Dietary Curcumin Promotes Gilthead Seabream Larvae Digestive Capacity and Modulates Oxidative Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Husbandry and Experimental Set-up
2.2. Experimental Diets and Feeding Protocol
2.3. Growth and Survival
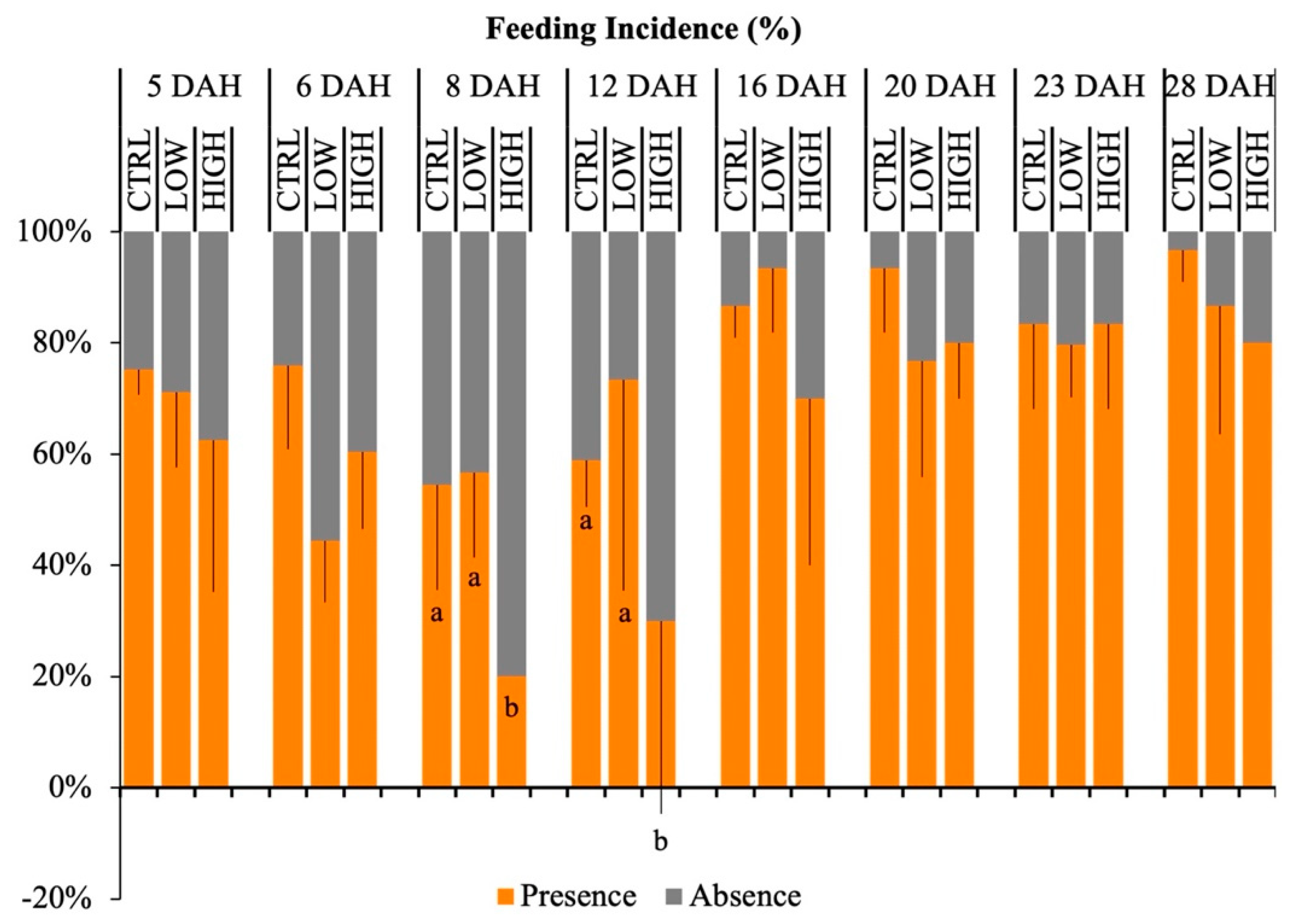
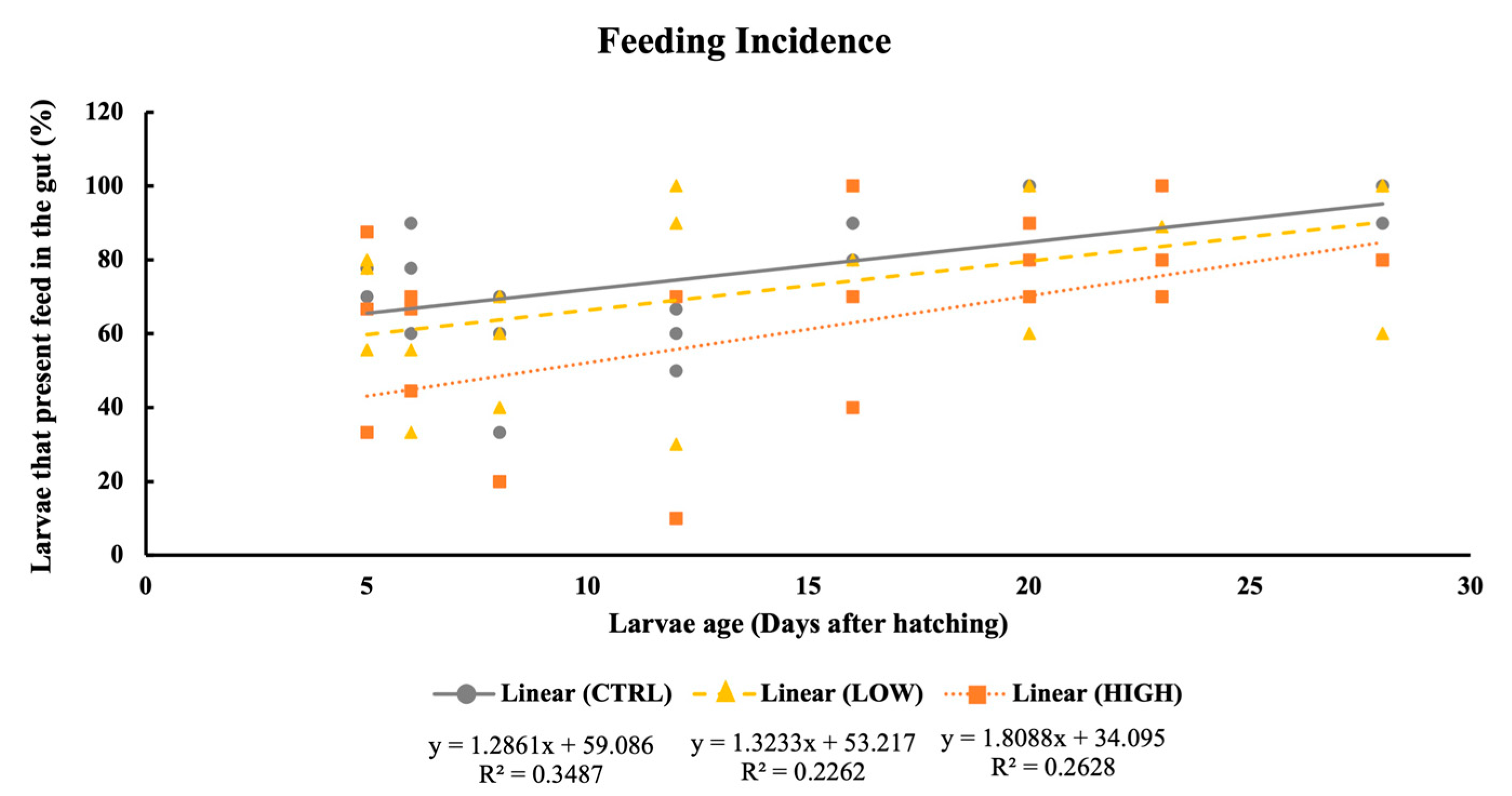
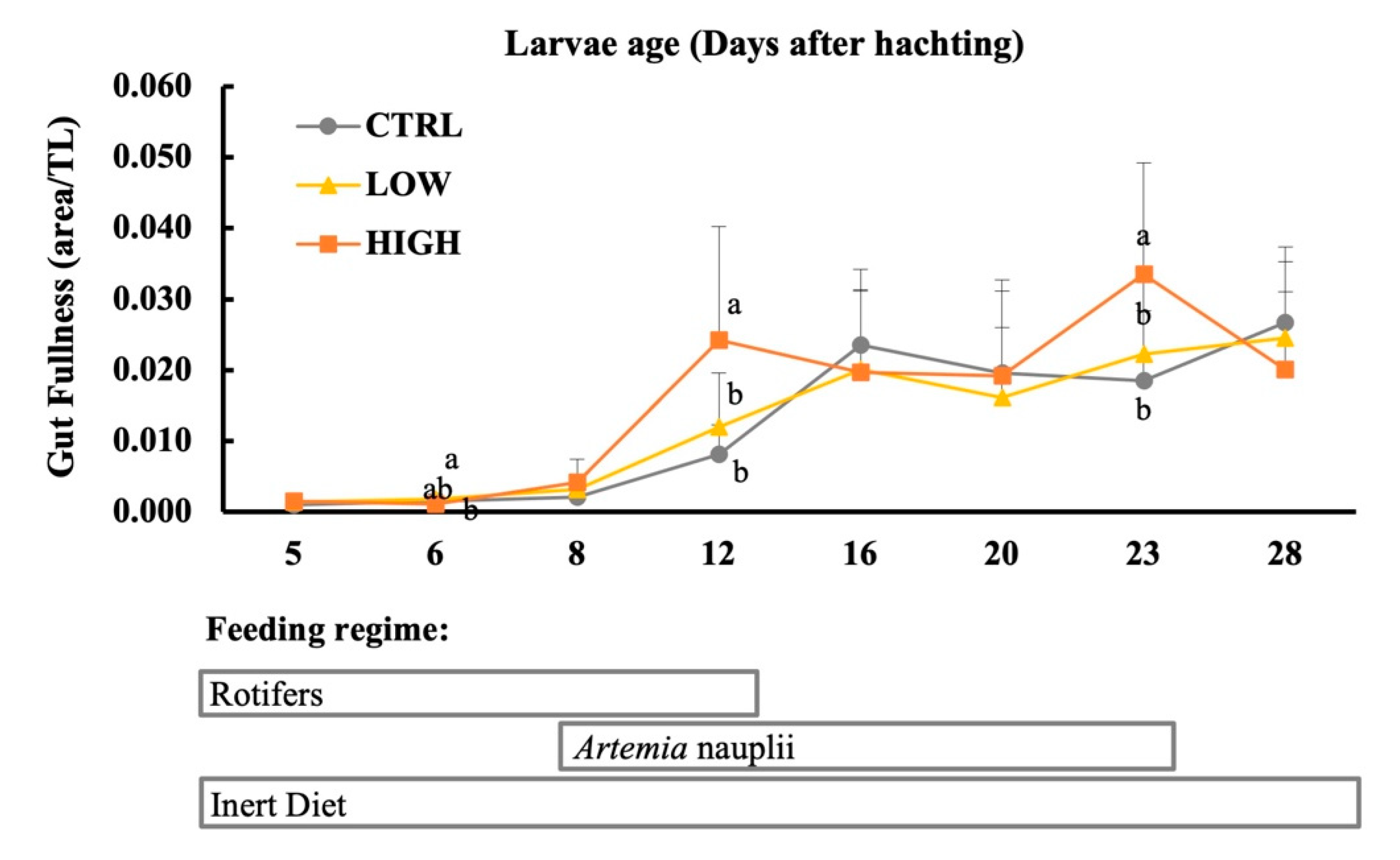
2.4. Feeding Incidence and Gut Fullness
2.5. Gut Maturation
2.6. Antioxidant Status
2.6.1. Sample Preparation for Biomarker Analysis
2.6.2. Oxidative Status Biomarker Measurements
2.7. Statistical Analysis
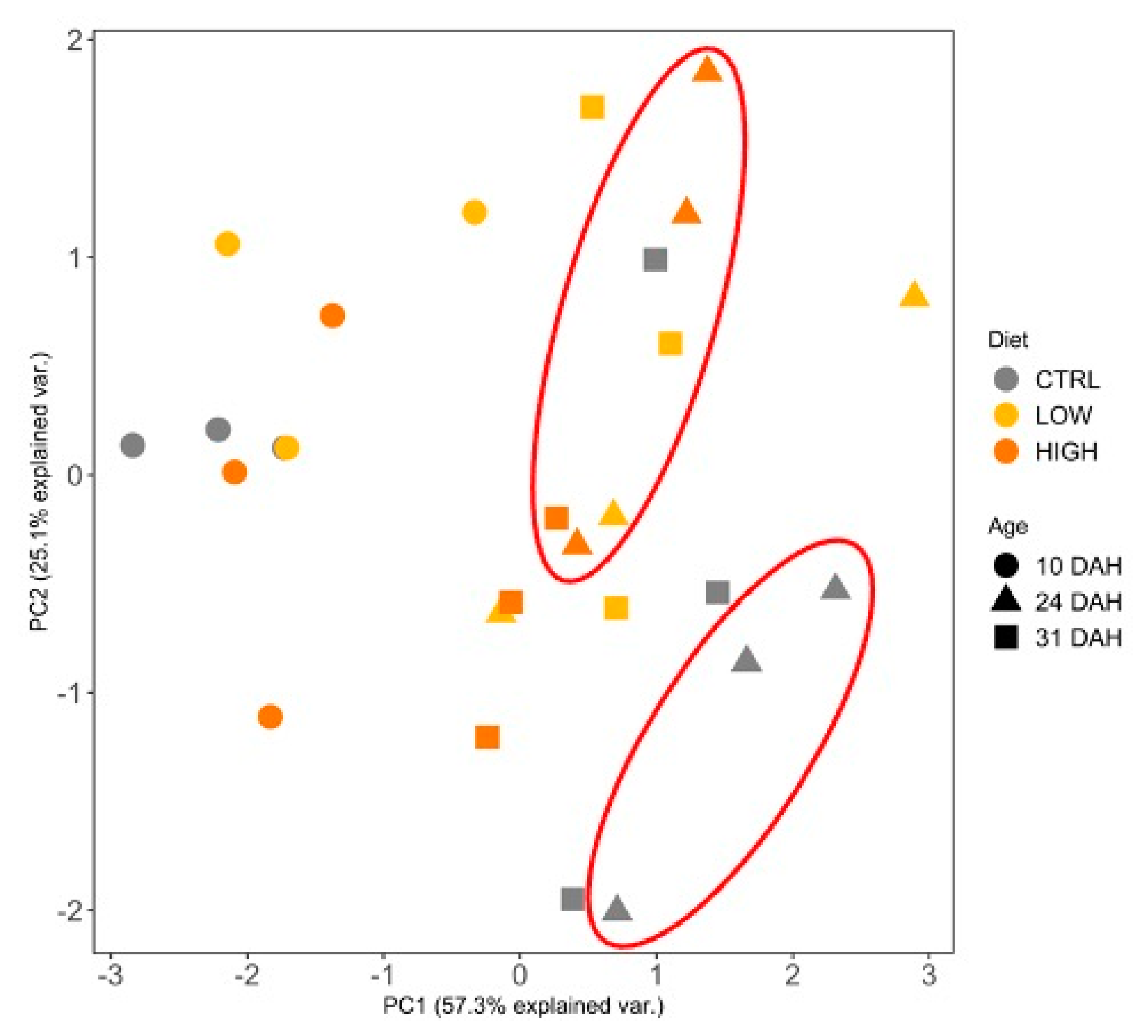
3. Results
3.1. Growth Performance
3.2. Feeding Incidence
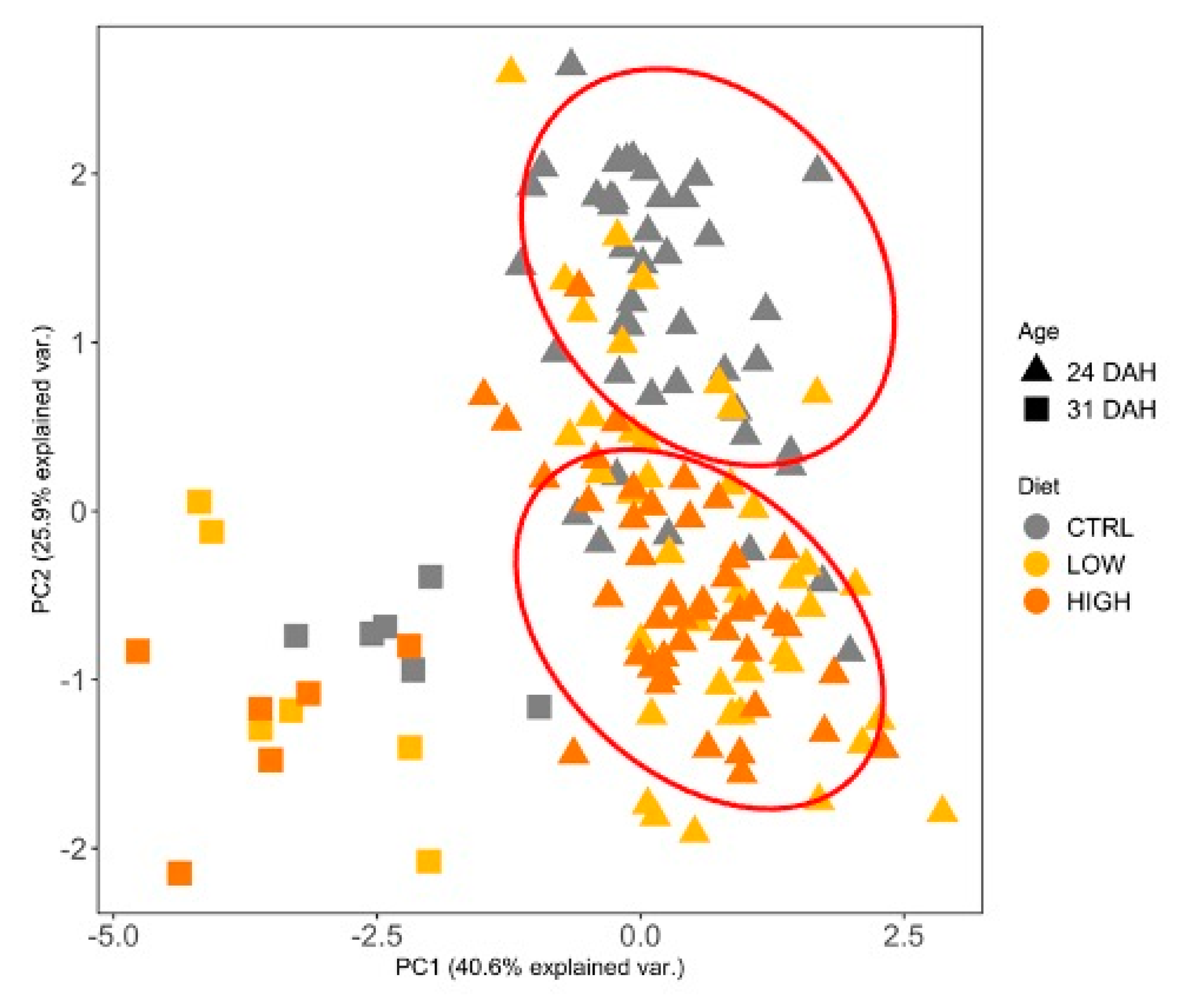
3.3. Digestive Enzymes
3.4. Antioxidant Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rønnestad, I.; Kamisaka, Y.; Conceição, L.; Morais, S.; Tonheim, S. Digestive physiology of marine fish larvae: Hormonal control and processing capacity for proteins, peptides and amino acids. Aquaculture 2007, 268, 82–97. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Saele, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquacult. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Hamre, K.; Nordgreen, A.; Grøtan, E.; Breck, O. A holistic approach to development of diets for Ballan wrasse (Labrus berggylta)—A new species in aquaculture. PeerJ 2013, 1, e99. [Google Scholar] [CrossRef]
- Canada, P.; Engrola, S.; Mira, S.; Teodósio, R.; del Mar Yust, M.; Sousa, V.; Pedroche, J.; Fernandes, J.M.; Conceição, L.E.; Valente, L.M. Larval dietary protein complexity affects the regulation of muscle growth and the expression of DNA methyltransferases in Senegalese sole. Aquaculture 2018, 491, 28–38. [Google Scholar] [CrossRef]
- Engrola, S. Improving Growth Performance of Senegalese Sole Postlarvae. Ph.D. Thesis, Universidade do Algarve, Faro, Portugal, 2008. [Google Scholar]
- Pinto, W.; Engrola, S.; Conceição, L.E. Towards an early weaning in Senegalese sole: A historical review. Aquaculture 2018, 496, 1–9. [Google Scholar] [CrossRef]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquacult. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Diniz, M.; Machado, J.; Pousao-Ferreira, P.; Peck, M.A.; Portner, H.O.; Rosa, R. Oxidative Stress and Digestive Enzyme Activity of Flatfish Larvae in a Changing Ocean. PLoS ONE 2015, 10, e0134082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H.; Ai, Q. Effects of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture 2015, 446, 48–56. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Pattern and variety in development. In Fish. Physiology Vol XI, The Physiology of Developing Fish. Part A: Eggs and Larvae; Hoar, W.S., Randall, D.J., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 1–58. [Google Scholar]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z. Substitution of live food by formulated diets in marine fish larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Conceição, L.; Dersjant-Li, Y.; Verreth, J. Cost of growth in larval and juvenile African catfish (Clarias gariepinus) in relation to growth rate, food intake and oxygen consumption. Aquaculture 1998, 161, 95–106. [Google Scholar] [CrossRef]
- Finn, R.N.; Rønnestad, I.; van der Meeren, T.; Fyhn, H.J. Fuel and metabolic scaling during the early life stages of Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser. 2002, 243, 217–234. [Google Scholar] [CrossRef]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of Vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef]
- Betancor, M.B.; Caballero, M.; Terova, G.; Saleh, R.; Atalah, E.; Benitez-Santana, T.; Bell, J.G.; Izquierdo, M. Selenium inclusion decreases oxidative stress indicators and muscle injuries in sea bass larvae fed high-DHA microdiets. Brit. J. Nutr. 2012, 108, 2115–2128. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhou, A.; Ye, Q.; Feng, Y.; Wang, Z.; Wang, S.; Xu, G.; Zou, J. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard Mater. 2021, 403, 123948. [Google Scholar] [CrossRef] [PubMed]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2-Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Toledo-Solís, F.J.; Larrán, A.M.; de Mercado, E.; Alarcón, F.J.; Rico, D.; Martín-Diana, A.B.; Fernández, I. Effects and Safe Inclusion of Narbonne Vetch (Vicia narbonensis) in Rainbow Trout (Oncorhynchus mykiss) Diets: Towards a More Sustainable Aquaculture. Animals 2020, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Pourhanifeh, M.H.; Mirzaei, H.R.; Sahebkar, A.; Asemi, Z.; Mirzaei, H. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacol. Res. 2019, 147, 104353. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2020, 532, 736030. [Google Scholar] [CrossRef]
- Cui, H.; Liu, B.; Ge, X.-P.; XiE, J.; Xu, P.; Miao, L.-H.; Sun, S.; Liao, Y.; Chen, R.; Ren, M. Effects of dietary curcumin on growth performance, biochemical parameters, HSP70 gene expression and resistance to Streptococcus iniae of juvenile Gift Tilapia, Oreochromis niloticus. Isr. J. Aquac. 2013, 66, 986–996. [Google Scholar]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Park, S.C. Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish. Shellfish Immunol. 2019, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-kappaB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish. Shellfish Immunol. 2020, 97, 540–553. [Google Scholar]
- Yonar, M.E.; Mise Yonar, S.; Ispir, U.; Ural, M.S. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish. Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Moustafa, A.A.; Rahman Mohamed, A.A. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, X.-Y.; Zhou, X.-Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, Y. Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 2016, 463, 174–180. [Google Scholar] [CrossRef]
- Manju, M.; Vijayasree, A.S.; Akbarsha2æ, M.A.; Oommen, O.V. Effect of curcumin supplementation on hepatic, renal and intestinal organization of Anabas testudineus (Bloch): Light and electron microscopic studies. J. Endocrinal. Reprod. 2013, 17, 83–98. [Google Scholar]
- Xavier, M.J.; Engrola, S.; Conceição, L.E.C.; Manchado, M.; Carballo, C.; Gonçalves, R.; Colen, R.; Figueiredo, V.; Valente, L.M.P. Dietary Antioxidant Supplementation Promotes Growth in Senegalese Sole Postlarvae. Front. Physiol. 2020, 11, 580600. [Google Scholar] [CrossRef]
- Xavier, M.J.; Conceição, L.E.C.; Valente, L.M.P.; Engrola, S. Natural plant extracts modulate growth performance, oxidative status and stress resistance of Senegalese sole postlarvae. In Proceedings of the EAS2020—European Aquaculture Society, 12–15 April 2021. Online. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Parra, G.; Yúfera, M. Comparative energetics during early development of two marine fish species, Solea senegalensis (Kaup) and Sparus aurata (L.). J. Exp. Biol. 2001, 204, 2175–2183. [Google Scholar] [CrossRef]
- Pavlidis, M.A.; Mylonas, C.C. Sparidae: Biology and Aquaculture of Gilthead Sea Bream and Other Species. John Wiley & Sons: Oxford, UK, 2011; pp. 133–169. [Google Scholar]
- Rocha, F.; Dias, J.; Geurden, I.; Dinis, M.T.; Panserat, S.; Engrola, S. High-glucose feeding of gilthead seabream (Sparus aurata) larvae: Effects on molecular and metabolic pathways. Aquaculture 2016, 451, 241–253. [Google Scholar] [CrossRef]
- Ricker, W.E. Handbook of Computations for Biological Statistics of Fish Populations. Can. Fish. Res. Board Bull. 1958, 119, 1–300. [Google Scholar]
- Romero-Romero, S.; Yúfera, M. Contribution of gut content to the nutritional value of Brachionus plicatilis used as prey in larviculture. Aquaculture 2012, 364, 124–129. [Google Scholar] [CrossRef]
- Mata-Sotres, J.A.; Martínez-Rodríguez, G.; Pérez-Sánchez, J.; Sánchez-Vázquez, F.J.; Yufera, M. Daily rhythms of clock gene expression and feeding behavior during the larval development in gilthead seabream, Sparus aurata. Chronobiol. Int. 2015, 32, 1061–1074. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Sanz, Y.; Toldrá, F. Purification and characterization of an arginine aminopeptidase from Lactobacillus sakei. Appl. Environ. Microb. 2002, 68, 1980–1987. [Google Scholar] [CrossRef]
- Rotllant, G.; Moyano, F.J.; Andrés, M.; Díaz, M.; Estévez, A.; Gisbert, E. Evaluation of fluorogenic substrates in the assessment of digestive enzymes in a decapod crustacean Maja brachydactyla larvae. Aquaculture 2008, 282, 90–96. [Google Scholar] [CrossRef]
- Fernley, H.; Walker, P. Kinetic behaviour of calf-intestinal alkaline phosphatase with 4-methylumbelliferyl phosphate. Biochem. J. 1965, 97, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Braz, G.; Pedroza, A.; Nascimento, L.; Freitas, C.; Ferreira, D.; De Castro, R.M.; Lagranha, C. Fluoxetine induces lean phenotype in rat by increasing the brown/white adipose tissue ratio and UCP1 expression. J. Bioenerg. Biomembr. 2015, 47, 309–318. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.; Pestana, J.L. Exposure to chlorantraniliprole affects the energy metabolism of the caddisfly Sericostoma vittatum. Environ. Toxicol. Chem. 2017, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2, 4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- van der Toorn, M.; Kauffman, H.F.; van der Deen, M.; Slebos, D.J.; Koëter, G.H.; Gans, R.O.; Bakker, S.J. Cyclosporin A-induced oxidative stress is not the consequence of an increase in mitochondrial membrane potential. FEBS J. 2007, 274, 3003–3012. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Colell, A.; Morales, A.; Kaplowitz, N.; Fernández-Checa, J.C. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: Studies with isolated mitochondria and rat hepatocytes. Mol. Pharmacol. 1995, 48, 825–834. [Google Scholar]
- Ennos, A.R. Statistical and data Handling Skills in Biology, 2nd ed.; Pearson Prentice Hall: Harlow, UK, 2007; pp. 55–60. [Google Scholar]
- Herbinger, C.; Friars, G. Correlation between condition factor and total lipid content in Atlantic salmon, Salmo salar L., parr. Aquac. Res. 1991, 22, 527–529. [Google Scholar] [CrossRef]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative weight (Wr) status and current use in fisheries assessment and management. Rev. Fish. Sci. 2000, 8, 1–44. [Google Scholar] [CrossRef]
- Vicente, P.N. Variability in Growth and Condition of Juvenile Common Two-Banded Sea Bream (Diplodus vulgaris). Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2015. [Google Scholar]
- Pinto, W.; Figueira, L.; Santos, A.; Barr, Y.; Helland, S.; Dinis, M.T.; Aragão, C. Is dietary taurine supplementation beneficial for gilthead seabream (Sparus aurata) larvae? Aquaculture 2013, 384, 1–5. [Google Scholar] [CrossRef]
- Izquierdo, M.; Domínguez, D.; Jiménez, J.I.; Saleh, R.; Hernández-Cruz, C.M.; Zamorano, M.J.; Hamre, K. Interaction between taurine, vitamin E and vitamin C in microdiets for gilthead seabream (Sparus aurata) larvae. Aquaculture 2019, 498, 246–253. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Domínguez, D.; Roo, J.; Hernandez-Cruz, C.M.; Zamorano, M.J.; Castro, P.; Hamre, K.; Izquierdo, M. Effect of dietary microminerals in early weaning diets on growth, survival, mineral contents and gene expression in gilthead sea bream (Sparus aurata, L) larvae. Aquac. Nutr. 2020, 26, 1760–1770. [Google Scholar] [CrossRef]
- Eid, A.; Eldahrawy, A.A.; Salama, F.; El-Naby, A.; Asma, S. Growth performance and survival of gilthead seabream Sparus aurata larvae fed rotifer and artemia. Egypt. J. Nutr. Feeds 2018, 21, 899–907. [Google Scholar] [CrossRef][Green Version]
- Venkatramalingam, K.; Christopher, J.G.; Citarasu, T. Zingiber officinalis an herbal appetizer in the tiger shrimp Penaeus monodon (Fabricius) larviculture. Aquac. Nutr. 2007, 13, 439–443. [Google Scholar] [CrossRef]
- Nyadjeu, P.; Ekemeni, R.; Tomedi, M. Growth Perfor-mance, Feed Utilization and Survival of Clarias gariepinus Post-larvae Fed with a Dietary Supplementation of Zingiber officinale-Allium sativum Mixture. J. Aquac. Fish. 2020, 4, 028. [Google Scholar]
- Radhakrishnan, S.; Saravana Bhavan, P.; Seenivasan, C.; Shanthi, R.; Poongodi, R. Influence of medicinal herbs (Alteranthera sessilis, Eclipta alba and Cissus quadrangularis) on growth and biochemical parameters of the freshwater prawn Macrobrachium rosenbergii. Aquac. Int. 2014, 22, 551–572. [Google Scholar] [CrossRef]
- Harada, K. Attraction activities of spices for oriental weatherfish and yellowtail. Bull. Jpn. Soc. Sci. Fish. 1990, 56, 2029–2033. [Google Scholar] [CrossRef][Green Version]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Medl. Res. 2004, 119, 167. [Google Scholar]
- Syahidah, A.; Saad, C.; Daud, H.; Abdelhadi, Y. Status and potential of herbal applications in aquaculture: A review. Iran. J. Fish. Sci 2015, 14, 27–44. [Google Scholar]
- Rajput, N.; Muhammad, N.; Yan, R.; Zhong, X.; Wang, T. Effect of Dietary Supplementation of Curcumin on Growth Performance, Intestinal Morphology and Nutrients Utilization of Broiler Chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar] [CrossRef]
- Corring, T. The adaptation of digestive enzymes to the diet: Its physiological significance. Reprod. Nutr. Dev. 1980, 20, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquac. Rep. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Midhun, S.J.; Arun, D.; Edatt, L.; Sruthi, M.V.; Thushara, V.V.; Oommen, O.V.; Sameer Kumar, V.B.; Divya, L. Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, Tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac. Intl. 2016, 24, 1277–1286. [Google Scholar] [CrossRef]
- Srinivasan, K. Spices as influencers of body metabolism: An overview of three decades of research. Food Res. Int. 2005, 38, 77–86. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z. Maturation of the pancreatic and intestinal digestive functions in sea bass (Dicentrarchus labrax): Effect of weaning with different protein sources. Fish. Physiol. Biochem. 1995, 14, 431–437. [Google Scholar] [CrossRef] [PubMed]
| Feeding Plan | ||||
|---|---|---|---|---|
| Age (DAH) | Rotifers | Artemia Nauplii | Artemia Metanauplii | Inert Diet |
| 3 | 12 | 500 | ||
| 4 | 16 | 500 | ||
| 5 | 16 | 500 | ||
| 6 | 16 | 500 | ||
| 7 | 12 | 500 | ||
| 8 | 13 | 500 | ||
| 9 | 14 | 500 | ||
| 10 | 14 | 0.3 | 500 | |
| 11 | 7 | 0.3 | 500 | |
| 12 | 7 | 0.3 | 500 | |
| 13 | 4 | 0.3 | 500 | |
| 14 | 0.3 | 750 | ||
| 15 | 0.2 | 1000 | ||
| 16 | 0.2 | 1000 | ||
| 17 | 0.2 | 1000 | ||
| 18 | 0.2 | 1200 | ||
| 19 | 0.2 | 1200 | ||
| 20 | 0.2 | 1200 | ||
| 21 | 0.2 | 1500 | ||
| 22 | 0.2 | 1500 | ||
| 23 | 0.2 | 1600 | ||
| 24 | 2000 | |||
| 25 | 2000 | |||
| 26 | 2100 | |||
| 27 | 2200 | |||
| 28 | 2300 | |||
| 29 | 2400 | |||
| 30 | 2600 | |||
| 31 | 2800 | |||
| Treatments | 1-Way Anova | ||||
|---|---|---|---|---|---|
| CTRL | LOW | HIGH | p Value | ||
| DW (mg) | |||||
| 4 DAH | 0.026 ± 0.007 | ||||
| 10 DAH | 0.020 ± 0.005 | 0.020 ± 0.006 | 0.019 ± 0.004 | 0.059 | |
| 24 DAH | 0.143 ± 0.061 | 0.140 ± 0.068 | 0.142 ± 0.059 | 0.973 | |
| 31 DAH | 0.207 ± 0.082 | 0.243 ± 0.102 | 0.221 ± 0.089 | 0.238 | |
| TL (mm) | |||||
| 10 DAH | 3.544 ± 0.264 | 3.593 ± 0.179 | 3.574 ± 0.159 | 0.529 | |
| 24 DAH | 5.504 ± 0.691 | 5.434 ± 0.645 | 5.525 ± 0.659 | 0.795 | |
| 31 DAH | 5.909 ± 0.617 | 6.029 ± 0.566 | 5.943 ± 0.675 | 0.697 | |
| K | |||||
| 10 DAH | 0.219 ± 0.059 | 0.216 ± 0.074 | 0.202 ± 0.028 | 0.583 | |
| 24 DAH | 0.421 ± 0.067 | 0.393 ± 0.073 | 0.402 ± 0.062 | 0.183 | |
| 31 DAH | 0.476 ± 0.081 b | 0.538 ± 0.115 a | 0.517 ± 0.099 ab | 0.016 | |
| RGR (% day−1) | |||||
| 10–24 DAH | 14.904 ± 1.674 | 14.910 ± 2.232 | 15.977 ± 1.033 | 0.694 | |
| 24–31 DAH | 5.807 ± 4.739 | 7.720 ± 4.123 | 5.565 ± 1.98 | 0.784 | |
| 4–31 DAH | 8.069 ± 0.501 | 8.509 ± 0.809 | 8.020 ± 0.896 | 0.698 | |
| Survival (%) | 4–31 DAH | 2.069 ± 0.536 | 2.045 ± 0.691 | 1.539 ± 0.104 | 0.381 |
| Treatments | 1-Way Anova | ||||
|---|---|---|---|---|---|
| CTRL | LOW | HIGH | p Value | ||
| Trypsin (RFU/ mg protein) | |||||
| 10 DAH | 30,459.3 ± 13,867.0 | 31,570.1 ± 14,050.5 | 54,140.9 ± 43,501.8 | 0.158 | |
| 24 DAH | 5337.5 ± 2949.4 ab | 4053.2 ± 2962.1 b | 6722.3 ± 3916.7 a | 0.003 | |
| 31 DAH | 58,992.9 ± 12,290.3 b | 78,515.8 ± 12,833.5 ab | 102,968.8 ± 24,976.4 b | 0.002 | |
| Chymotrypsin (RFU/mg protein) | |||||
| 10 DAH | n.d | n.d | n.d | ||
| 24 DAH | n.d | n.d | n.d | ||
| 31 DAH | 55,218.2 ± 17,427.5 b | 73,100.8 ± 17,260.1 ab | 95,252.2 ± 175,789 a | 0.004 | |
| Aminopeptidase (RFU/mg protein) | |||||
| 10 DAH | n.d | n.d | n.d | ||
| 24 DAH | n.d | n.d | n.d | ||
| 31 DAH | 2806.4 ± 280.9 | 2169.3 ± 594.3 | 2716.4 ± 940.9 | 0.463 | |
| 4C-like lipase (RFU/mg protein) | |||||
| 10 DAH | 15,523.4 ± 3479.0 ab | 18,834.0 ± 2671.4 a | 11,843.0 ± 3328.1b | 0.001 | |
| 24 DAH | 3638.0 ± 1109.1 a | 2852.2 ± 1268.9 b | 2726.1 ± 1031.6 b | 0.001 | |
| 31 DAH | 4297.7 ± 610.7 | 4564.5 ± 1104.4 | 4842.7 ± 436.3 | 0.537 | |
| 18C-like lipase (RFU/mg protein) | |||||
| 10 DAH | n.d | n.d | n.d | ||
| 24 DAH | 67,138.1 ± 11,514.5 a | 42,311.6 ± 15,716.9 b | 38,381.8 ± 9563.8 b | <0.001 | |
| 31 DAH | 26,624.9 ± 2486.3 | 25,552.0 ± 10,114.4 | 26,330.4 ± 5902.6 | 0.966 | |
| Alk phosphatase (RFU/mg protein) | |||||
| 10 DAH | n.d | n.d | n.d | ||
| 24 DAH | 156,951.4 ± 56,199.1 a | 123,276.2 ± 43,477.0 b | 139,037.9 ± 55,263 ab | 0.016 | |
| 31 DAH | 207,951.5 ± 36,001.9 | 237,011.1 ± 53,083.7 | 221,804.8 ± 72,089.3 | 0.705 | |
| Amylase (RFU/mg protein) | |||||
| 10 DAH | n.d | n.d | n.d | ||
| 24 DAH | 78,820.7 ± 38,379.5 | 782,09.6 ± 39,566.6 | 71,996.3 ± 27,531.5 | 0.637 | |
| 31 DAH | 43,469.5 ± 17,852.6 | 28,195.8 ± 7710.2 | 37,535.2 ± 11,410.4 | 0.201 | |
| Larvae Age (Days after Hatching) | 1-Way Anova | ||||
|---|---|---|---|---|---|
| 10 DAH | 24 DAH | 31 DAH | p Value | ||
| GSH (μM/min/mg protein) | |||||
| CTRL | 8.1 ± 3.8 β | 68.6 ± 29.0 α | 68.3 ± 1.9 α | 0.007 | |
| LOW | 17.9 ± 8.1 | 68.6 ± 33.2 | 73.7 ± 5.5 | 0.134 | |
| HIGH | 12.9 ± 1.4 β | 69.1 ± 20.7 α | 67.1 ± 14.5 α | 0.022 | |
| TAC (mM Trolox equivalents/mg protein) | |||||
| CTRL | 687.1 ± 59.1 α | 497.2 ± 36.8 β | 560.3 ± 28.7 β | 0.005 | |
| LOW | 667.1 ± 63.3 α | 512.2 ± 41.8 β | 534.4 ± 57.8 αβ | 0.027 | |
| HIGH | 681.3 ± 63.2 | 539.6 ± 32.9 | 620.2 ± 61.8 | 0.051 | |
| PC (nmol carbonyl/mg protein) | |||||
| CTRL | 27.8 ± 6.9 | 33.1 ± 15.0 | 40.6 ± 36.2 | 0.924 | |
| LOW | 52.9 ± 10.7 | 51.8 ± 29.1 | 58.6 ± 24.3 | 0.927 | |
| HIGH | 23.7 ± 17.5 | 70.2 ± 23.6 | 26.4 ± 13.5 | 0.058 | |
| mtROS (RFU/mg protein) | |||||
| CTRL | 23.2 ± 16.1 β | 172.7 ± 17.8 α | 144.0 ± 25.7 α | 0.000 | |
| LOW | 29.7 ± 7.6 | 116.9 ± 66.4 | 81.0 ± 16.8 | 0.092 | |
| HIGH | 34.8 ± 6.2 | 98.5 ± 21.0 | 119.7 ± 65.2 | 0.065 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, M.J.; Dardengo, G.M.; Navarro-Guillén, C.; Lopes, A.; Colen, R.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S. Dietary Curcumin Promotes Gilthead Seabream Larvae Digestive Capacity and Modulates Oxidative Status. Animals 2021, 11, 1667. https://doi.org/10.3390/ani11061667
Xavier MJ, Dardengo GM, Navarro-Guillén C, Lopes A, Colen R, Valente LMP, Conceição LEC, Engrola S. Dietary Curcumin Promotes Gilthead Seabream Larvae Digestive Capacity and Modulates Oxidative Status. Animals. 2021; 11(6):1667. https://doi.org/10.3390/ani11061667
Chicago/Turabian StyleXavier, Maria J., Gian Marco Dardengo, Carmen Navarro-Guillén, André Lopes, Rita Colen, Luisa M. P. Valente, Luís E. C. Conceição, and Sofia Engrola. 2021. "Dietary Curcumin Promotes Gilthead Seabream Larvae Digestive Capacity and Modulates Oxidative Status" Animals 11, no. 6: 1667. https://doi.org/10.3390/ani11061667
APA StyleXavier, M. J., Dardengo, G. M., Navarro-Guillén, C., Lopes, A., Colen, R., Valente, L. M. P., Conceição, L. E. C., & Engrola, S. (2021). Dietary Curcumin Promotes Gilthead Seabream Larvae Digestive Capacity and Modulates Oxidative Status. Animals, 11(6), 1667. https://doi.org/10.3390/ani11061667







