Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Koalas
2.2. Faecal Sample Collection Regime
2.3. Sample Preparation, Extraction and EIAs
2.4. FCM Variation within Defecations
2.5. Data Analyses
3. Results
3.1. FCM Variation within Defecations
3.2. Overview of FCM Values Measured by EIAs
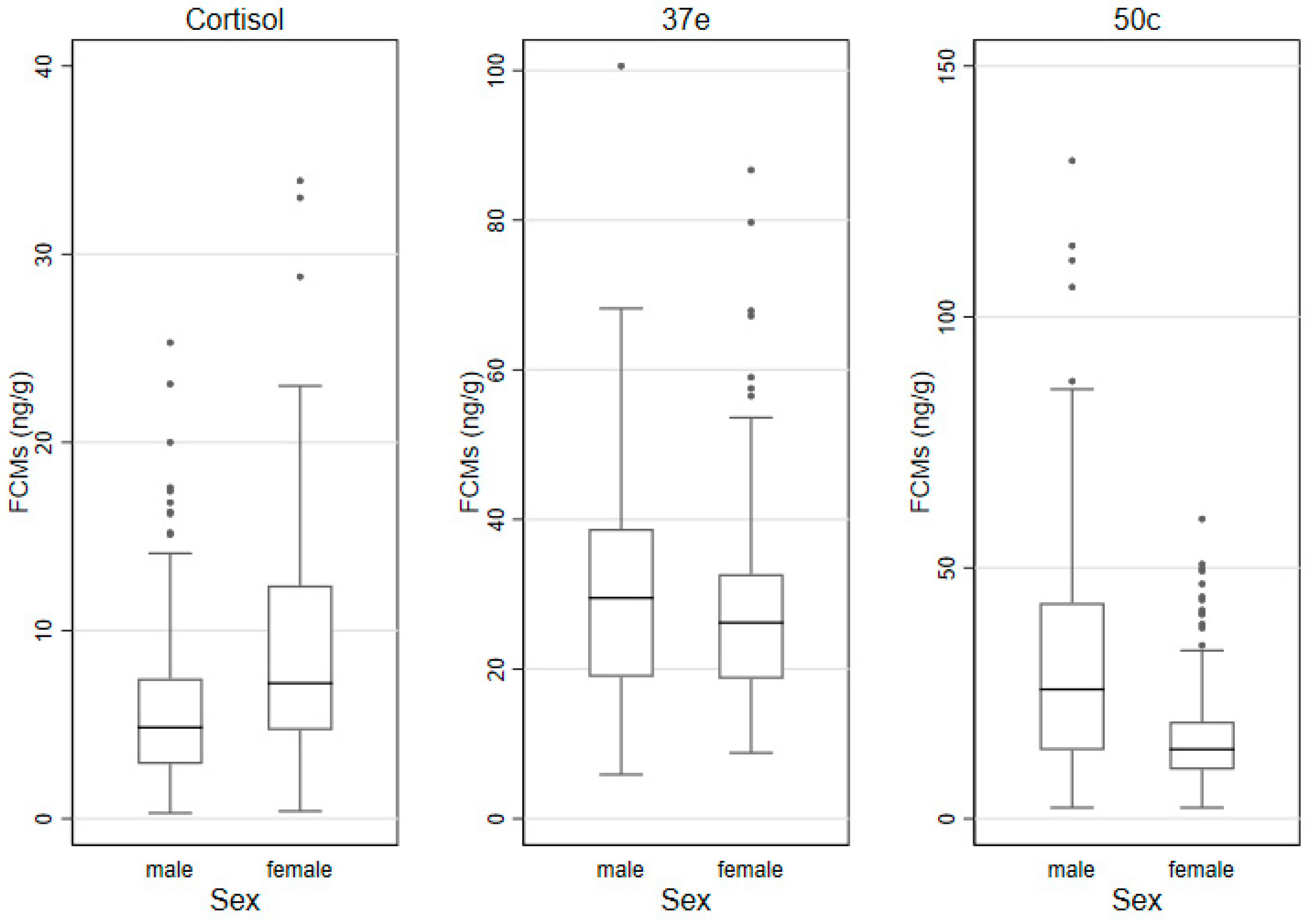
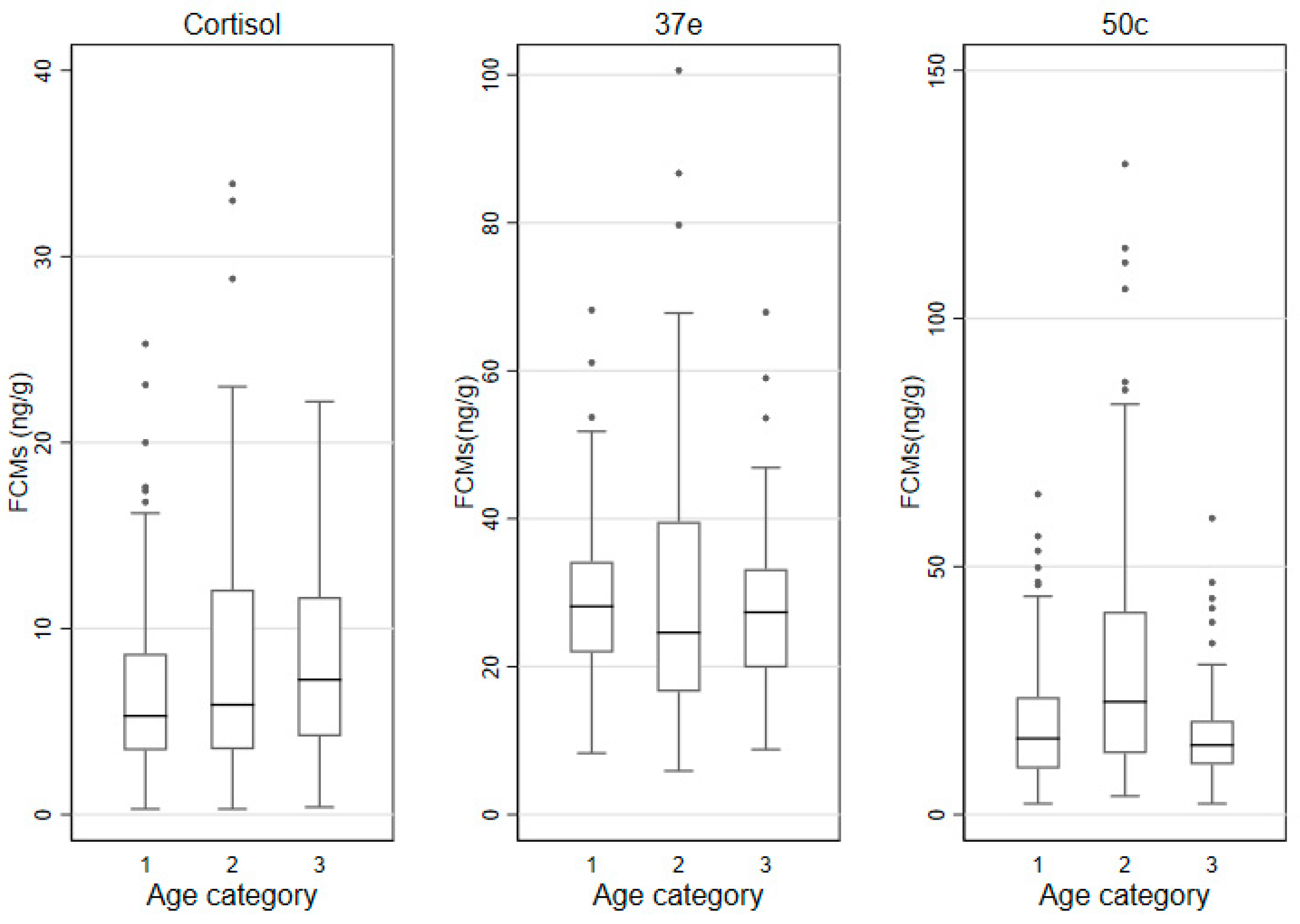
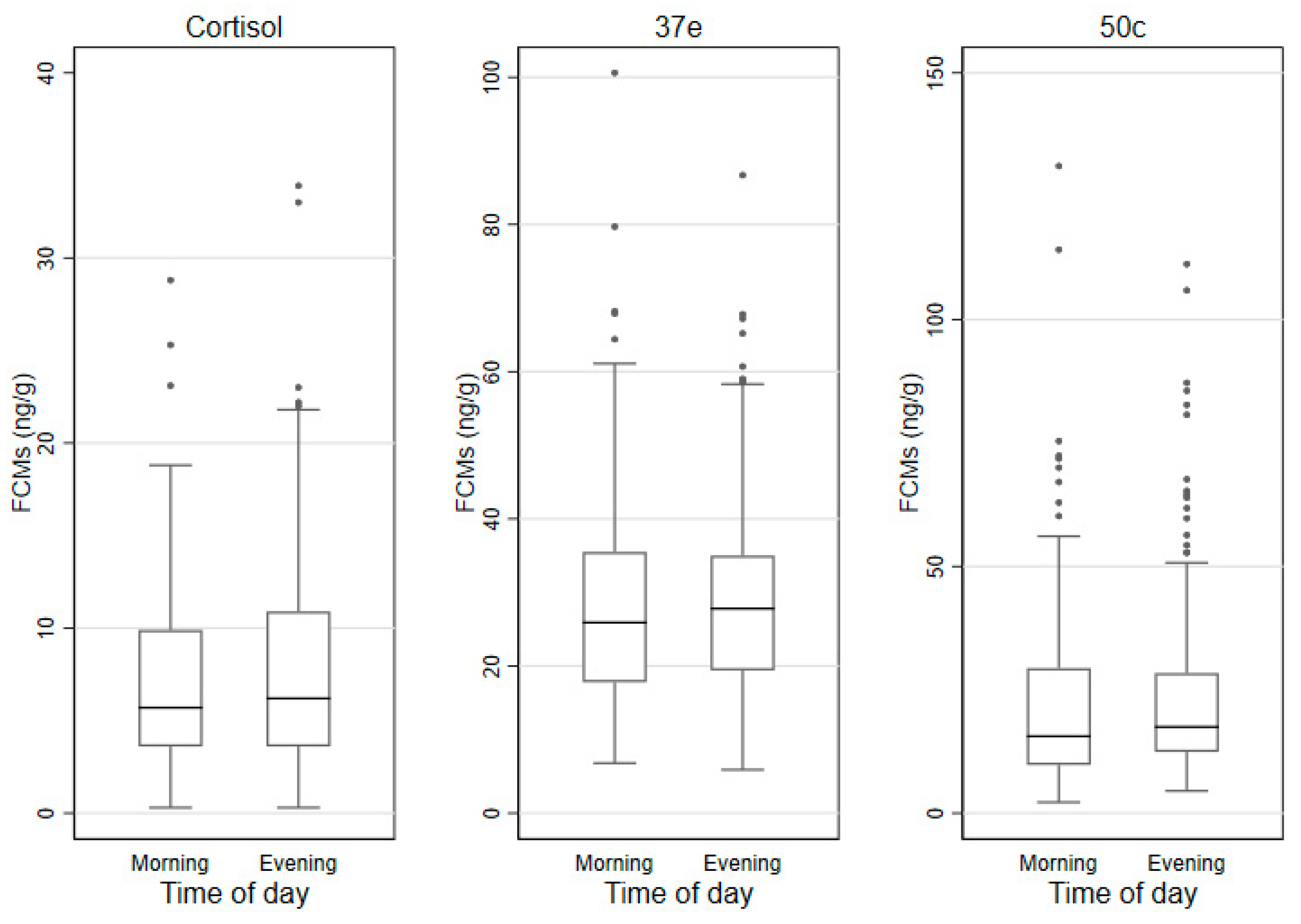
3.3. FCMs by Sex, Age Categories and Time of Sampling
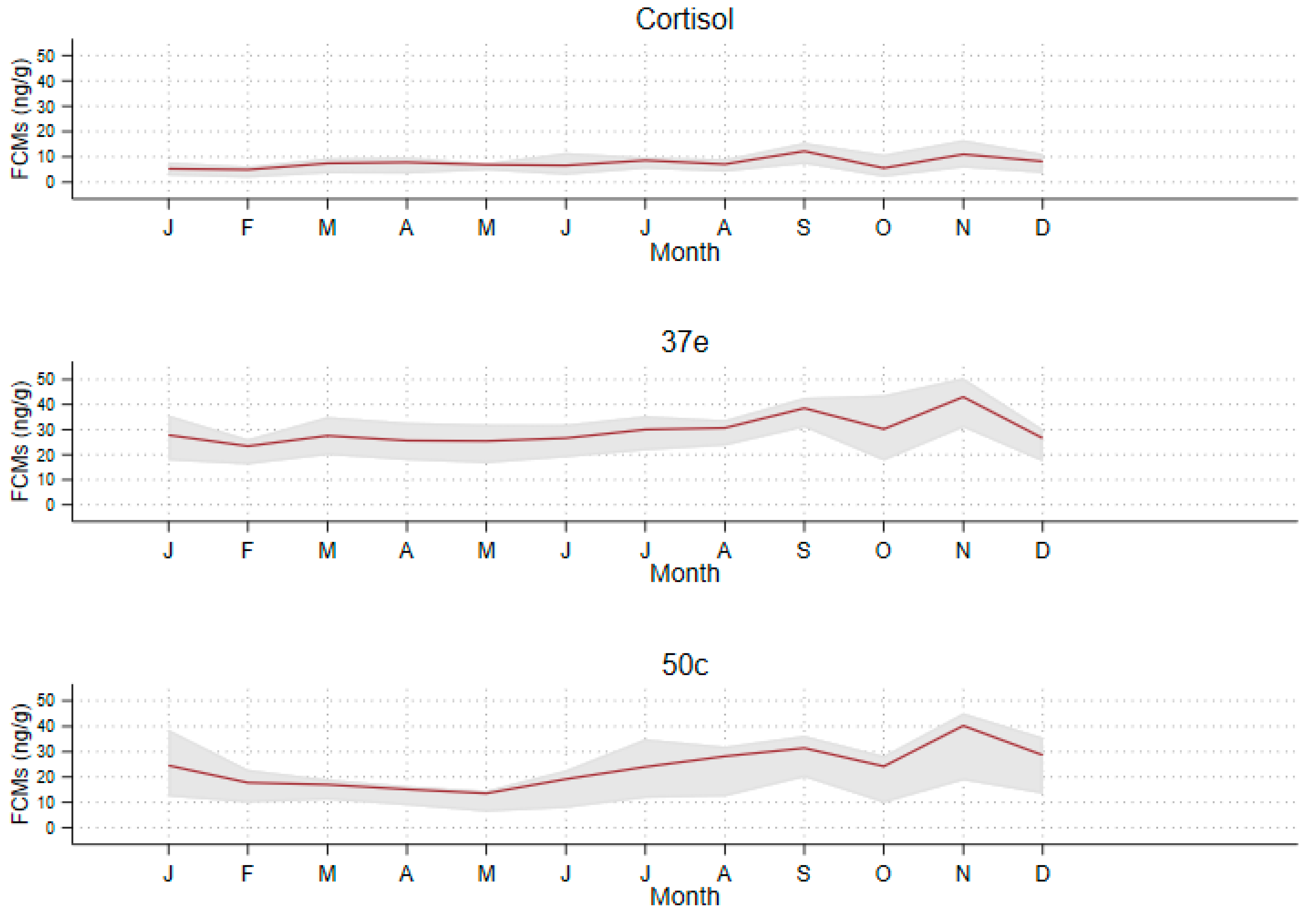
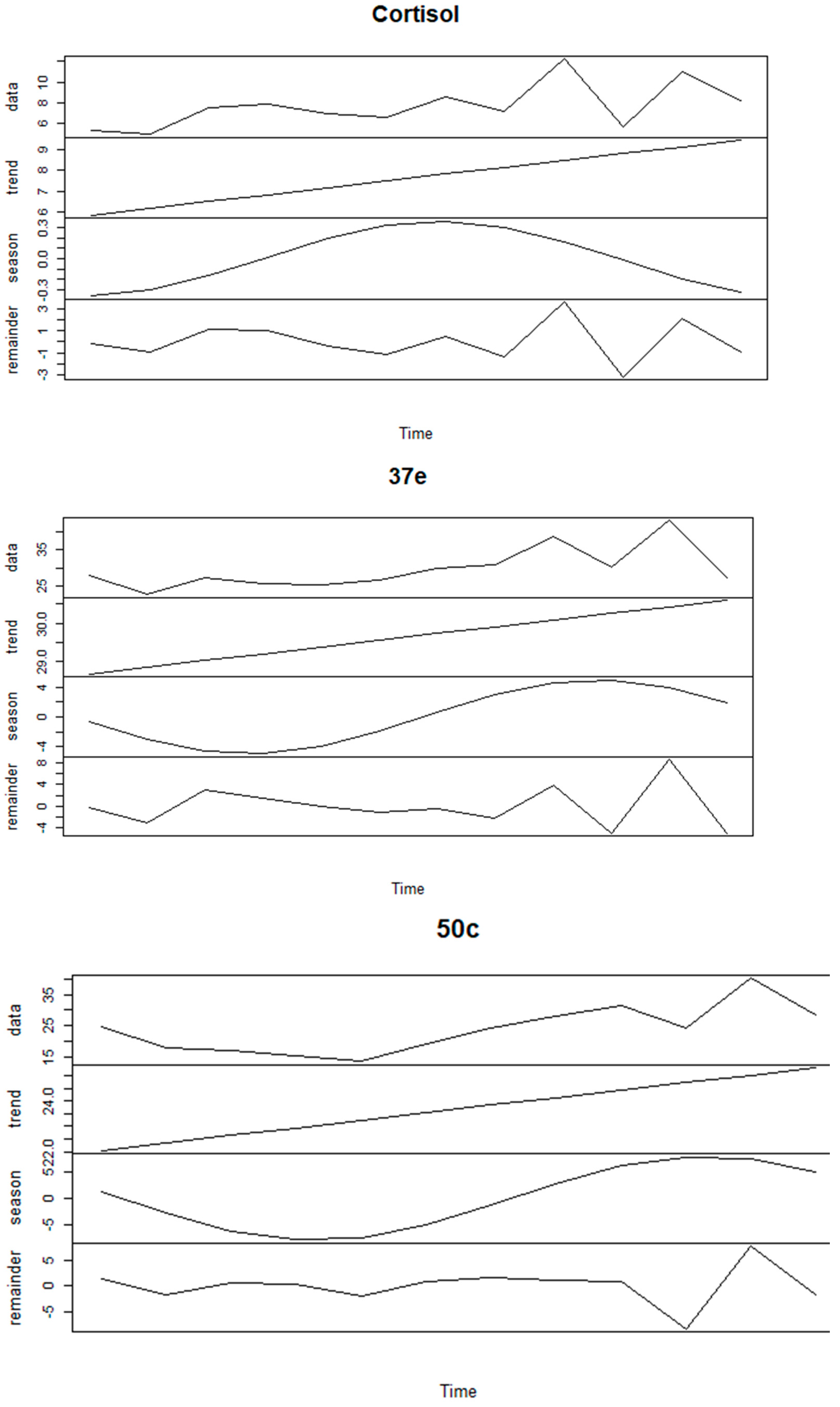
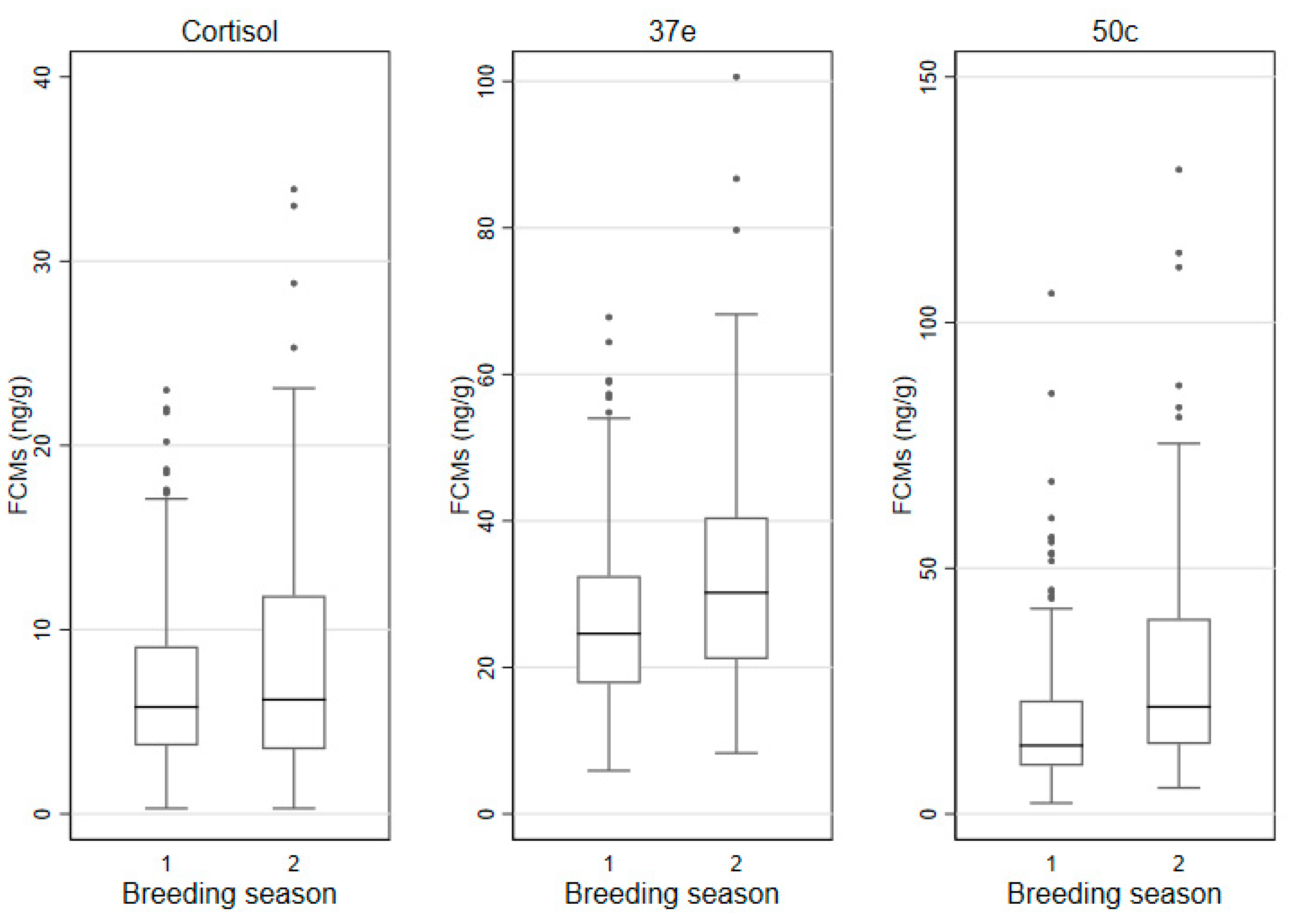
3.4. Seasonality of FCM Values
3.5. Correlation of FCM Values between EIAs
3.6. Factors Affecting FCM Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koala (Phascolarctos cinereus) Listing. Available online: https://www.environment.gov.au/biodiversity/threatened/species/koala (accessed on 11 May 2020).
- Gonzalez-Astudillo, V.; Allavena, R.; McKinnon, A.; Larkin, R.; Henning, J. Decline causes of Koalas in South East Queensland, Australia: A 17-year retrospective study of mortality and morbidity. Sci. Rep. 2017, 7, srep42587. [Google Scholar] [CrossRef]
- Koala Expert Panel Interim Report. Available online: http://www.ehp.qld.gov.au/wildlife/koalas/review-conservation-measures.html (accessed on 2 October 2020).
- Henning, J.; Hannon, C.; McKinnon, A.; Larkin, R.; Allavena, R. The causes and prognoses of different types of fractures in wild koalas submitted to wildlife hospitals. Prev. Veter Med. 2015, 122, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Schlagloth, R.; Santamaria, F.; Golding, B.; Thomson, H. Why is it important to use flagship species in community education? The Koala as a case study. Anim. Stud. J. 2018, 7, 127–148. [Google Scholar]
- Penn, A.M.; Sherwin, W.B.; Gordon, G.; Lunney, D.; Melzer, A.; Lacy, R.C. Demographic Forecasting in Koala Conservation. Conserv. Biol. 2000, 14, 629–638. [Google Scholar] [CrossRef]
- Seabrook, L.; McAlpine, C.; Baxter, G.; Rhodes, J.; Bradley, A.; Lunney, D. Drought-driven change in wildlife distribution and numbers: A case study of koalas in south west Queensland. Wildl. Res. 2011, 38, 509–524. [Google Scholar] [CrossRef]
- Reckless, H.J.; Murray, M.; Crowther, M. A review of climatic change as a determinant of the viability of koala populations. Wildl. Res. 2017, 44, 458–470. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 2041–2049. [Google Scholar] [CrossRef]
- Considine, M.-L. Moving on: Relocating species in response to climate change. ECOS 2011, 160, 11. [Google Scholar]
- Gallagher, R.V.; Makinson, R.O.; Hogbin, P.M.; Hancock, N. Assisted colonization as a climate change adaptation tool. Austral Ecol. 2014, 40, 12–20. [Google Scholar] [CrossRef]
- Minteer, B.A.; Collins, J.P. Move it or lose it? The ecological ethics of relocating species under climate change. Ecol. Appl. 2010, 20, 1801–1804. [Google Scholar] [CrossRef]
- Chipman, R.; Slate, D.; Rupprecht, C.; Mendoza, M. Downside Risk of Wildlife Translocation. Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/1896?utm_source=digitalcommons.unl.edu%2Ficwdm_usdanwrc%2F1896&utm_medium=PDF&utm_campaign=PDFCoverPages (accessed on 10 December 2020).
- Waugh, C.; Hanger, J.; Timms, P.; Polkinghorne, A. Koala translocations and Chlamydia: Managing risk in the effort to conserve native species. Biol. Conserv. 2016, 197, 247–253. [Google Scholar] [CrossRef]
- Boyce, W.M.; Weisenberger, M.E.; Penedo, M.C.T.; Johnson, C.K. Wildlife translocation: The conservation implications of pathogen exposure and genetic heterozygosity. BMC Ecol. 2011, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress and translocation: Alterations in the stress physiology of translocated birds. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2009, 276, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Short, J. The Characteristics and Success of Vertebrate Translocations within Australia, Perth, and Australian Government Department of Agriculture, Fisheries and Forestry; Wildlife Research and Management Pty Ltd: Canberra, Australia, 2009. [Google Scholar]
- Santamaria, F.; Schlagloth, R. The effect of Chlamydia on translocated Chlamydia-naïve koalas: A case study. Aust. Zool. 2016, 38, 192–202. [Google Scholar] [CrossRef]
- McAlpine, C. Relationships between human-induced habitat disturbance, stressors and disease in Koala. In Proceedings of the Koala Research Network Disease Workshop, Queensland University of Technology, Brisbane, Australia, 19 September 2011. [Google Scholar]
- Brearley, G.; Rhodes, J.; Bradley, A.; Baxter, G.; Seabrook, L.; Lunney, D.; Liu, Y.; McAlpine, C. Wildlife disease prevalence in human-modified landscapes. Biol. Rev. 2012, 88, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. International Textbook of Obesity; Wiley: Chichester, UK, 2001. [Google Scholar]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress Hormones in Mammals and Birds: Comparative Aspects Regarding Metabolism, Excretion, and Noninvasive Measurement in Fecal Samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring Fecal Glucocorticoid Metabolites in Mammals and Birds: The Importance of Validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA axis-rhythms. Compr. Physiol. 2011, 4, 1273–1298. [Google Scholar]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, E.D.; Fukushima, D.; Nogeire, C.; Roffwarg, H.; Gallagher, T.F.; Hellman, L. Twenty-four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J. Clin. Endocrinol. Metab. 1971, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda, L.; Kowarski, A.; Migeon, C.J. Integrated Concentration and Diurnal Variation of Plasma Cortisol1. J. Clin. Endocrinol. Metab. 1973, 36, 227–238. [Google Scholar] [CrossRef]
- Hunninck, L.; May, R.; Jackson, C.R.; Palme, R.; Røskaft, E.; Sheriff, M.J. Consequences of climate-induced vegetation changes exceed those of human disturbance for wild impala in the Serengeti ecosystem. Conserv. Physiol. 2020, 8, coz117. [Google Scholar] [CrossRef] [PubMed]
- Merl, S.; Scherzer, S.; Palme, R.; Möstl, E. Pain causes increased concentrations of glucocorticoid metabolites in horse feces. J. Equine Veter Sci. 2000, 20, 586–590. [Google Scholar] [CrossRef]
- Fureix, C.; Benhajali, H.; Henry, S.; Bruchet, A.; Prunier, A.; Ezzaouïa, M.; Coste, C.; Hausberger, M.; Palme, R.; Jego, P. Plasma cortisol and faecal cortisol metabolites concentrations in stereotypic and non-stereotypic horses: Do stereotypic horses cope better with poor environmental conditions? BMC Veter Res. 2013, 9, 3. [Google Scholar] [CrossRef]
- Dalerum, F.; Ganswindt, A.; Palme, R.; Bettega, C.; Delgado, M.D.M.; Dehnhard, M.; Freire, S.; González, R.G.; Marcos, J.; Miranda, M.; et al. Methodological Considerations for Using Fecal Glucocorticoid Metabolite Concentrations as an Indicator of Physiological Stress in the Brown Bear (Ursus arctos). Physiol. Biochem. Zool. 2020, 93, 227–234. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Palme, R.; Robia, C.; Messmann, S.; Hofer, J.; Möstl, E. Measurement of faecal cortisol metabolites in ruminants: A non-invasive parameter of adrenocortical function. Wien. Tierärztliche Mon. 1999, 86, 237–241. [Google Scholar]
- Palme, R. Measuring Fecal Steroids: Guidelines for Practical Application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Hogan, L.A.; Lisle, A.T.; Johnston, S.D.; Robertson, H. Non-invasive assessment of stress in captive numbats, Myrmecobius fasciatus (Mammalia: Marsupialia), using faecal cortisol measurement. Gen. Comp. Endocrinol. 2012, 179, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.J.; Evans, N.; Hero, J.-M. Monitoring physiological stress in semi-free ranging populations of an endangered Australian marsupial, the Greater Bilby (Macrotis lagotis). Eur. J. Wildl. Res. 2014, 60, 727–735. [Google Scholar] [CrossRef]
- Davies, N.; Gillett, A.; McAlpine, C.; Seabrook, L.; Baxter, G.; Lunney, D.; Bradley, A. The effect of ACTH upon faecal glucocorticoid excretion in the koala. J. Endocrinol. 2013, 219, 1–12. [Google Scholar] [CrossRef]
- Narayan, E.J.; Webster, K.; Nicolson, V.; Mucci, A.; Hero, J.-M. Non-invasive evaluation of physiological stress in an iconic Australian marsupial: The Koala (Phascolarctos cinereus). Gen. Comp. Endocrinol. 2013, 187, 39–47. [Google Scholar] [CrossRef]
- Weiss, M.; Richards, P.G. Adrenal Steroid Secretion in The Koala (Phascolarctos cinereus). J. Endocrinol. 1970, 48, 145–146. [Google Scholar] [CrossRef]
- McDonald, I.; Than, K.; Handasyde, K.; Michaelides, J. Factors Affecting Plasma Adrenocortical Hormone Concentration of Koalas. In Biology of the Koala; Lee, A.K., Handasyde, K.A., Sanson, G.D., Eds.; Surrey Beatty and Sons: Sydney, Australia, 1990; pp. 289–294. [Google Scholar]
- Johnston, S.; Booth, R.; Pyne, M.; Keeley, T.; Mackie, J.; Hulse, L.; Ellis, W. Preliminary study of faecal cortisol and corticosterone as an index of acute cortisol secretion in the koala (Phascolarctos cinereus). Aust. Veter J. 2013, 91, 534–537. [Google Scholar] [CrossRef]
- Keeley, T.; O’Brien, J.; Fanson, B.; Masters, K.; McGreevy, P. The reproductive cycle of the Tasmanian devil (Sarcophilus harrisii) and factors associated with reproductive success in captivity. Gen. Comp. Endocrinol. 2012, 176, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Fanson, K.V.; Best, E.C.; Bunce, A.; Fanson, B.G.; Hogan, L.A.; Keeley, T.; Narayan, E.; Palme, R.; Parrott, M.L.; Sharp, T.M.; et al. One size does not fit all: Monitoring faecal glucocorticoid metabolites in marsupials. Gen. Comp. Endocrinol. 2017, 244, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, F.; Barlow, C.K.; Schlagloth, R.; Schittenhelm, R.B.; Palme, R.; Henning, J. Identification of koala (Phascolarctos cinereus) faecal cortisol metabolites using liquid chromatography-mass spectrometry and enzyme immunoassays. Metabolites 2021, 11, 393. [Google Scholar] [CrossRef]
- Quillfeldt, P.; Möstl, E. Resource allocation in Wilson’s storm-petrels Oceanites oceanicus determined by measurement of glucocorticoid excretion. Acta Ethol. 2003, 5, 115–122. [Google Scholar] [CrossRef]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierärztliche Mon. 2013, 100, 238–246. [Google Scholar]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Z. Säugetierkunde Int. J. Mamm. Biol. 1997, 62, 192–197. [Google Scholar]
- Ian, D.; Wayne, M.; Stryhyn, H. Veterinary Epidemiologic Research, 2nd ed.; Charlotte, P.E.I., Ed.; VER Inc.: Phoenix, AZ, USA, 2009. [Google Scholar]
- Rob, J.H. Seasonal Decomposition of Short Time Series. Available online: https://robjhyndman.com/hyndsight/tslm-decomposition/ (accessed on 5 February 2021).
- Cheung, Y.-W.; Lai, K.S. Lag order and critical values of the augmented Dickey-Fuller test. J. Bus. Econ. Stat. 1995, 13, 277–280. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dupoué, A.; Rutschmann, A.; Le Galliard, J.F.; Clobert, J.; Blaimont, P.; Sinervo, B.; Miles, D.B.; Haussy, C.; Meylan, S. Reduction in baseline corticosterone secretion correlates with climate warming and drying across wild lizard populations. J. Anim. Ecol. 2018, 87, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.M.; Adams, A.G.; Invik, R.M.; Wynne-Edwards, K.; Smits, J. Hair as a Meaningful Measure of Baseline Cortisol Levels over Time in Dogs. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 189–196. [Google Scholar]
- Davies, N.A.; Gramotnev, G.; McAlpine, C.; Seabrook, L.; Baxter, G.; Lunney, D.; Rhodes, J.R.; Bradley, A. Physiological Stress in Koala Populations near the Arid Edge of Their Distribution. PLoS ONE 2013, 8, e79136. [Google Scholar] [CrossRef]
- Narayan, E. Physiological stress levels in wild koala sub-populations facing anthropogenic induced environmental trauma and disease. Sci. Rep. 2019, 9, 6031. [Google Scholar] [CrossRef]
- Hogan, L.A.; Johnston, S.D.; Lisle, A.T.; Keeley, T.; Wong, P.; Nicolson, V.; Horsup, A.B.; Janssen, T.; Phillips, C.J. Behavioural and physiological responses of captive wombats (Lasiorhinus latifrons) to regular handling by humans. Appl. Anim. Behav. Sci. 2011, 134, 217–228. [Google Scholar] [CrossRef]
- Flanagan, C. Koala Rehabilitation Manual; Koala Preservation Society Austaralia: Port Macquarie, NSW, Australia, 2015. [Google Scholar]
- Noti, M.; Sidler, D.; Brunner, T. Extra-adrenal glucocorticoid synthesis in the intestinal epithelium: More than a drop in the ocean? Semin. Immunopathol. 2009, 31, 237–248. [Google Scholar] [CrossRef]
- McKenzie, S.; Deane, E.M. The effects of age, season, and gender on serum cortisol levels in the tammar wallaby, Macropus eugenii. Gen. Comp. Endocrinol. 2003, 133, 273–278. [Google Scholar] [CrossRef]
- Blas, J.; Baos, R.; Bortolotti, G.R.; Marchant, T.A.; Hiraldo, F. Age-related variation in the adrenocortical response to stress in nestling white storks (Ciconia ciconia) supports the developmental hypothesis. Gen. Comp. Endocrinol. 2006, 148, 172–180. [Google Scholar] [CrossRef]
- Smith, B.; Flavel, M.; Simpson, B. Quantification of salivary cortisol from captive dingoes (Canis dingo) in relation to age, sex, and breeding season: Implications for captive management. Aust. Mammal. 2016, 38, 21. [Google Scholar] [CrossRef]
- Edwards, P.D.; Boonstra, R. Glucocorticoids and CBG during pregnancy in mammals: Diversity, pattern, and function. Gen. Comp. Endocrinol. 2018, 259, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.-I.; Tsukamura, H. The Impact of Stress on Reproduction: Are Glucocorticoids Inhibitory or Protective to Gonadotropin Secretion? Endocrinology 2006, 147, 1085–1086. [Google Scholar] [CrossRef]
- Rhodes, J.R.; Beyer, H.; Preece, H.; McAlpine, C. South East Queensland Koala Population Modelling Study; UniQuest: Brisbane, Australia, 2015. [Google Scholar]
- Ballantyne, K.; Lisle, A.; Mucci, A.; Johnston, S.D. Seasonal oestrous cycle activity of captive female koalas in south-east Queensland. Aust. Mammal. 2015, 37, 245. [Google Scholar] [CrossRef]
- Cristescu, R.H.; Goethals, K.; Banks, P.B.; Carrick, F.N.; Frere, C. Experimental Evaluation of Koala Scat Persistence and Detectability with Implications for Pellet-Based Fauna Census. Int. J. Zool. 2012, 2012, 631856. [Google Scholar] [CrossRef]


| EIA Code | Details | Description |
|---|---|---|
| Cortisol | Standard | 4-pregnene-11β,17α,21-triol-3,20-dione(cortisol) |
| Targeted structure | 11β,17α,21-triol-20-one | |
| Antibody against | cortisol-3-CMO:BSA | |
| Label | cortisol-3-CMO-DADOO-biotin | |
| Reference | Palme and Möstl [51] | |
| 37e | Standard | 5α-pregnane-3β,11β,21-triol-20-one (3β-allotetrahydrocorticosterone) |
| Targeted structure | 5α-3ß,11ß-diol | |
| Antibody against | 5α-pregnane-3β,11β,21-triol-20-one-CMO-BSA | |
| Label | 5α-pregnane-3β,11β,21-triol-20-one-CMO-biotinyl-LC | |
| Reference | Touma et al. [49] | |
| 50c | Standard | 5β-pregnane-3α,11β,21-triol-20-one(tetrahydrocorticosterone) |
| Targeted structure | 5β-3α,11β-diol | |
| Antibody against | 5β-pregnane-3α,11β,21-triol-20-one-CMO-BSA | |
| Label | 5β-pregnane-3α,11β,21-triol-20-one-21-HS-biotinyl-LC | |
| Reference | Quillfeldt and Möstl [48] |
| EIA | Mean | Median | p25 | p75 | IQR | Min | Max |
|---|---|---|---|---|---|---|---|
| Cortisol | 7.6 | 5.9 | 3.6 | 10.6 | 7.0 | 0.3 | 33.9 |
| 37e | 29.3 | 27.3 | 18.8 | 35.3 | 16.5 | 5.9 | 100.6 |
| 50c | 23.3 | 16.5 | 11.1 | 29.4 | 18.3 | 2.2 | 131.1 |
| Variable | Sub-Groups | FCM (ng/g) | ||
|---|---|---|---|---|
| Cortisol EIA | 37e EIA | 50c EIA | ||
| Sex | Male | 5.9 (4.6) | 31.0 (19.8) | 31.1 (29.4) |
| Female | 9.0 (7.7) | 27.9 (14.0) | 16.4 (9.6) | |
| Age | ≤2 years | 6.5 (5.2) | 28.8 (12.3) | 18.4 (14.4) |
| >2–5 years | 8.0 (8.6) | 30.2 (23.0) | 29.9 (28.6) | |
| >5 years | 8.5 (7.5) | 27.8 (13.6) | 16.6 (8.9) | |
| Time | Evening | 8.1 (7.3) | 29.5 (15.6) | 24. 1 (16.0) |
| of day | Morning | 7.1 (6.3) | 29.1 (17.7) | 22.5 (19.6) |
| EIA | Mean | Median | p25 | p75 | IQR |
|---|---|---|---|---|---|
| Non-breeding (February–August) | |||||
| Cortisol | 7.0 | 5.8 | 3.7 | 9.1 | 5.4 |
| 37e | 26.8 | 24.6 | 17.8 | 32.5 | 14.7 |
| 50c | 18.7 | 13.9 | 9.8 | 23.1 | 13.3 |
| Breeding (September–January) | |||||
| Cortisol | 8.2 | 6.2 | 3.5 | 11.9 | 8.4 |
| 37e | 32.7 | 30.2 | 21.1 | 40.5 | 19.4 |
| 50c | 29.2 | 21.8 | 14.2 | 39.7 | 25.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaria, F.; Palme, R.; Schlagloth, R.; Klobetz-Rassam, E.; Henning, J. Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland. Animals 2021, 11, 1622. https://doi.org/10.3390/ani11061622
Santamaria F, Palme R, Schlagloth R, Klobetz-Rassam E, Henning J. Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland. Animals. 2021; 11(6):1622. https://doi.org/10.3390/ani11061622
Chicago/Turabian StyleSantamaria, Flavia, Rupert Palme, Rolf Schlagloth, Edith Klobetz-Rassam, and Joerg Henning. 2021. "Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland" Animals 11, no. 6: 1622. https://doi.org/10.3390/ani11061622
APA StyleSantamaria, F., Palme, R., Schlagloth, R., Klobetz-Rassam, E., & Henning, J. (2021). Seasonal Variations of Faecal Cortisol Metabolites in Koalas in South East Queensland. Animals, 11(6), 1622. https://doi.org/10.3390/ani11061622







