Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PMECs Isolation, Cell Culture, and Treatments
2.2. Cell Viability Assay and Flow Cytometric Analysis
2.3. Real-Time PCR
2.4. Measurement of Inflammatory Factor Levels
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
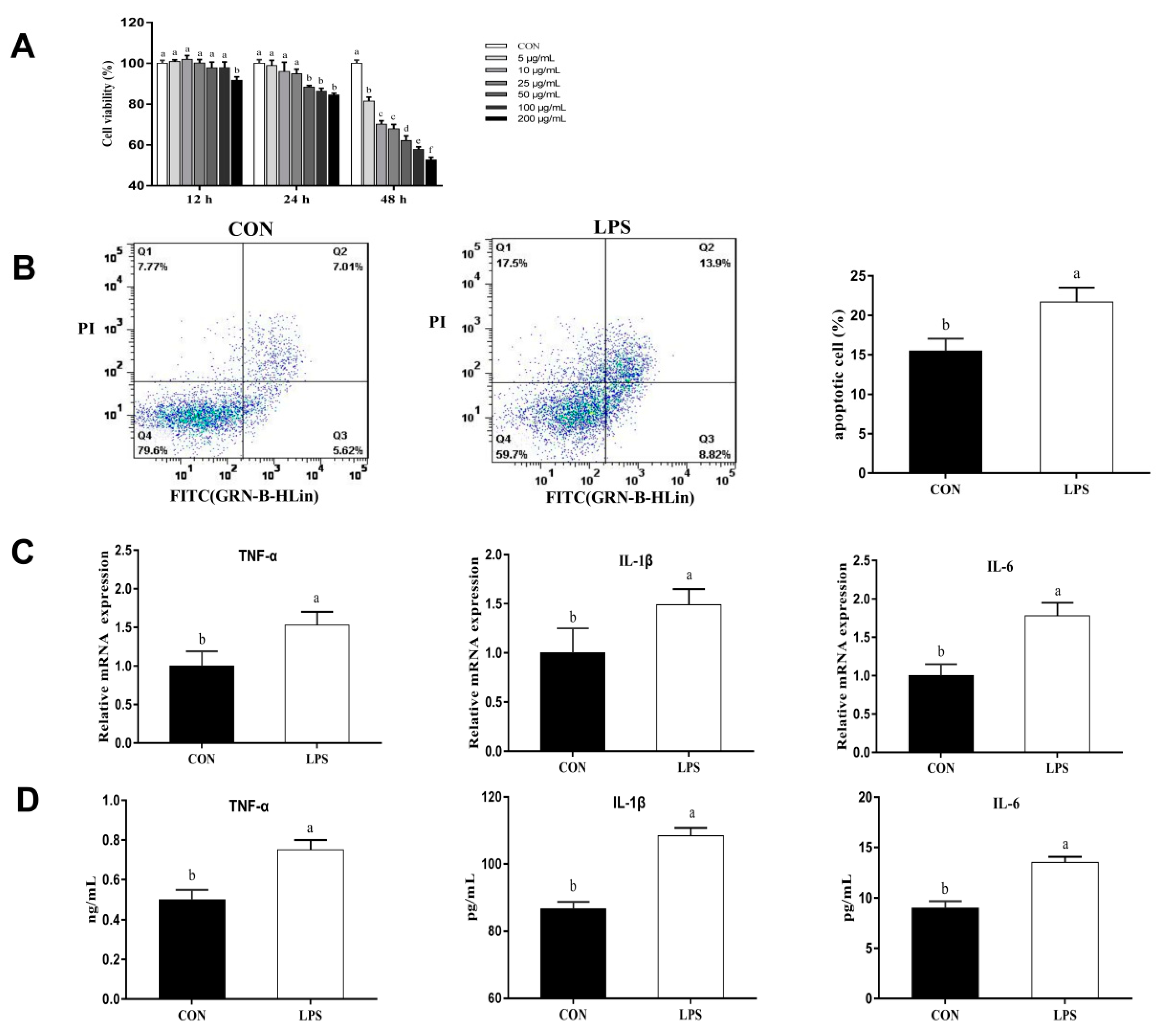
3.1. Inflammatory Injury in LPS-Induced PMECs
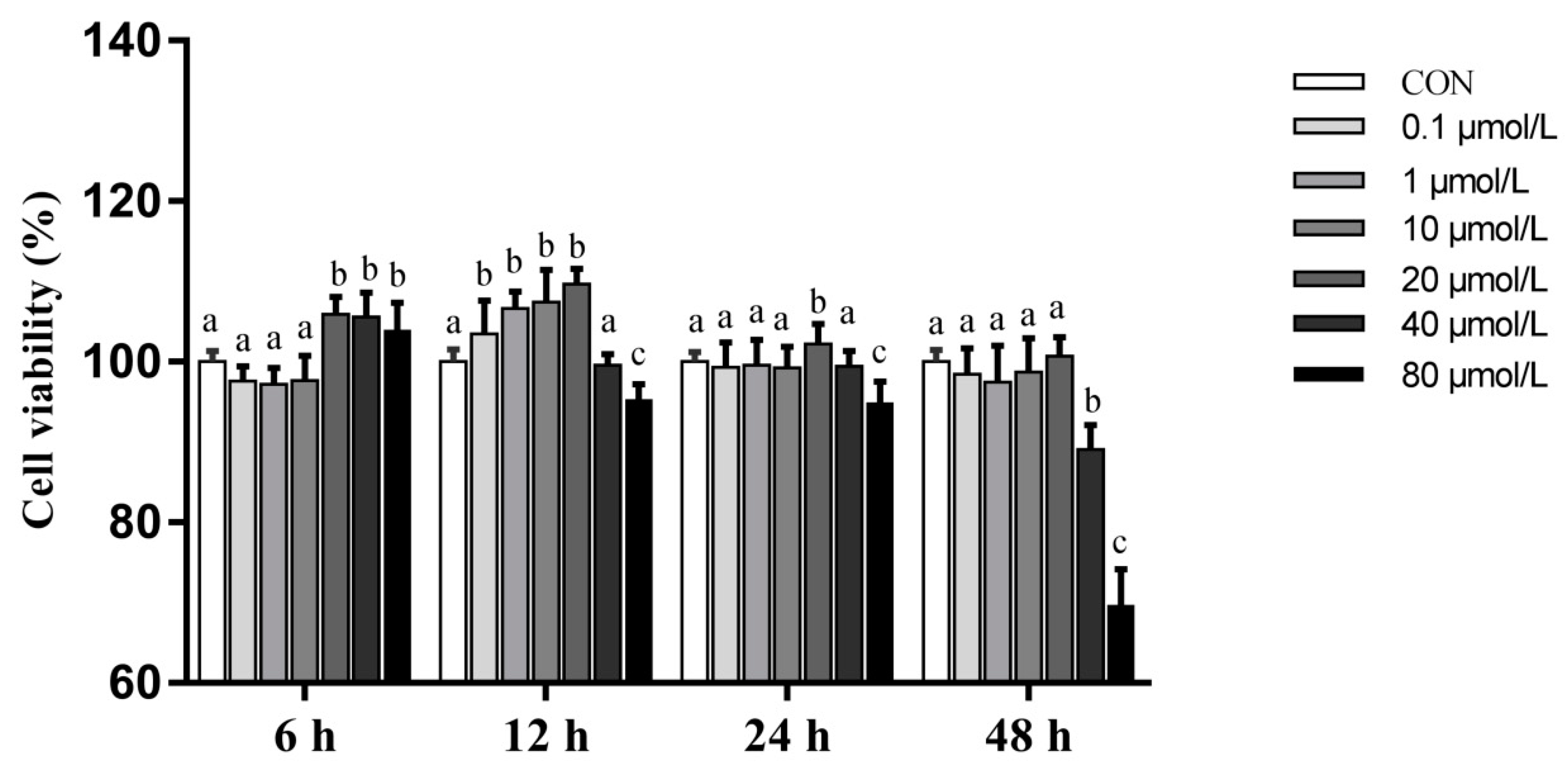
3.2. Effects of Artemisinin on the Viability of PMECs
3.3. Effects of Artemisinin on Viability and Apoptosis in LPS-Induced PMECs
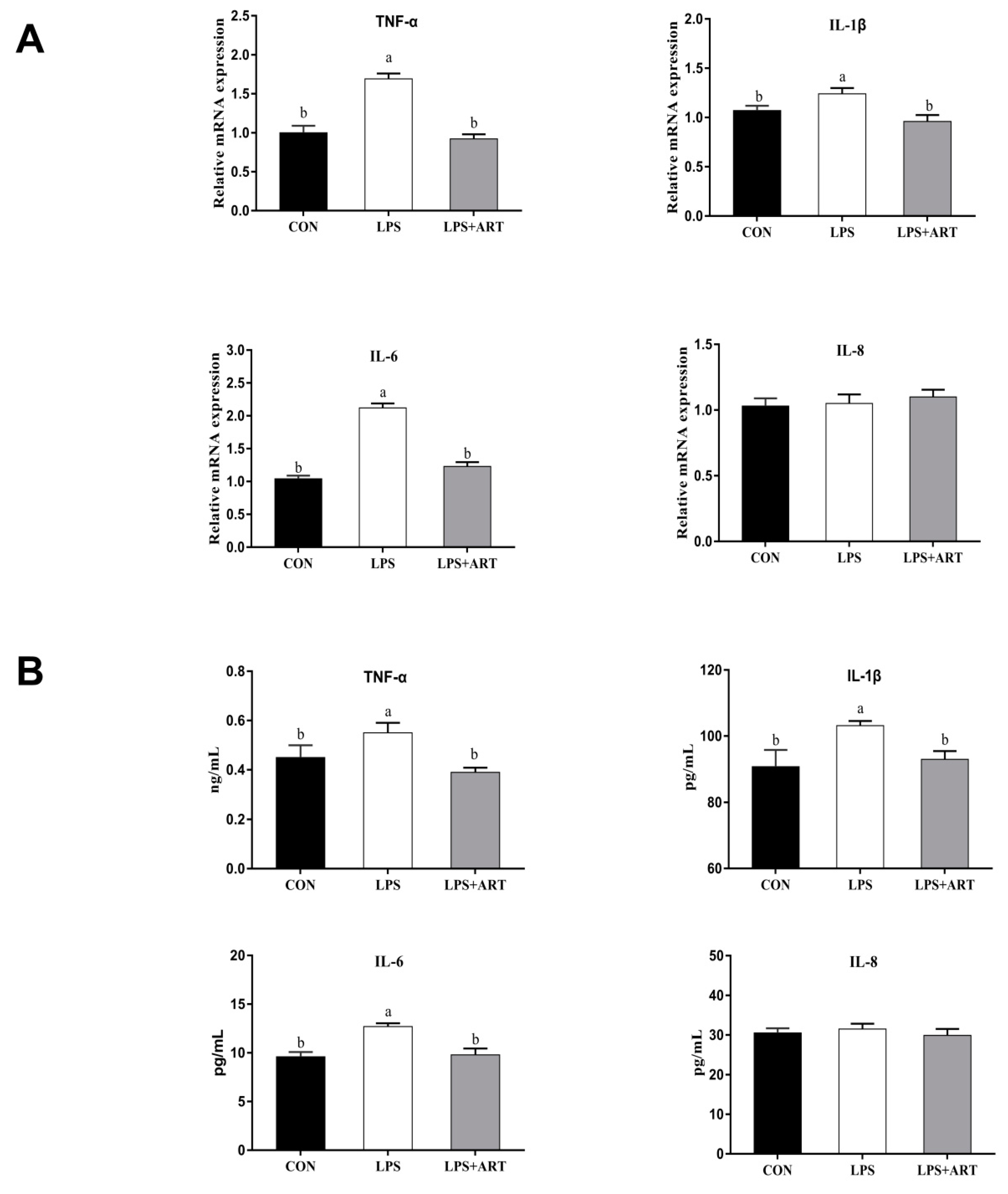
3.4. Effects of Artemisinin on LPS-Induced Inflammatory Factors
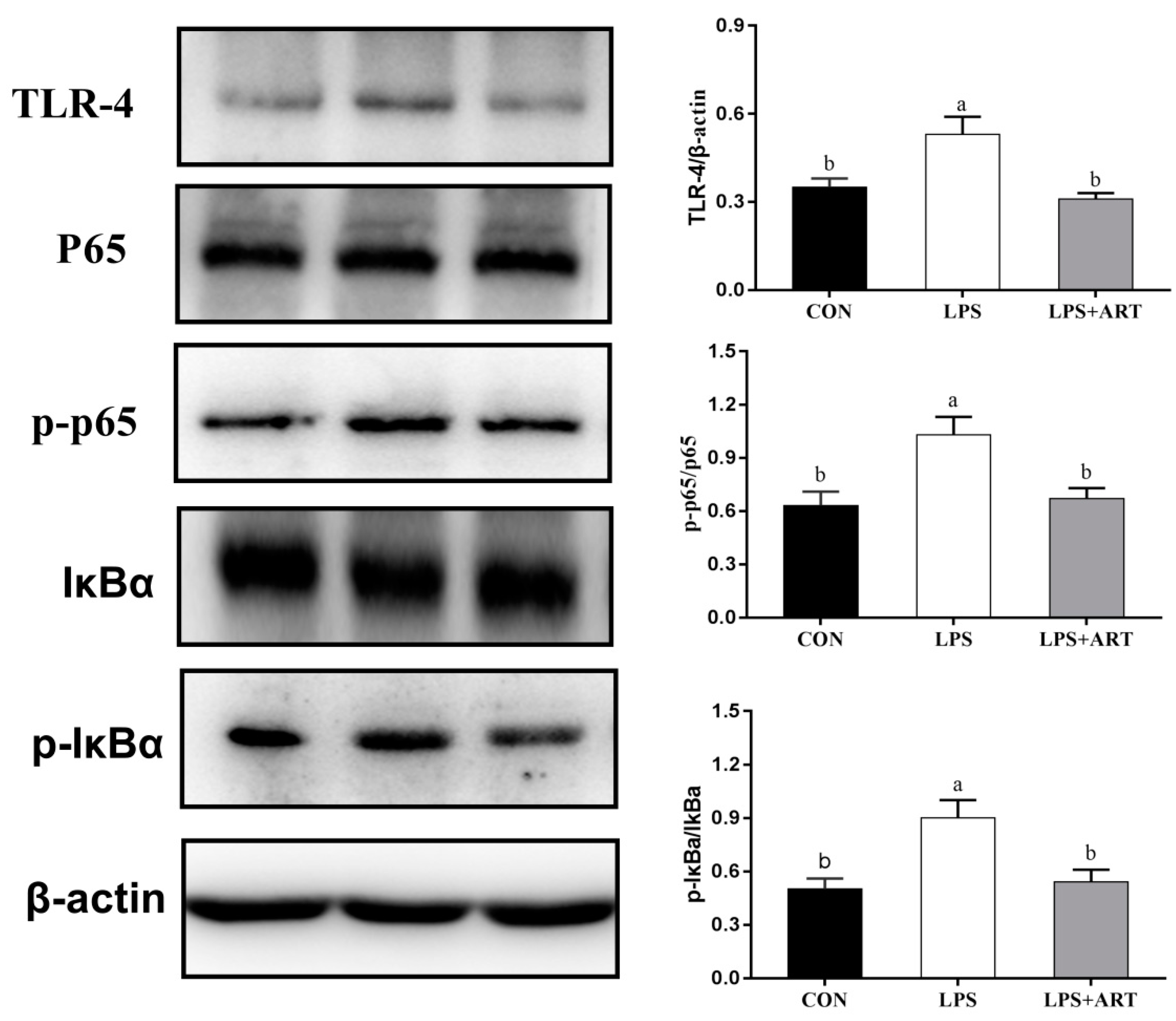
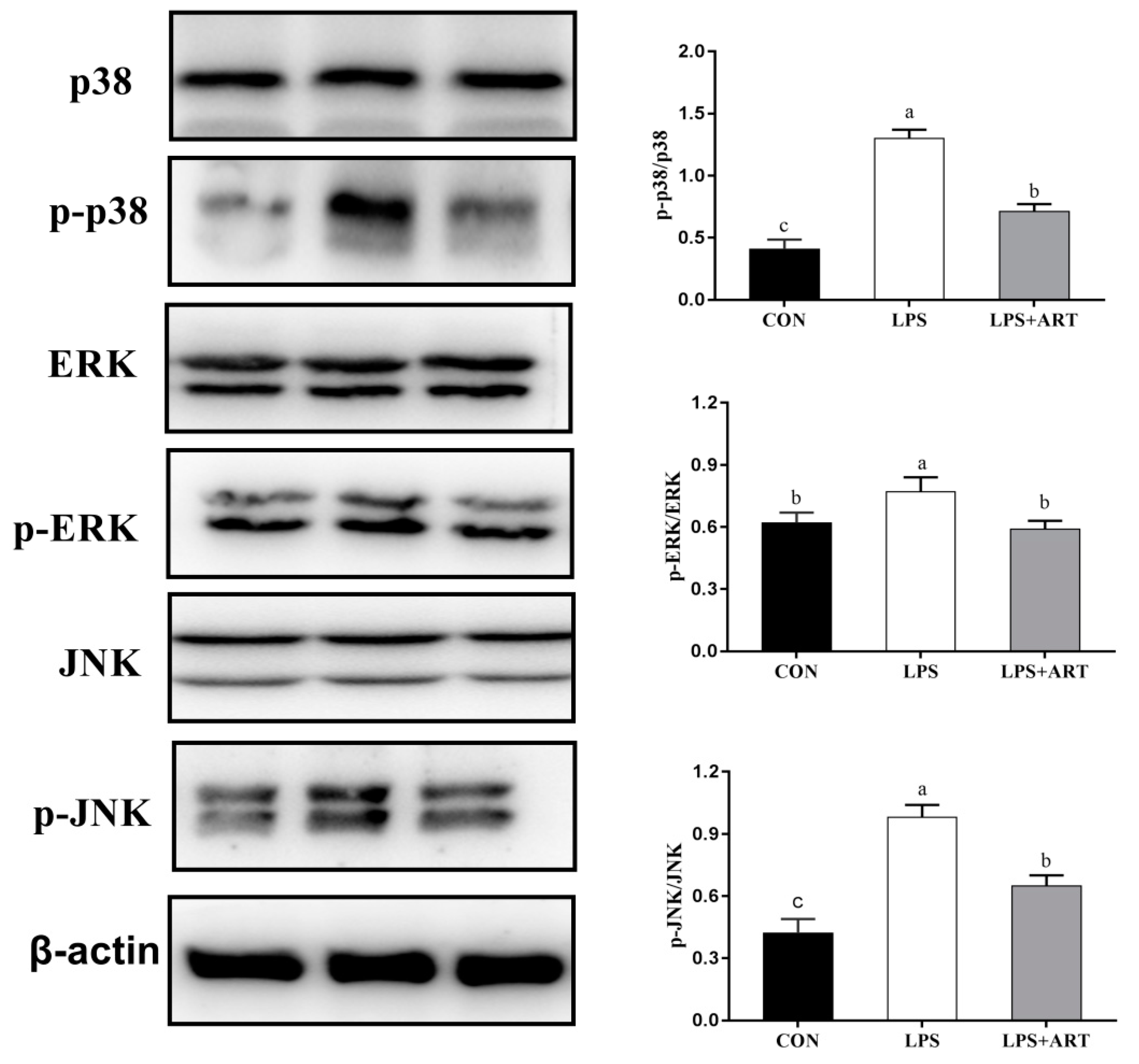
3.5. Effects of Artemisinin on the NF-κB and MAPK Signaling Pathways in LPS-Induced PMECs
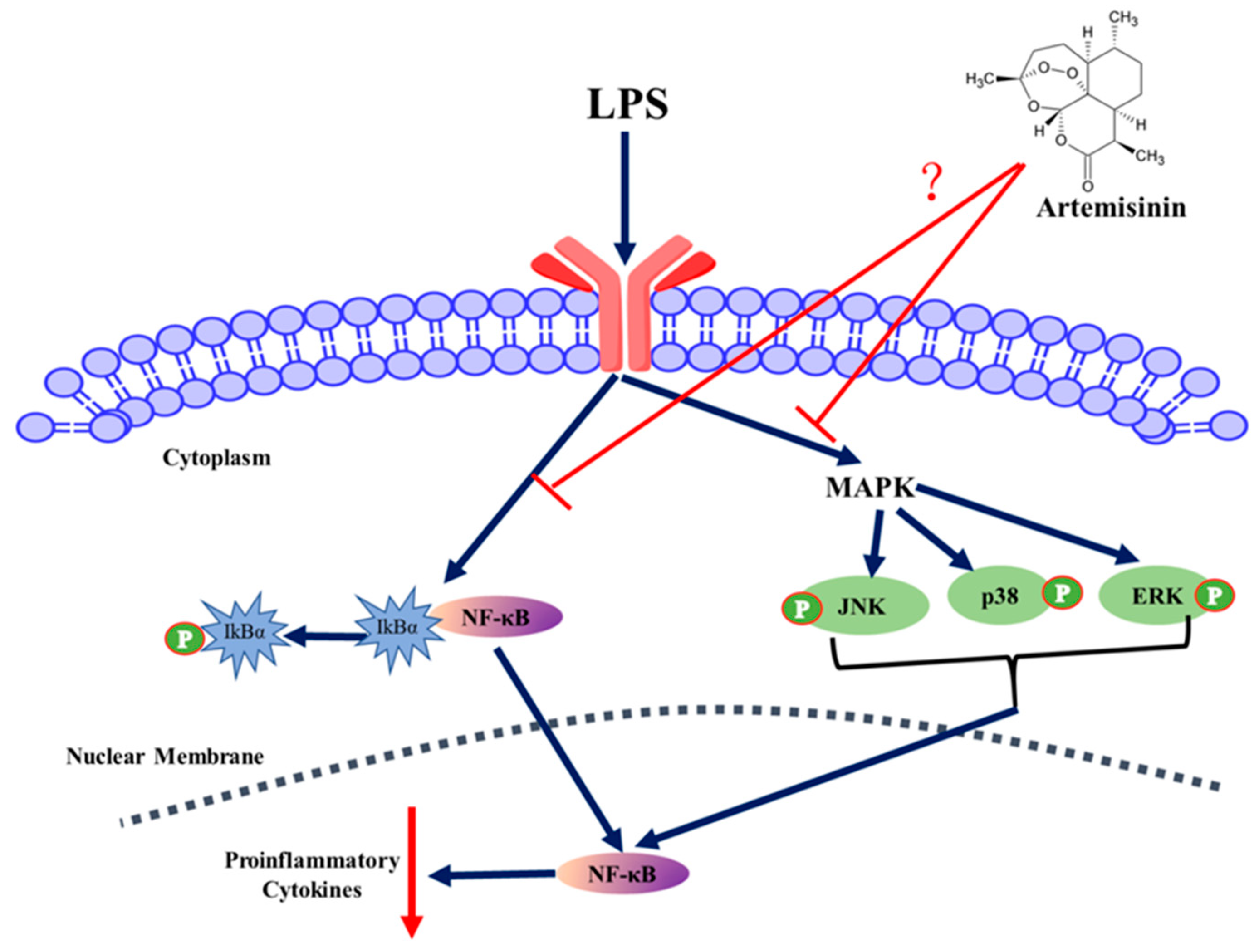
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Ellis, R.P.; Magnuson, R.J. Escherichia coli isolates’ serotypes, genotypes, and virulence genes and clinical coliform mastitis severity. J. Dairy Sci. 2006, 89, 3408–3412. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Tian, M.; Li, L.; Guan, W. Recent progress of porcine milk components and mammary gland function. J. Anim. Sci. Biotechnol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Wu, Z.; Heng, J.; Tian, M.; Song, H.; Zhang, S. Amino acid transportation, sensing and signal transduction in the mammary gland: Key molecular signaling pathways in the regulation of milk synthesis. Nutr. Res. Rev. 2020, 33, 287–297. [Google Scholar] [CrossRef]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of mastitis: Insights into disease recognition and resolution. J. Mammary Gland Biol. Neoplasia. 2011, 16, 291–304. [Google Scholar] [CrossRef]
- Ning, L.T.; Dong, G.Z.; Ao, C.; Zhang, D.G.; Erdene, K.; Zhang, F.Q.; Zhang, T.L. Effects of continuous low dose infusion of lipopolysaccharide on inflammatory responses, milk production and milk quality in dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, 262–269. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, G.; Xu, T.; Xu, L.; Guo, J.; Jin, D.; Shen, X. Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget 2016, 7, 9652. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.I.; Lin, Y.E.; Bian, Y.; Gao, X.; Qu, B.O.; Li, Q. 14-3-3γ regulates cell viability and milk fat synthesis in lipopolysaccharide-induced dairy cow mammary epithelial cells. Exp. Ther. Med. 2016, 11, 1279–1287. [Google Scholar] [CrossRef]
- Strandberg, Y.; Gray, C.; Vuocolo, T.; Donaldson, L.; Broadway, M.; Tellam, R. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 2005, 31, 72–86. [Google Scholar] [CrossRef]
- Liu, H.W.; Tong, J.M.; Zhou, D.W. Utilization of Chinese herbal feed additives in animal production. Agri Sci. China 2011, 10, 1262–1272. [Google Scholar] [CrossRef]
- Meshnick, S.R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasit. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Meshnick, S.R.; Thomas, A.; Ranz, A.; Xu, C.M.; Pan, H.Z. Artemisinin (qinghaosu): The role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 1991, 49, 181–189. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P.; Kumar, S. Secondary metabolites of Artemisia annua and their biological activity. Curr. Sci. 2001, 80, 35–48. [Google Scholar]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.; Luthria, D.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Hou, K.; Tong, J.J.; Zhang, H.; Gao, S.; Guo, Y.Q.; Niu, H.; Xiong, B.H.; Jiang, L.S. Microbiome and metabolic changes in milk in response to artemisinin supplementation in dairy cows. AMB Express 2020, 10, 154–172. [Google Scholar] [CrossRef]
- Park, K.H.; Yoon, Y.D.; Han, S.B.; Oh, S.J.; Yun, J.; Lee, C.W.; Kang, J.S. Artemisinin inhibits lipopolysaccharide-induced interferon-β production in RAW 264.7 cells: Implications on signal transducer and activator of transcription-1 signaling and nitric oxide production. Int. Immunopharmacol. 2012, 14, 580–584. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Zhang, J.L.; Wu, X.H.; Zhou, H.J. Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio 2012, 2, 103–112. [Google Scholar] [CrossRef]
- Lin, S.P.; Li, W.; Winters, A.; Liu, R.; Yang, S.H. Artemisinin prevents glutamate-induced neuronal cell death via Akt pathway activation. Front. Cell. Neurosci. 2018, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, H.; Gao, Y.; Xu, J.; Zheng, W. Artemisinin protects retinal neuronal cells against oxidative stress and restores rat retinal physiological function from light exposed damage. ACS Chem. Neurosci. 2017, 8, 1713–1723. [Google Scholar] [CrossRef]
- Yang, S.X.; Xie, S.S.; Gao, H.L.; Long, Z.Z. Artemisinin and its derivatives enhance T lymphocyte-mediated immune responses in normal mice and accelerate immunoreconstitution of mice with syngeneic bone marrow transplantation. Clin. Immunol. Immunopathol. 1993, 69, 143–148. [Google Scholar] [CrossRef]
- Yang, Z.S.; Zhou, W.L.; Sui, Y.; Wang, J.X.; Wu, J.M.; Zhou, Y.; Zuo, J.P. Synthesis and immunosuppressive activity of new artemisinin derivatives. 1.[12 (β or α)-dihydroartemisininoxy] phen (ox) yl aliphatic acids and esters. J. Med. Chem. 2005, 48, 4608–4617. [Google Scholar] [CrossRef]
- Zheng, Y.M.; He, X.Y. Characteristics and EGFP expression of porcine mammary gland epithelial cells. Res. Vet. Sci. 2010, 89, 383–390. [Google Scholar] [CrossRef]
- Liu, M.J.; Song, S.X.; Li, H.; Jiang, X.Y.; Yin, P.; Wan, C.R.; Liu, X.X.; Liu, F.H.; Xu, J.Q. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J. Dairy Sci. 2014, 97, 2856–2865. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, S.H.; Guan, W.T.; Chen, F.; Chen, L.; Lv, Y.T.; Chen, J. GLUT1 and lactose synthetase are critical genes for lactose synthesis in lactating sows. Nutr. Metab. 2018, 15, 40–53. [Google Scholar] [CrossRef]
- Kenneth, J.; Thomas, D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar]
- Lv, Y.; Zhang, S.; Guan, W.; Chen, F.; Cheng, L.; Lv, Y.; Chen, J. Metabolic transition of milk triacylglycerol synthesis in response to varying levels of palmitate in porcine mammary epithelial cells. Genes Nutr. 2018, 13, 18–30. [Google Scholar] [CrossRef]
- Tian, M.; Chen, J.; Wu, Z.; Song, H.; Yang, F.; Cui, C.; Guan, W. Fat encapsulation reduces diarrhea in piglets partially by repairing the intestinal barrier and improving fatty acid transport. Animals 2021, 11, 28. [Google Scholar] [CrossRef]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Yang, Z. Salidroside attenuates inflammatory responses by suppressing nuclear factor-κB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm. Res. 2013, 62, 9–15. [Google Scholar] [CrossRef]
- Günther, J.; Petzl, W.; Zerbe, H.; Schuberth, H.J.; Koczan, D.; Goetze, L.; Seyfert, H.M. Lipopolysaccharide priming enhances expression of effectors of immune defence while decreasing expression of pro-inflammatory cytokines in mammary epithelia cells from cows. BMC Genom. 2012, 13, 1–13. [Google Scholar] [CrossRef]
- Porcherie, A.; Cunha, P.; Trotereau, A.; Roussel, P.; Gilbert, F.B.; Rainard, P.; Germon, P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet. Res. 2012, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, M.; Klein, B.; Merkle, H.; Schulz, M.; Fritsch, R.; Greten, F.R.; Schmid, R.M. IκBβ is an essential co-activator for LPS-induced IL-1β transcription in vivo. J. Exp. Med. 2010, 207, 2621–2630. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Zheng, J.; Cheng, J.; Liu, W.; Ding, G.; Zhang, L. The antimalarial artemisinin synergizes with antibiotics to protect against lethal live Escherichia coli challenge by decreasing proinflammatory cytokine release. Antimicrob. Agents Chemother. 2006, 50, 2420–2427. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Li, W.D.; Dong, Y.J.; Tu, Y.Y.; Lin, Z.B. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int. Immunopharmacol. 2006, 6, 1243–1250. [Google Scholar] [CrossRef]
- Risco, A.; del Fresno, C.; Mambol, A.; Alsina-Beauchamp, D.; MacKenzie, K.F.; Yang, H.T.; Cuenda, A. p38γ and p38δ kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Pro. Natl. Acad. Sci. USA 2012, 109, 11200–11205. [Google Scholar] [CrossRef]
- Meylan, E.; Dooley, A.L.; Feldser, D.M.; Shen, L.; Turk, E.; Ouyang, C.; Jacks, T. Requirement for NF-κB signalling in a mouse model of lung adenocarcinoma. Nature 2009, 462, 104–107. [Google Scholar] [CrossRef]
- Gantke, T.; Sriskantharajah, S.; Sadowski, M.; Ley, S.C. IκB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol. Rev. 2012, 246, 168–182. [Google Scholar] [CrossRef]
- DiDonato, J.; Mercurio, F.; Rosette, C.; Wu-Li, J.; Suyang, H.; Ghosh, S.; Karin, M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 1996, 16, 1295–1304. [Google Scholar] [CrossRef]
- Aldieri, E.; Atragene, D.; Bergandi, L.; Riganti, C.; Costamagna, C.; Bosia, A.; Ghigo, D. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-κB activation. FEBS Lett. 2003, 552, 141–144. [Google Scholar] [CrossRef]
- Chen, H.; Sun, B.; Wang, S.; Pan, S.; Gao, Y.; Bai, X.; Xue, D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: Involvement of cell cycle arrest and inactivation of nuclear factor-κB. J. Cancer Res. Clin. Oncol. 2010, 136, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Chong, C.M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef]
- Cao, Q.; Jiang, Y.; Shi, J.; Xu, C.; Liu, X.; Yang, T.; Niu, T. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J. Surg. Res. 2015, 194, 667–678. [Google Scholar] [CrossRef]
- Zhu, C.; Xiong, Z.; Chen, X.; Peng, F.; Hu, X.; Chen, Y.; Wang, Q. Artemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κB pathway in microglia cells. PLoS ONE 2012, 7, e35125. [Google Scholar] [CrossRef]

| Genes | Accession | Sequence Primers (5′-3′) | Size (bp) |
|---|---|---|---|
| TNF-α | NM_214022.1 | F-ATGGGCTGTACCTCATCTACTC | 141 |
| R- GGCTCTTGATGGCAGAGAGG | |||
| IL-1β | XM_021081828.1 | F-CCGAAGAGGGACATGGAGAA | 88 |
| R-AGTTGGGGTACAGGGCAGAC | |||
| IL-6 | NM_214399.1 | F-TGGCTACTGCCTTCCCTACC | 132 |
| R-CAGAGATTTTGCCGAGGATG | |||
| IL-8 | NM_213867.1 | F-AGGACCAGAGCCAGGAAGAGAC | 108 |
| R-CACAGAGAGCTGCAGAAAGCAG | |||
| β-actin | XM_021086047.1 | F-TGCGGGACATCAAGGAGAAG | 176 |
| R-AGTTGAAGGTGGTCTCGTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xiong, L.; Chen, J.; Tian, Z.; Liu, J.; Chen, F.; Ren, M.; Guan, W.; Zhang, S. Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways. Animals 2021, 11, 1528. https://doi.org/10.3390/ani11061528
Zhang W, Xiong L, Chen J, Tian Z, Liu J, Chen F, Ren M, Guan W, Zhang S. Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways. Animals. 2021; 11(6):1528. https://doi.org/10.3390/ani11061528
Chicago/Turabian StyleZhang, Wenfei, Liang Xiong, Jiaming Chen, Zhezhe Tian, Jiaxin Liu, Fang Chen, Man Ren, Wutai Guan, and Shihai Zhang. 2021. "Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways" Animals 11, no. 6: 1528. https://doi.org/10.3390/ani11061528
APA StyleZhang, W., Xiong, L., Chen, J., Tian, Z., Liu, J., Chen, F., Ren, M., Guan, W., & Zhang, S. (2021). Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways. Animals, 11(6), 1528. https://doi.org/10.3390/ani11061528








