–256. [Google Scholar] [CrossRef]
- Hinkovska-Galcheva, V.; Boxer, L.; Mansfield, P.J.; Schreiber, A.D.; Shayman, J.A. Enhanced phagocytosis through inhibition of de novo ceramide synthesis. J. Biol. Chem. 2003, 278, 974–982. [Google Scholar] [CrossRef]
- Seumois, G.; Fillet, M.; Gillet, L.; Faccinetto, C.; DPSmet, C.; Francois, C.; Dewals, B.; Oury, C.; Vanderplasschen, A.; Lekeux, P.; et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. Leukoc. Biol. 2007, 81, 1477–1486. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Grassme, H.; Doring, G.; Gulbins, E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J. Immunol. 2010, 184, 5104–5111. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Sly, L.M.; Lopez, M.; Nauseef, W.M.; Reiner, N.E. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001, 276, 35482–35493. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules. 2020, 25, 3219. [Google Scholar] [CrossRef]
- Leventis, P.; Patel, S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology 2008, 47, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J.L. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, P.-C.; Lin, P.-C.; Wu, S.-W.; Wang, C.-K.; Chung, T.-K.; Walzem, R.L.; Lai, L.-S.; Chen, S.-E. Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens. Animals 2021, 11, 1742. https://doi.org/10.3390/ani11061742
Chou P-C, Lin P-C, Wu S-W, Wang C-K, Chung T-K, Walzem RL, Lai L-S, Chen S-E. Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens. Animals. 2021; 11(6):1742. https://doi.org/10.3390/ani11061742
Chicago/Turabian StyleChou, Pao-Chia, Pei-Chi Lin, Shu-Wei Wu, Chien-Kai Wang, Thau-Kiong Chung, Rosemary L. Walzem, Lih-Shiuh Lai, and Shuen-Ei Chen. 2021. "Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens" Animals 11, no. 6: 1742. https://doi.org/10.3390/ani11061742
APA StyleChou, P.-C., Lin, P.-C., Wu, S.-W., Wang, C.-K., Chung, T.-K., Walzem, R. L., Lai, L.-S., & Chen, S.-E. (2021). Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens. Animals, 11(6), 1742. https://doi.org/10.3390/ani11061742





