Survey on the Presence of Bacterial, Fungal and Helminthic Agents in Off-Leash Dog Parks Located in Urban Areas in Central-Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Areas
2.2. Sampling
2.3. Bacteriological Examinations
2.3.1. Salmonella spp.
2.3.2. Campylobacter spp.
2.3.3. Yersinia spp.
2.3.4. Listeria spp.
2.4. Parasitological Examinations
2.5. Mycological Examination
3. Results
3.1. Bacteriological Examinations
3.2. Parasitological Examinations
3.3. Mycological Examinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rock, M.J.; Degeling, C.; Graham, T.M.; Toohey, A.M.; Rault, D.; McCormack, G.R. Public engagement and com-munity participation in governing urban parks: A case study in changing and implementing a policy addressing off-leash dogs. Crit. Public Health 2016, 26, 588–601. [Google Scholar] [CrossRef]
- Carrier, L.O.; Cyr, A.; Anderson, R.E.; Walsh, C.J. Exploring the dog park: Relationships between social behaviours, personality and cortisol in companion dogs. Appl. Anim. Behav. Sci. 2013, 146, 96–106. [Google Scholar] [CrossRef]
- Rahim, T.; Barrios, P.R.; McKee, G.; McLaws, M.; Kosatsky, T. Public Health Considerations Associated with the Location and Operation of Off-Leash Dog Parks. J. Community Health 2018, 43, 433–440. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar]
- Skirrow, M.B. Campylobacter enteritis: A “new” disease. Br. Med. J. 1977, 2, 9–11. [Google Scholar] [CrossRef]
- Blaser, M.J.; LaForce, F.M.; Wilson, N.A.; Wang, W.L. Reservoirs for human campylobacteriosis. J. Infect. Dis. 1980, 141, 665–669. [Google Scholar] [CrossRef]
- Acke, E. Campylobacteriosis in dogs and cats: A review. N. Z. Vet. J. 2018, 66, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Tirziu, E.; Cumpanasoiu, C.; Gros, R.V.; Seres, M. Yersinia enterocolitica Monographic Study. J. Anim. Sci. Biotechnol. 2011, 44, 144–149. [Google Scholar]
- Quereda, J.J.; Leclercq, A.; Moura, A.; Vales, G.; Gómez-Martín, Á.; García-Muñoz, Á.; Thouvenot, P.; Tessaud-Rita, N.; Bracq-Dieye, H.; Lecuit, M. Listeria valentina sp. nov. isolated from a water trough and the faeces of healthy sheep. Int. J. Syst. Evol. Microbiol. 2020, 70, 5868–5879. [Google Scholar] [CrossRef]
- Abay, S.; Bayramb, L.C.; Aydina, F.; Muştakc, H.K.; Dikerc, K.S.; Erold, İ. Pathogenicity, genotyping and antibacterial susceptibility of the Listeria spp. recovered from stray dogs. Microb. Pathog. 2019, 126, 123–133. [Google Scholar] [CrossRef]
- Traversa, D.; Frangipane di Regalbono, A.; Di Cesare, A.; La Torre, F.; Drake, J.; Pietrobelli, M. Environmental contamination by canine geohelminths. Parasites Vectors 2014, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rebel, G.; Taplin, D. Dermatophytes, Their Recognition and Identification; University of Miami Press: Coral Gables, FL, USA, 1970. [Google Scholar]
- Falcaro, C.; Furlanello, T.; Binanti, D.; Fondati, A.; Bonfanti, U.; Krockenberger, M.; Malik, R.; Danesi, P. Molecular characterization of Prototheca in 11 symptomatic dogs. J. Vet. Diagn. Investig. 2021, 33, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kudinha, T.; Kong, F.; Zhang, Q.Q. Comparative Genome and Transcriptome Study of the Gene Expression Difference Between Pathogenic and Environmental Strains of Prototheca zopfii. Front. Microbiol. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Kano, R. Emergence of Fungal-Like Organisms: Prototheca. Mycopathologia 2020, 185, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Bakuła, Z.; Siedlecki, P.; Gromadka, R.; Gawor, J.; Gromadka, A.; Pomorski, J.J.; Panagiotopoulou, H.; Jagielski, T. A first insight into the genome of Prototheca wickerhamii, a major causative agent of human protothecosis. BMC Genom. 2021, 22, 168. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, M.; Fu, Y. Multiple cutaneous infections caused by Prototheca wickerhamii. J. Clin. Lab. Anal. 2020, 34, e23492. [Google Scholar] [CrossRef]
- Nardoni, S.; Sorrentino, V.; Mancianti, F. Occurrence and In Vitro Antifungal Susceptibility of Candida Spp. Isolated from Decayed Tree Parts in Green Urban Areas from Pisa (Central Italy). Biomed. J. Sci. Tech. Res. 2019, 19, 14516–14518. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef]
- Bertelloni, F.; Chemaly, M.; Cerri, D.; LeGall, F.; Ebani, V.V. Salmonella infection in healthy pet reptiles: Bacteriological isolation and study of some pathogenic characters. Acta Microbiol. Immunol. Hungarica 2016, 63, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.P. The occurrence of Campylobacter jejuni in dog faeces from a public park. J. Hyg. 1982, 89, 191–194. [Google Scholar] [CrossRef]
- Murphy, B.P.; Drummond, N.; Ringwood, T.; O’Sullivan, E.; Buckley, J.F.; Whyte, P.; Prentice, M.B.; Fanning, S. First report: Yersinia enterocolitica recovered from canine tonsils. Vet. Microbiol. 2010, 146, 336–339. [Google Scholar] [CrossRef]
- Bottone, E.J. Yersinia enterocolitica: The charisma continues. Clin. Microbiol. Rev. 1997, 10, 257–276. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Lan, R.; Salazar, J.K.; Liu, J.; Xu, J.; Ye, C. Isolation and characterization of Listeria species from rodents in natural environments in China. Emerg. Microbes Infect. 2017, 6, e44. [Google Scholar] [CrossRef]
- Riggio, F.; Mannella, R.; Ariti, G.; Perrucci, S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 2013, 193, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Caretta, G.; Mangiarotti, A.M.; Piontelli, E. Keratinophilic fungi isolated from soil of Italian parks in the province of Pavia. Eur. J. Epidemiol. 1992, 8, 330–339. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Ahmed, S.; Al-Hatmiu, A.M.S.; Figueras, M.J.; Vitale, R.G. Atlas of Clinical Fungi 2021, 4th ed.; Westerdijk Institute: Utrecht, The Netherlands; Universitat Rovira i Virgili: Reus, Spain, 2021; ISBN 9070351439. [Google Scholar]
- Carmichael, J.W. Chrysosporium and some other aleuriosporic hyphomycetes. Can. J. Bot. 1962, 40, 1137–1173. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Rinaldi, L.; Biggeri, A.; Carbone, S.; Musella, V.; Catelan, D.; Veneziano, V.; Cringoli, G. Canine faecal contamination and parasitic risk in the city of Naples (southern Italy). BMC Vet. Res. 2006, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Zanzani, S.A.; Di Cerbo, A.R.; Gazzonis, A.L.; Genchi, M.; Rinaldi, L.; Musella, V.; Cringoli, G.; Manfredi, M.T. Canine fecal contamination in a metropolitan area (Milan, north-western Italy): Prevalence of intestinal parasites and evaluation of health risks. Sci. World J. 2014, 2014, 132361. [Google Scholar] [CrossRef]
- Stamm, I.; Hailer, M.; Depner, B.; Kopp, P.A.; Rau, J. Yersinia enterocolitica in diagnostic fecal samples from european dogs and cats: Identification by fourier transform infrared spectroscopy and matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 887–893. [Google Scholar] [CrossRef]
- Bucher, M.; Meyer, C.; Grötzbach, B.; Wacheck, S.; Stolle, A.; Fredriksson-Ahomaa, M. Epidemiological data on pathogenic Yersinia enterocolitica in southern Germany during 2000–2006. Foodborne Pathog. Dis. 2008, 5, 273–280. [Google Scholar] [CrossRef]
- Nastasi, A.; Massenti, M.F.; Scarlata, G.; Mammina, C.; Calcò, C.; Villafrate, M.R. Salmonella and Yersinia enterocolitica in soil and dog faeces. Boll. Dell’istituto Sieroter. Milan. 1986, 65, 150–152. [Google Scholar]
- Christensen, S.G. The Yersinia enterocolitica situation in Denmark. Contrib. Microbiol. Immunol. 1987, 9, 93–97. [Google Scholar] [PubMed]
- Fantasia, M.; Mingrone, M.G.; Crotti, D.; Boscato, C. Isolation of Yersinia enterocolitica biotype 4 serotype O3 from canine sources in Italy. J. Clin. Microbiol. 1985, 22, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Cinquepalmi, V.; Monno, R.; Fumarola, L.; Ventrella, G.; Calia, C.; Greco, M.F.; de Vito, D.; Soleo, L. Environmental Contamination by Dog’s Faeces: A Public Health problem? Int. J. Environ. Res. Public Health 2013, 10, 72–84. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Z.; Wang, H.; Tang, L.; Yang, J.; Gu, L.; Jin, D.; Luo, L.; Qiu, H.; Xiao, Y.; et al. Pathogenic strains of Yersinia enterocolitica isolated from domestic dogs (Canis familiaris) belonging to farmers are of the same subtype as pathogenic Y. enterocolitica strains isolated from humans and may be a source of human infection in Jiangsu province, China. J. Clin. Microbiol. 2010, 48, 1604–1610. [Google Scholar] [PubMed]
- Fenwick, S.G.; Madie, P.; Wilks, C.R. Duration of carriage and transmission of Yersinia enterocolitica biotype 4, serotype O:3 in dogs. Epidemiol. Infect. 1994, 113, 471–477. [Google Scholar] [CrossRef][Green Version]
- Fredriksson-Ahomaa, M.; Korte, T.; Korkeala, H. Transmission of Yersinia enterocolitica 4/O: 3 to pets via contaminated pork. Lett. Appl. Microbiol. 2001, 32, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Lida, T.; Kanzaki, M.; Nakama, A.; Kokubo, Y.; Maruyama, T.; Kaneuchi, C. Detection of Listeria monocytogenes in humans, animals and foods. J. Vet. Med. Sci. 1998, 60, 1341–1343. [Google Scholar]
- Yoshida, T.; Sugimoto, T.; Sato, M.; Hirai, K. Incidence of Listeria monocytogenes in wild animals in Japan. J. Vet. Med. Sci. 2000, 62, 673–675. [Google Scholar] [CrossRef][Green Version]
- Kocabıyık, A.L.; Çetin, C. Faecal carriage of Listeria monocytogenes in stray dogs in Bursa province, Turkey. Turk. J. Vet. Anim. Sci. 2005, 29, 1357–1359. [Google Scholar]
- Alidoosti, H.A.; Razzaghi Manesh, M.R.; Mashhady Rafie, S.I.; Dadar, M.O. Prevalence of Listeria monocytogenes in dogs in Isfahan, Iran. Bulg. J. Vet. Med. 2014, 1, 69. [Google Scholar]
- Karli, A.; Sensoy, G.; Unal, N.; Yanik, K.; Cigdem, H.; Belet, N.; Sofuoğlu, A. Ventriculoperitoneal shunt infection with Listeria innocua. Pediatrics Int. 2014, 56, 621–623. [Google Scholar] [CrossRef]
- Walker, J.K.; Morgan, J.H.; McLauchlin, J.; Grant, K.A.; Shallcross, J.A. Listeria innocua isolated from a case of ovine meningoencephalitis. Vet. Microbiol. 1994, 42, 245–253. [Google Scholar] [CrossRef]
- Rocha, P.R.; Dalmasso, A.; Grattarola, C.; Casalone, C.; Del Piero, F.; Bottero, M.T.; Capucchio, M.T. Atypical cerebral listeriosis associated with Listeria innocua in a beef bull. Res. Vet. Sci. 2013, 94, 111–114. [Google Scholar] [CrossRef]
- Tarsitano, E.; Greco, G.; Decaro, N.; Nicassio, F.; Lucente, M.S.; Buonavoglia, C.; Tempesta, M. Environmental Monitoring and Analysis of Faecal Contamination in an Urban Setting in the City of Bari (Apulia Region, Italy): Health and Hygiene Implications. Int. J. Environ. Res. Public Health 2010, 7, 3972–3986. [Google Scholar] [CrossRef] [PubMed]
- Hackett, T.; Lappin, M.R. Prevalence of enteric pathogens in dogs of North-Central Colorado. J. Am. Anim. Hosp. Assoc. 2003, 39, 52–56. [Google Scholar] [CrossRef]
- Procter, T.D.; Pearl, D.L.; Finley, R.L.; Leonard, E.K.; Janecko, N.; Reid-Smith, R.J.; Weese, J.S.; Peregrine, A.S.; Sargeant, J.M. A cross-sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in South-Western Ontario and risk factors for shedding of Campylobacter spp. Zoonoses Public Health 2014, 61, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hascall, K.L.; Kass, P.H.; Saksen, J.; Ahlmann, A.; Scorza, A.V.; Lappin, M.R.; Marks, S.L. Prevalence of Enteropathogens in dogs attending 3 regional dog parks in Northern California. J. Vet. Int. Med. 2016, 30, 1838–1845. [Google Scholar] [CrossRef]
- Buss, J.E.; Thacker, E.; Santiago, M. Culture Methods to Determine the Limit of Detection and Survival in Transport Media of Campylobacter Jejuni in Human Fecal Specimens. J. Vis. Exp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Otero, D.; Alho, A.M.; Nijsse, R.; Roelfsema, J.; Overgaauw, P.; Madeira de Carvalho, L. Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Lisbon, Portugal. J. Infect. Public Health 2018, 11, 94–98. [Google Scholar] [CrossRef]
- Tamponi, C.; Knoll, S.; Tosciri, G.; Salis, F.; Dessì, G.; Cappai, M.G.; Varcasia, A.; Scala, A. Environmental Contamination by Dog Feces in Touristic Areas of Italy: Parasitological Aspects and Zoonotic Hazards. Am. J. Trop. Med. Hyg. 2020, 103, 1143–1149. [Google Scholar] [CrossRef]
- Simonato, G.; Cassini, R.; Morelli, S.; Di Cesare, A.; La Torre, F.; Marcer, F.; Traversa, D.; Pietrobelli, M.; Frangipane di Regalbono, A. Contamination of Italian parks with canine helminth eggs and health risk perception of the public. Prev. Vet. Med. 2019, 172, 104788. [Google Scholar] [CrossRef]
- Ferreira, A.; Alho, A.M.; Otero, D.; Gomes, L.; Nijsse, R.; Overgaauw, P.A.M.; Madeira de Carvalho, L. Urban dog parks as sources of canine parasites: Contamination rates and pet owner behaviours in Lisbon, Portugal. J. Environ. Public Health 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mercantini, R.; Marsella, R.; Caprilli, F.; Dovgiallo, G. Isolation of dermatophytes and correlated species from the soil of public gardens and parks in Rome. Sabouraudia 1980, 18, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Massai, L.; Gallo, A.; Fimiani, M. Microsporum gypseum infection in the Siena area in 2005–2006. Mycoses 2009, 52, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Dolenc-Voljč, M.; Gasparič, J. Human Infections with Microsporum gypseum Complex (Nannizzia gypsea) in Slovenia. Mycopathologia 2017, 182, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Caretta, G.; Mancianti, F.; Ajello, L. Dermatophytes and keratinophilic fungi in cats and dogs. Mycoses 1989, 32, 620–626. [Google Scholar] [CrossRef]
- Cafarchia, C.; Romito, D.; Sasanelli, M.; Lia, R.; Capelli, G.; Otranto, D. The epidemiology of canine and feline dermatophytoses in southern Italy. Mycoses 2004, 47, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Mugnaini, L.; Papini, R.; Fiaschi, M.; Mancianti, F. Canine and feline dermatophytosis due to Microsporum gypseum: A retrospective study of clinical data and therapy outcome with griseofulvin. J. Mycol. Med. 2013, 23, 164–167. [Google Scholar] [CrossRef]
- Quaife, R.A.; Lutwyche, P. Microsporum cookei as the suspected cause of ringworm in a dog. Vet. Rec. 1981, 109, 311. [Google Scholar] [CrossRef]
- Scott, E.M.; Carter, R.T. Canine keratomycosis in 11 dogs: A case series (2000–2011). J. Am. Anim. Hosp. Assoc. 2014, 50, 112–118. [Google Scholar] [CrossRef]
- Mancianti, F.; Papini, R.A. Isolation of keratinophilic fungi from the floors of private veterinary clinics in Italy. Vet. Res. Commun. 1996, 20, 161–166. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Werrett, G.; Stokes, C.R.; Gruffydd-Jones, T.J. Microsporum canis: Inapparent carriage by cats and the viability of arthrospores. J. Small Anim. Pract. 1994, 35, 397–401. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Zięba, P. Tinea corporis by Microsporum canis in mycological laboratory staff: Unexpected results of epidemiological investigation. Mycoses 2018, 61, 945–953. [Google Scholar] [CrossRef]
- Ziegler, W.; Lempert, S.; Goebeler, M.; Kolb-Mäurer, A. Tinea capitis: Temporal shift in pathogens and epidemiology. J. Dtsch. Dermatol. Ges. 2016, 14, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Pagano, L.; Martino, B.; D’Antonio, D.; Fanci, R.; Specchia, G.; Melillo, L.; Buelli, M.; Pizzarelli, G.; Venditti, M.; et al. GIMEMA Infection Program. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: A retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005, 43, 1818–1828. [Google Scholar] [CrossRef]
- Navarro, B.S.; Auler, M.E.; Dos Santos, R.L.O.; da Silva Ruiz, L.; Nascimento, D.C.; Felippe, P.A.N.; Domaneschi, C.; Moreira, D.; Baroni, F.A.; Piresg, M.F.C.; et al. Antifungal sensitivity and species of yeasts in oral mucosa of street mixed-breed dogs. J. Mycol. Med. 2020, 30, 101010. [Google Scholar] [CrossRef] [PubMed]
- Biegańska, M.J.; Rzewuska, M.; Dąbrowska, I.; Malewska-Biel, B.; Ostrzeszewicz, M.; Dworecka-Kaszak, B. Mixed Infection of Respiratory Tract in a Dog Caused by Rhodotorula mucilaginosa and Trichosporon jirovecii: A Case Report. Mycopathologia 2018, 183, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Pal, M. Role of Geotrichum candidum in canine oral ulcers. Rev. Iberoam Micol. 2005, 22, 183. [Google Scholar] [CrossRef]
- Reppas, G.P.; Snoeck, T.D. Cutaneous geotrichosis in a dog. Aust. Vet. J. 1999, 77, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Rhyan, J.C.; Stackhouse, L.L.; Davis, E.G. Disseminated geotrichosis in two dogs. J. Am. Vet. Med. Assoc. 1990, 197, 358–360. [Google Scholar] [PubMed]
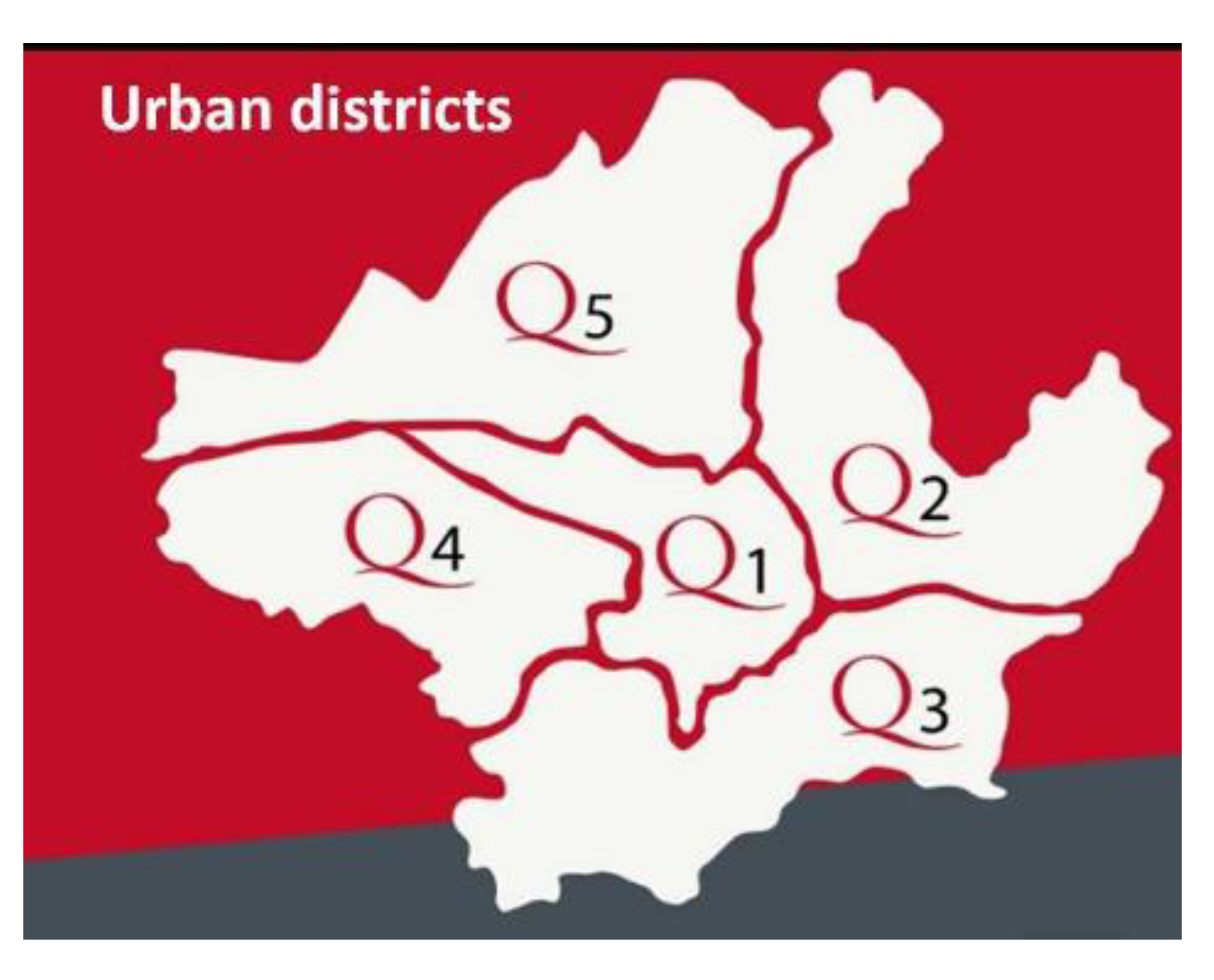
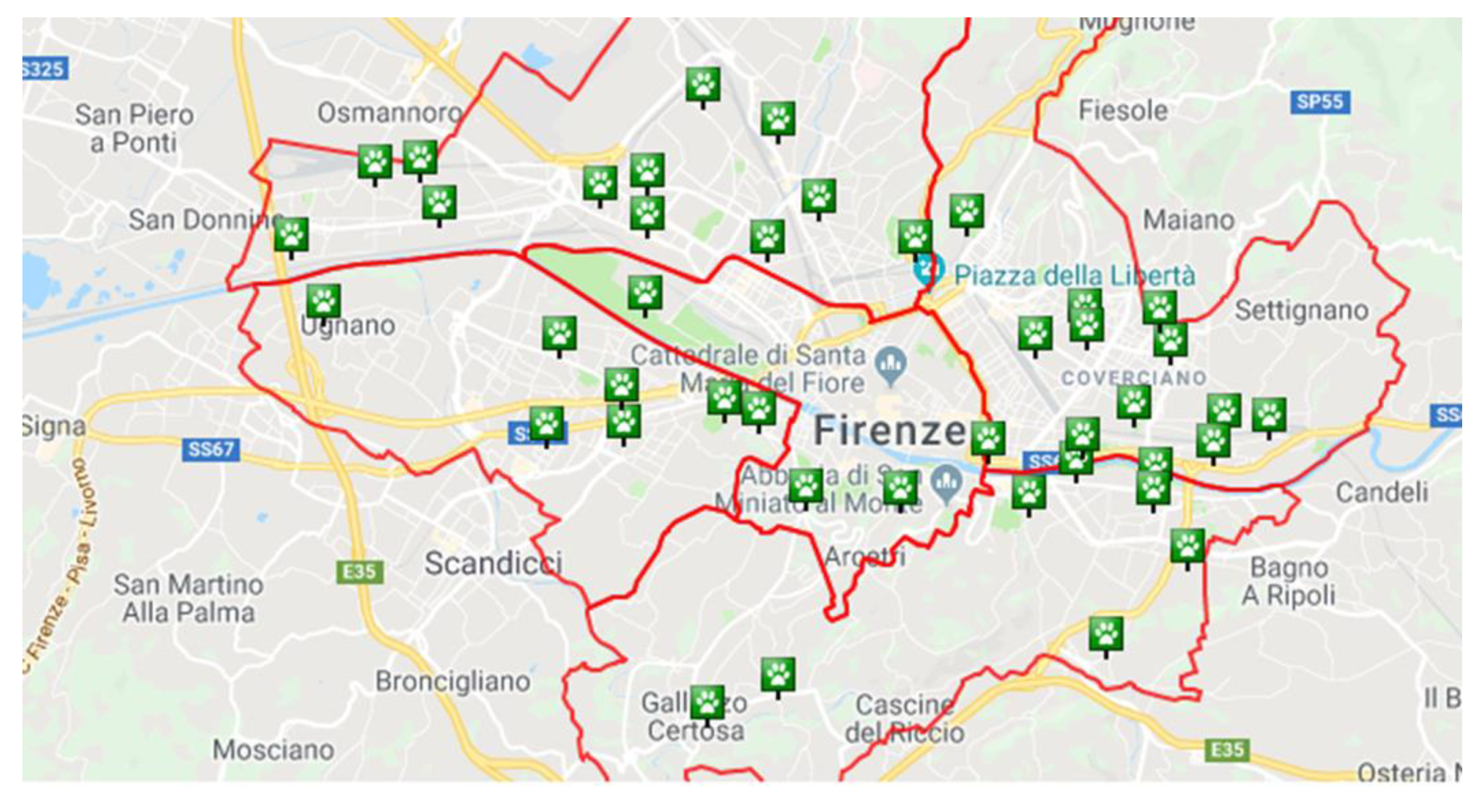
| Urban District | Off-Leash Park | M2 | Exposure | Trees | Soil |
|---|---|---|---|---|---|
| Q1 | Porta Romana | 553 | Shady | maples | clay |
| Q2 | Mezzetta | 4141 | Sunny | pines, limes | grass |
| Q2 | Malta | 579 | Shady | pines, tuje | clay |
| Q2 | Villa Favard | 1300 | Shady | holm oaks | grass |
| Q2 | Tempio | 680 | Shady | holm oaks | clay |
| Q2 | Moro | 659 | Sunny | cedars | grass |
| Q2 | Venosta | 522 | Sunny | prunus | grass |
| Q2 | Rocca Tedalda | 100 | Shady | limes | grass |
| Q2 | Campo di Marte | 15,043 | Sunny | bangolari, limes, holm oaks | grass |
| Q2 | Fanti | 450 | Shady | plane trees | grass |
| Q2 | D’Ancona | 124 | Shady | maples | grass |
| Q3 | Anconella | 2361 | Sunny | poplars, maples | grass |
| Q3 | Villamagna | 4141 | Sunny | limes, Judas’s tree | grass |
| Q3 | Gran Bretagna | 873 | Shady | pines | grass |
| Q3 | Ponte a Ema | 1100 | Sunny | limes, cypresses, olive trees | grass |
| Q3 | Pozzolatico | 3235 | Shady | poplars | grass |
| Q3 | Isonzo | 715 | Sunny | mulberries, limes, olive trees | grass |
| Q3 | Galluzzo | 1345 | Shady | pines | grass |
| Q4 | Aleardi | 1059 | Sunny | limes, magnolia, holm oaks | grass |
| Q4 | Strozzi | 1500 | Sunny | olive trees, birches, oaks, pines, | grass |
| Q4 | Pisano | 1659 | Shady | plane trees | grass |
| Q4 | S. Lorenzo a Greve | 355 | Sunny | maples | grass |
| Q5 | Parnaso | 2078 | Sunny | holm oaks, birches, limes | grass |
| Q5 | Zucchi | 250 | Sunny | no | grass |
| Q5 | Circondaria | 872 | Shady | plane trees | grass |
| Q5 | Pisacane | 2919 | Sunny | holm oaks, cypresses, olive trees | grass |
| Urban District | Off-Leash Park | Fecal Samples | Microbial Strains (Positive Samples) | Parasite Eggs (Positive Samples) |
|---|---|---|---|---|
| Q1 | Porta Romana | 1 | ||
| Q2 | Mezzetta | 9 | Listeria innocua (3) | |
| Q2 | Malta | 2 | ||
| Q2 | Villa Favard | 5 | ||
| Q2 | Tempio | 1 | Yersinia frederiksenii (1) | |
| Q2 | Moro | 4 | ||
| Q2 | Venosta | 6 | Yersinia enterocolitica BT2 (1) | Toxocara canis (1), |
| Q2 | Rocca Tedalda | 5 | Ancylostoma caninum/Uncinaria stenocephala(1) | |
| Q2 | Campo di Marte | 1 | ||
| Q2 | Fanti | 3 | ||
| Q2 | D’Ancona | 1 | Toxocara canis (1) | |
| Q3 | Anconella | 7 | Listeria innocua (1) | |
| Q3 | Villamagna | 11 | ||
| Q3 | Gran Bretagna | 2 | Yersinia frederiksenii (1) | |
| Q3 | Ponte a Ema | no | ||
| Q3 | Pozzolatico | 1 | ||
| Q3 | Isonzo | 3 | ||
| Q3 | Galluzzo | 3 | ||
| Q4 | Aleardi | 5 | ||
| Q4 | Strozzi | 2 | Yersinia enterocolitica BT2 (1) | |
| Q4 | Pisano | no | ||
| Q4 | S. Lorenzo a Greve | 3 | ||
| Q5 | Parnaso | 1 | Yersinia enterocolitica BT1 (1) | |
| Q5 | Zucchi | no | ||
| Q5 | Circondaria | 3 | ||
| Q5 | Pisacane | 4 | Yersinia frederiksenii (2) |
| Urban District | Off-leash Park | N. Soil Samples | Fungal Isolates | Hair | Horsehair | Feathers |
|---|---|---|---|---|---|---|
| Q2 | Mezzetta | 1 | Trichophyton terrestre/ Arthroderma quadrifidum | 0/1 | 1/1 | 1/1 |
| Q2 | Malta | 1 | Microsporum gypseum/Arthroderma incurvatum | 1/1 | 1/1 | 1/1 |
| Q2 | Tempio | 1 | Microsporum gypseum/Arthroderma incurvatum | 1/1 | 1/1 | 1/1 |
| Q2 | Moro | 2 | Trichophyton terrestre/(Arthroderma quadrifidum | 1/2 | 1/2 | 2/2 |
| Q2 | Venosta | 1 | Microsporum gypseum/Arthroderma incurvatum Microsporum cookie/Arthroderma cajetani | 0/1 0/1 | 1/1 1/1 | 0/1 1/1 |
| Q2 | Rocca Tedalda | 1 | Trichophyton terrestre/ Arthroderma quadrifidum Microsporum gypseum/Arthroderma incurvatum | 0/1 1/1 | 1/1 1/1 | 0/1 1/1 |
| Q3 | Anconella | 1 | Microsporum gypseum/Arthroderma incurvatum | 0/1 | 1/1 | 1/1 |
| Q3 | Villamagna | 2 | Trichophyton ajelloi /Arthroderma uncinatum | 0/2 | 0/2 | 1/2 |
| Q3 | Ponte a Ema | 2 | Trichophyton ajelloi /Arthroderma uncinatum | 1/2 | 2/2 | 2/2 |
| Q3 | Pozzolatico | 1 | Chrysosporium keratinophilum/Aphanoascus fulvescens | 0/1 | 0/1 | 1/1 |
| Q3 | Isonzo | 1 | Microsporum gypseum/Arthroderma incurvatum | 0/1 | 1/1 | 1/1 |
| Q4 | Aleardi | 2 | Microsporum gypseum/Arthroderma incurvatum Microsporum cookie/Arthroderma cajetani | 0/2 0/2 | 2/2 2/2 | 1/2 1/2 |
| Q4 | Strozzi | 3 * | Microsporum gypseum/Arthroderma incurvatum | 0/3 | 3/3 | 2/3 |
| Q4 | Pisano | 2 | Chrysosporium indicum/Aphanoascus terreus Microsporum gypseum/Arthroderma incurvatum | 0/2 1/2 | 0/2 2/2 | 2/2 2/2 |
| Q5 | Parnaso | 3 | Microsporum gypseum/Arthroderma incurvatum | 2/3 | 3/3 | 2/3 |
| Q5 | Circondaria | 4 | Microsporum gypseum/Arthroderma incurvatum | 4/4 | 3/4 | 1/4 |
| total | 43 | 8 | 14 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebani, V.V.; Nardoni, S.; Ciapetti, S.; Guardone, L.; Loretti, E.; Mancianti, F. Survey on the Presence of Bacterial, Fungal and Helminthic Agents in Off-Leash Dog Parks Located in Urban Areas in Central-Italy. Animals 2021, 11, 1685. https://doi.org/10.3390/ani11061685
Ebani VV, Nardoni S, Ciapetti S, Guardone L, Loretti E, Mancianti F. Survey on the Presence of Bacterial, Fungal and Helminthic Agents in Off-Leash Dog Parks Located in Urban Areas in Central-Italy. Animals. 2021; 11(6):1685. https://doi.org/10.3390/ani11061685
Chicago/Turabian StyleEbani, Valentina Virginia, Simona Nardoni, Stefania Ciapetti, Lisa Guardone, Enrico Loretti, and Francesca Mancianti. 2021. "Survey on the Presence of Bacterial, Fungal and Helminthic Agents in Off-Leash Dog Parks Located in Urban Areas in Central-Italy" Animals 11, no. 6: 1685. https://doi.org/10.3390/ani11061685
APA StyleEbani, V. V., Nardoni, S., Ciapetti, S., Guardone, L., Loretti, E., & Mancianti, F. (2021). Survey on the Presence of Bacterial, Fungal and Helminthic Agents in Off-Leash Dog Parks Located in Urban Areas in Central-Italy. Animals, 11(6), 1685. https://doi.org/10.3390/ani11061685








