A Study on the Pathological Effects of Trypanorhyncha Cestodes in Dusky Groupers Epinephelus marginatus from the Canary Islands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
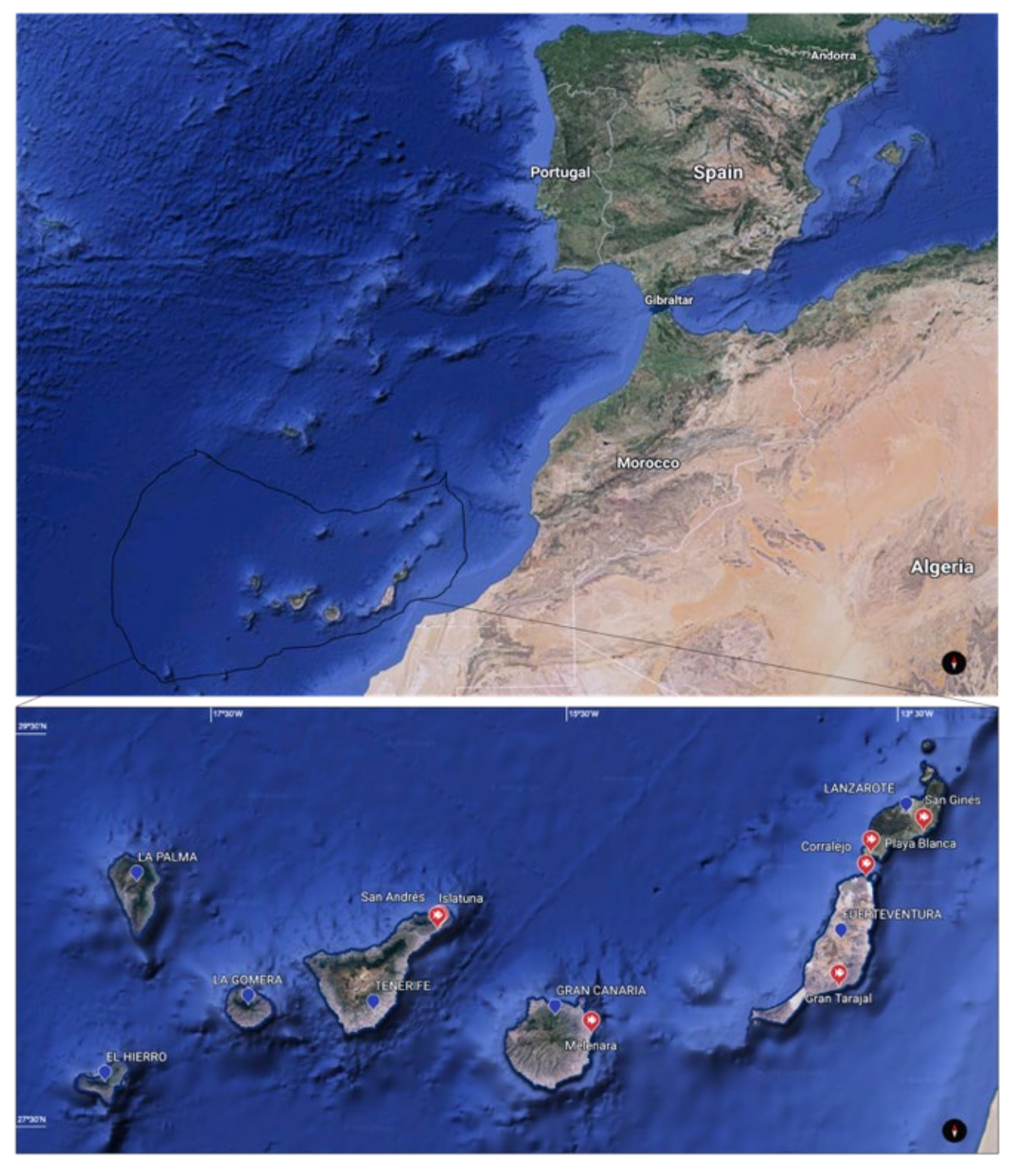
2.1. Sample Collection
2.2. Histology and Parasitology
3. Results
3.1. General Observations
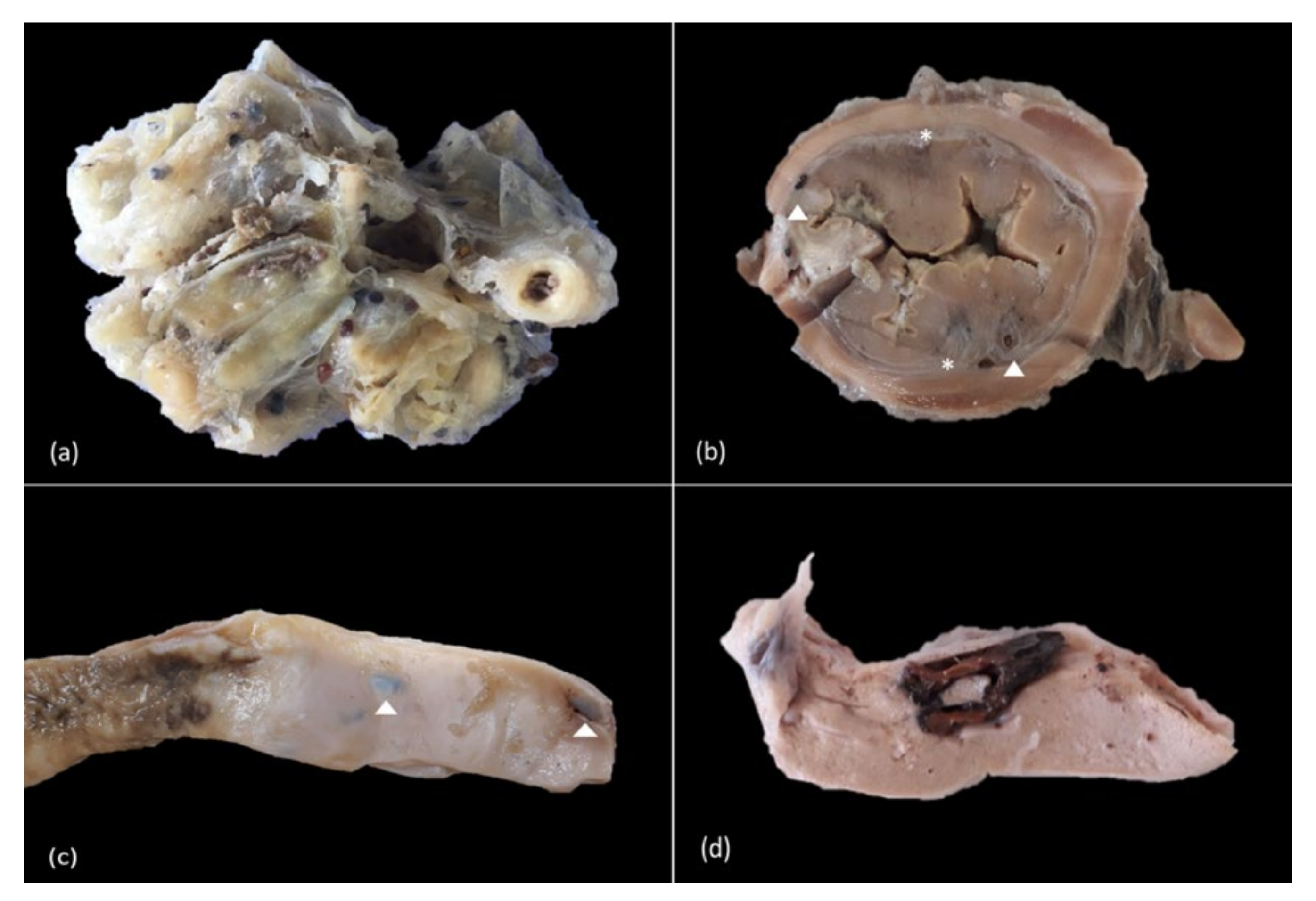
3.2. Gross Findings
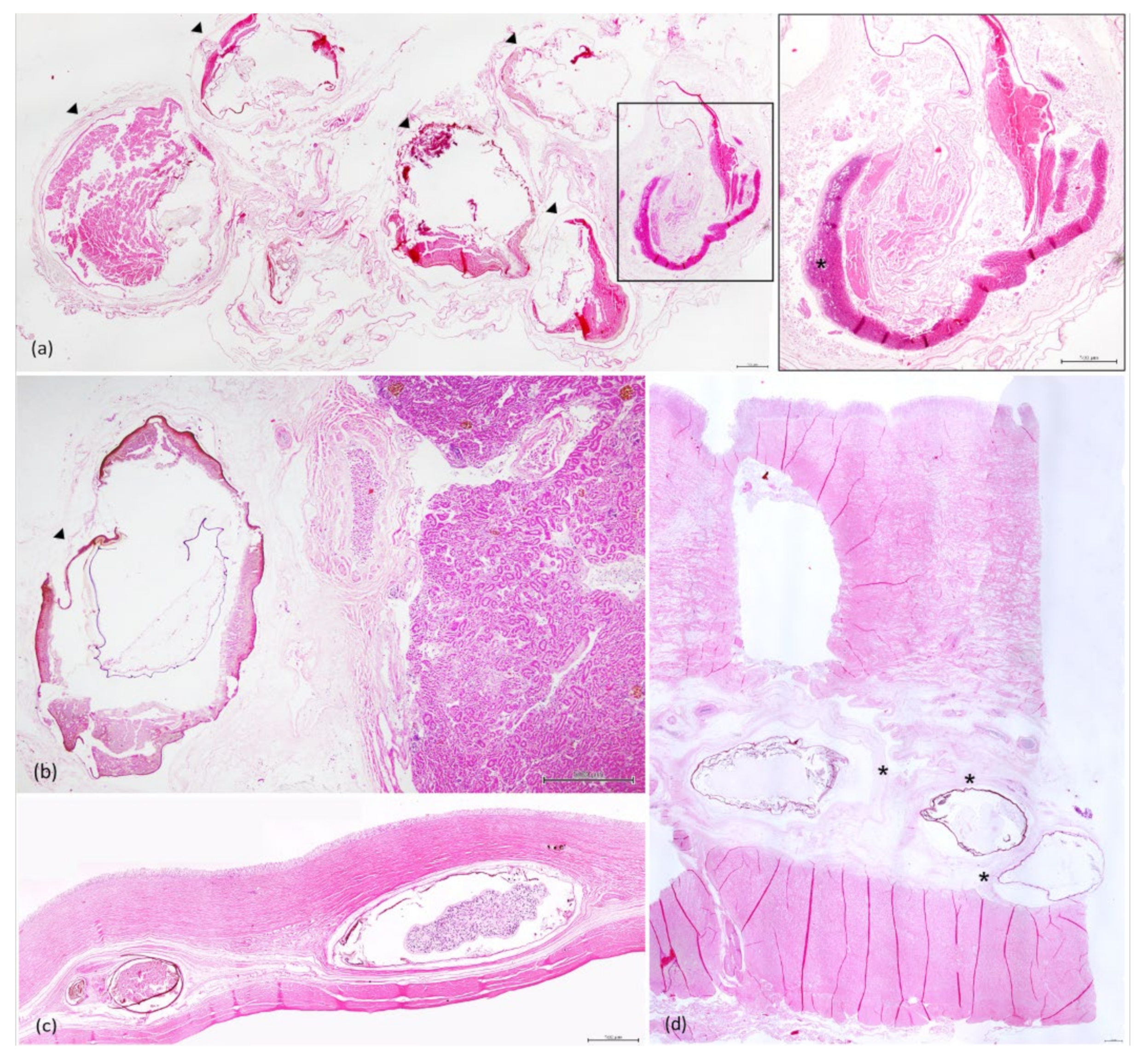
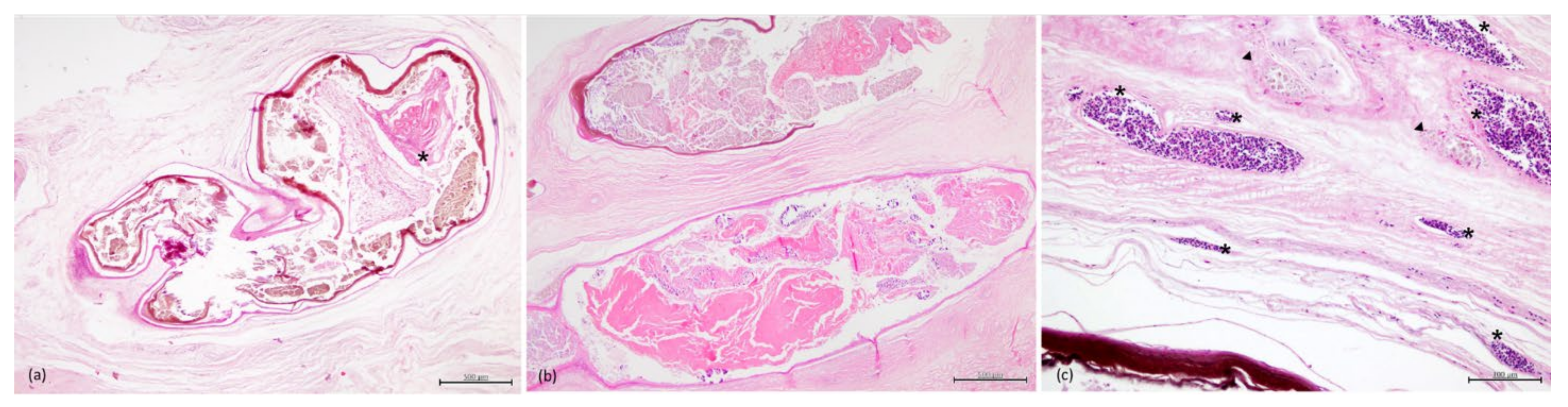
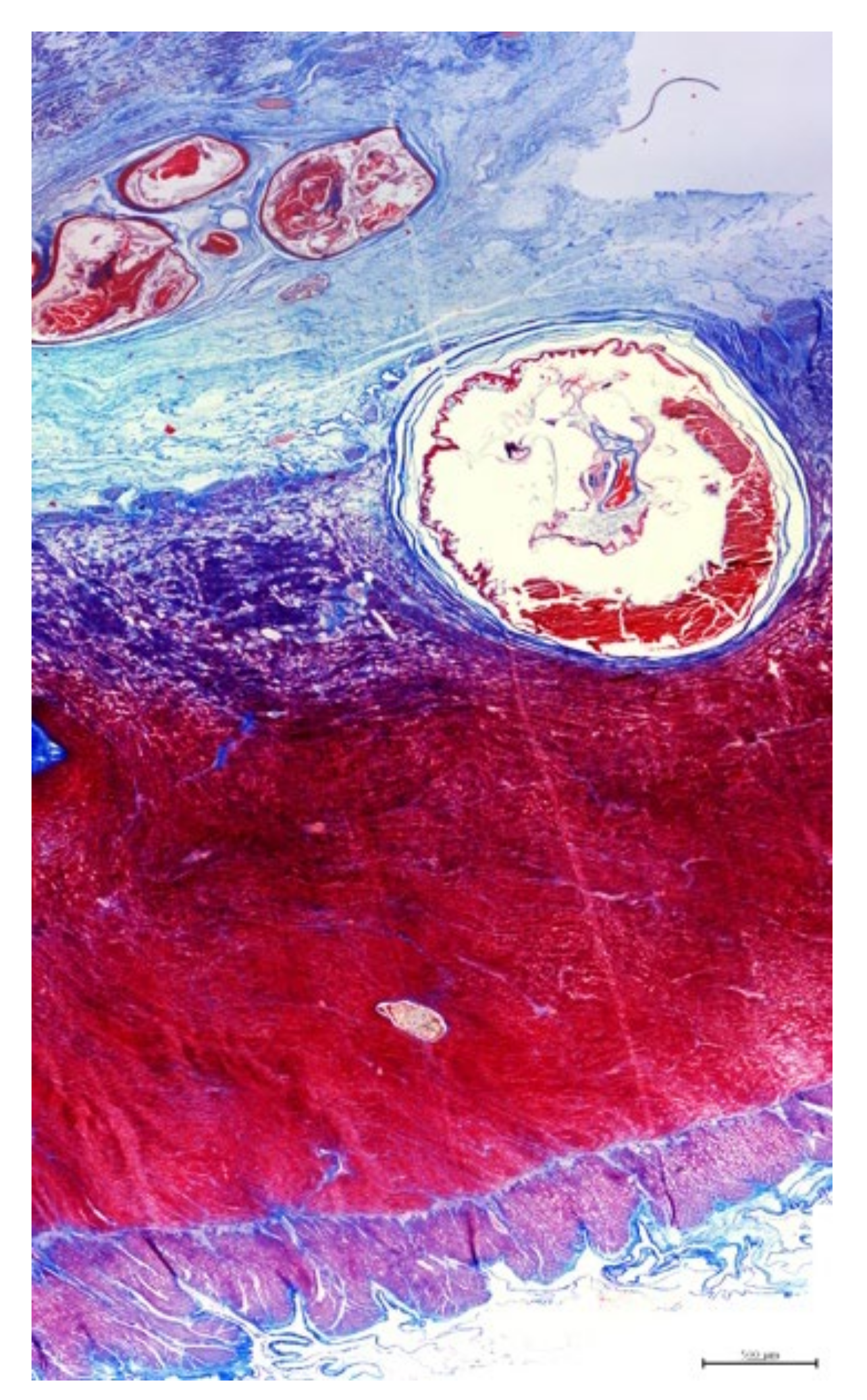
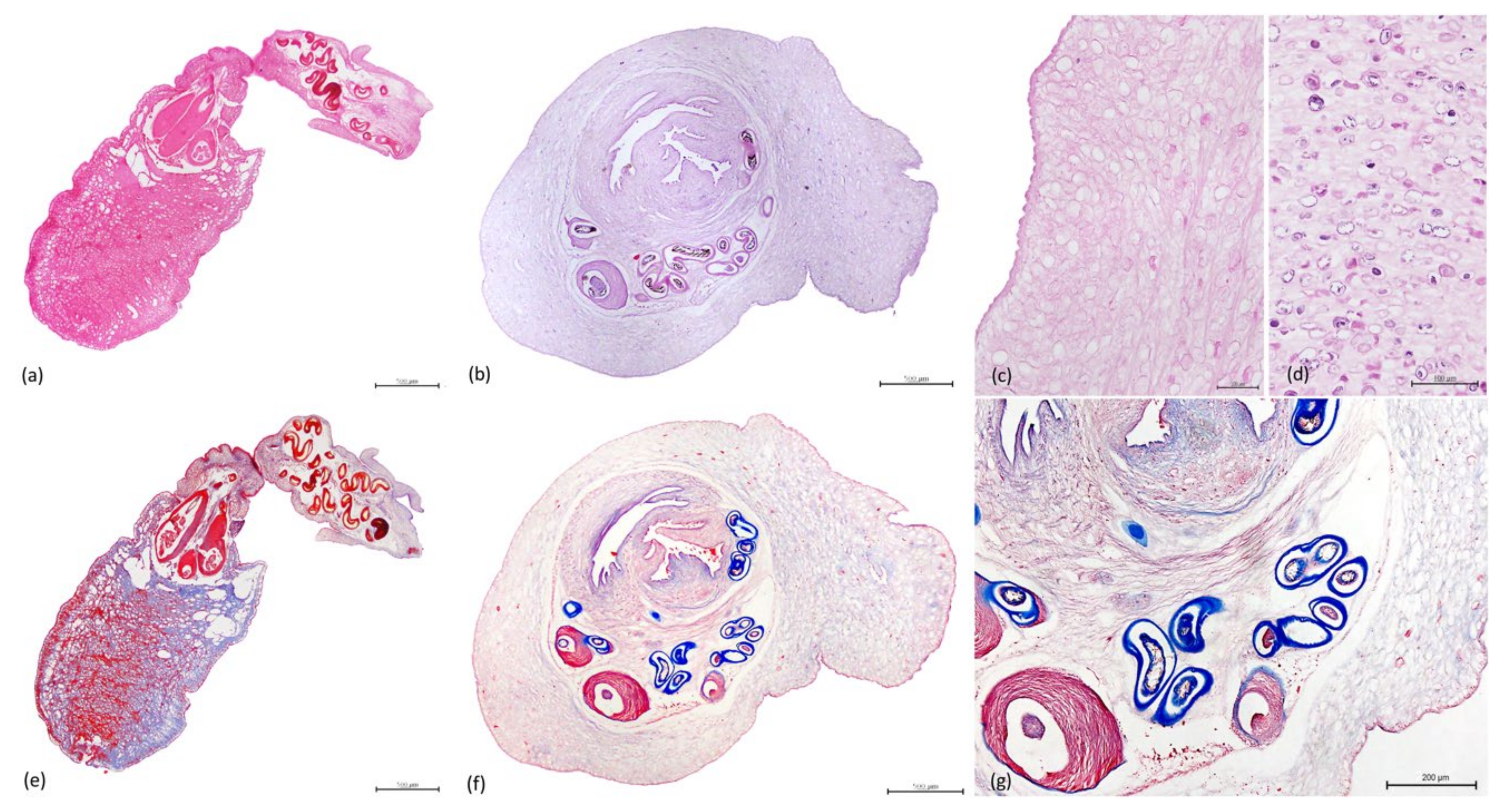
3.3. Histopathology
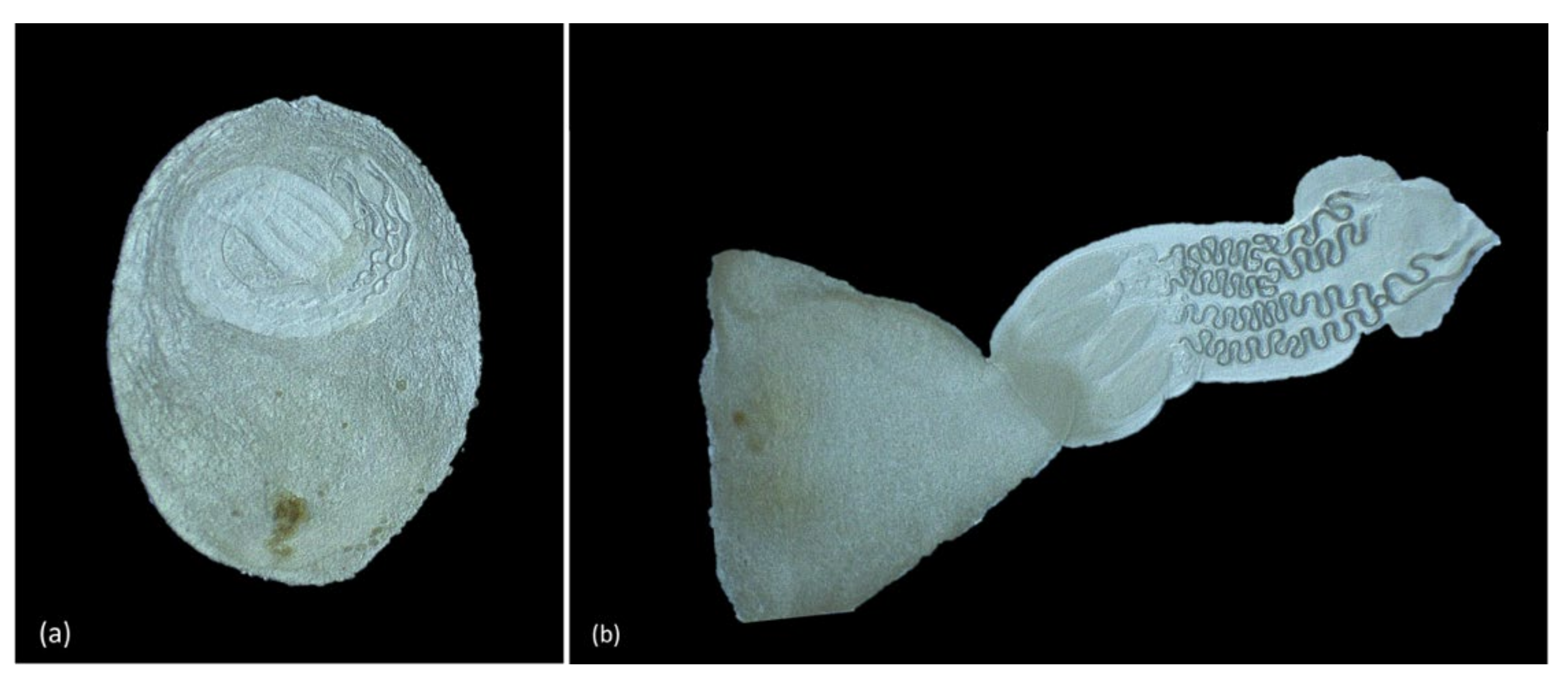
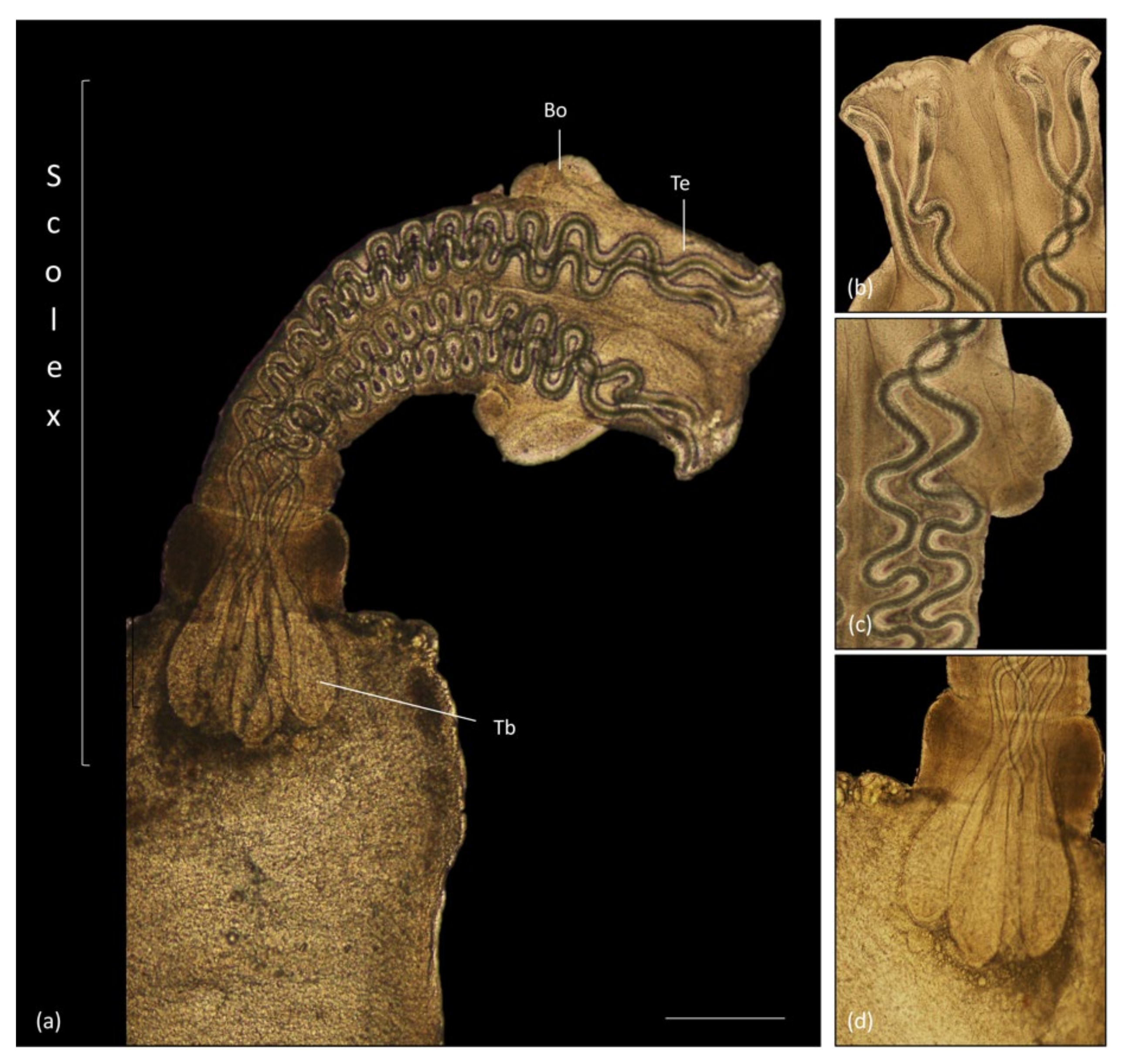
3.4. Parasitology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, M.; Aragort, W.; Leiro, J.; Sanmartín, M. Macroparasites of five species of ray (genus Raja) on the northwest coast of Spain. Dis. Aquat. Org. 2006, 70, 93–100. [Google Scholar] [CrossRef]
- Alves, P.V.; De Chambrier, A.; Scholz, T.; Luque, J. Annotated checklist of fish cestodes from South America. ZooKeys 2017, 650, 1–205. [Google Scholar] [CrossRef]
- Beveridge, I.; Bray, R.A.; Cribb, T.H.; Justine, J.-L. Diversity of trypanorhynch metacestodes in teleost fishes from coral reefs off eastern Australia and New Caledonia. Parasite 2014, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Haseli, M.; Malek, M.; Valinasab, T.; Palm, H. Trypanorhynch cestodes of teleost fish from the Persian Gulf, Iran. J. Helminthol. 2010, 85, 215–224. [Google Scholar] [CrossRef]
- Overstreet, R.M. Trypanorhynch Infections in the Flesh of Sciaenid Fishes. Mar. Fish. Rev. 1978, 40, 37. [Google Scholar]
- Palm, H.W. Trypanorhynch cestodes from Indonesian coastal waters (East Indian Ocean). Folia Parasitol. 2000, 47, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.; Obiekezie, A.; Möller, H. Trypanorhynchid cestodes of commercial inshore fishes of the West African coast. Aquat. Living Resour. 1994, 7, 153–164. [Google Scholar] [CrossRef]
- Palm, H.W.; Walter, T.; Schwerdtfeger, G.; Reimer, L.W. NybeliniaPoche, 1926 (Cestoda: Trypanorhyncha) from the Moçambique coast, with description ofN. beveridgeisp. nov. and systematic consideration of the genus. South Afr. J. Mar. Sci. 1997, 18, 273–285. [Google Scholar] [CrossRef]
- Scholz, T.; Garippa, G.; Scala, A. Grillotia epinepheli sp. n. (Cestoda: Trypanorhyncha) Plerocerci from the Teleost, Epinephelus Guaza, in Sardinia, Italy. Folia Parasitol. 1993, 40, 23–28. [Google Scholar]
- Rohde, K. Marine Parasitology; CSIRO: Collingwood, Australia, 2005; pp. 96–103.
- Palm, H.W.; Waeschenbach, A.; Olson, P.D.; Littlewood, D.T.J. Molecular phylogeny and evolution of the Trypanorhyncha Diesing, 1863 (Platyhelminthes: Cestoda). Mol. Phylogenetics Evol. 2009, 52, 351–367. [Google Scholar] [CrossRef]
- Mehlhorn, H. Encyclopaedia of Parasitology, 4th ed.; Springer: Berlin, Germany, 2016; pp. 1914–2913. [Google Scholar]
- Overstreet, R.M. Marine Maladies? Worms, Germs, and Other Symbionts from the Northern Gulf of Mexico; Blossman Printing: Ocean Springs, MI, USA, 1978; pp. 37–61. [Google Scholar]
- Mudry, D.R.; Dailey, M.D. Postembryonic development of certain tetraphyllidean and trypanorhynchan cestodes with a possible alternative life cycle for the order Trypanorhyncha. Can. J. Zool. 1971, 49, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.W. The Trypanorhyncha Diesing, 1863; PKsPl-iPB Press: Bogor, Indonesia, 2004. [Google Scholar]
- Tamaru, C.S.; Klinger-Bowen, R.C.; Ogawa, K.; Iwaki, T.; Kurashima, A.; Itoh, N. Prevalence and Species Identity of Trypanorhyncha in Cultured and Wild Amberjack, Seriola spp. in Hawaii-Implications for Aquaculture. J. World Aquac. Soc. 2016, 47, 42–50. [Google Scholar] [CrossRef]
- Palm, H.W.; Yulianto, I.; Piatkowski, U. Trypanorhynch Assemblages Indicate Ecological and Phylogenetical Attributes of Their Elasmobranch Final Hosts. Fishes 2017, 2, 8. [Google Scholar] [CrossRef]
- Roberts, R.J. Fish Pathology, 4th ed.; Wiley-Blackwell: West Sussex, UK, 2012; pp. 301–450. [Google Scholar]
- Condini, M.V.; García-Charton, J.A.; Garcia, A.M. A review of the biology, ecology, behavior and conservation status of the dusky grouper, Epinephelus marginatus (Lowe 1834). Rev. Fish Biol. Fish. 2017, 28, 301–330. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E.; FAO. Groupers of the World. Available online: http://www.fao.org/3/t0540e/t0540e27.pdf (accessed on 20 July 2020).
- De Mitcheson, Y.S.; Craig, M.T.; Bertoncini, A.; E Carpenter, K.; Cheung, W.W.L.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 2012, 14, 119–136. [Google Scholar] [CrossRef]
- Amorim, P.; Sousa, P.; Jardim, E.; Menezes, G.M. Sustainability Status of Data-Limited Fisheries: Global Challenges for Snapper and Grouper. Front. Mar. Sci. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Pollard, D.A.; Afonso, P.; Bertoncini, A.A.; Fennessy, S.; Francour, P.; Barreiros, J. The IUCN Red List of Threatened Species 2018. Dusky Grouper. Available online: https://www.iucnredlist.org/species/7859/100467602 (accessed on 20 July 2020).
- Neubert, K.; Yulianto, I.; Kleinertz, S.; Theisen, S.; Wiryawan, B.; Palm, H.W. Parasite fauna of white-streaked grouper, Epinephelus ongus (Bloch, 1790) (Epinephelidae) from Karimunjawa, Indonesia. Parasitol. Open 2016, 2, e12. [Google Scholar] [CrossRef][Green Version]
- Hassan, M.A.; Palm, H.W.; Mahmoud, M.A.; Jama, F.A. Trypanorhynch Cestodes from the Musculature of Commercial Fishes from the Arabian Gulf. Arab Gulf J. Sci. Res. 2002, 20, 74–86. [Google Scholar]
- Ibrahim, M. Histopathology of Trypanorhyncha plerocercoids (Cestodes) in some Marine Fish from Waters of the Arabian Gulf. J. King Abdulaziz Univ. Sci. 2000, 11, 59–73. [Google Scholar] [CrossRef]
- Rizgalla, J. An Investigation of the Health Status of Wild Libyan Dusky Grouper, Epinephelus Marginatus (Lowe), with Char-acterisation of a New Disease, Dusky Grouper Dermatitis (DGD). Ph.D. Thesis, University of Stirling, Stirling, UK, 2016. [Google Scholar]
- Genc, E.; Genc, M.A.; Genc, E.; Cengizler, I.; Can, M.F. Seasonal Variation and Pathology Associated with Helminthes In-fecting Two Serranids (Teleostei) of Iskenderun Bay (Northeast Mediterranean Sea), Turkey. Turkish J. Fish. Aquat. Sci. 2005, 5, 29–33. [Google Scholar]
- Beveridge, I.; Campbell, R.A. Review of the Rhopalothylacidae Guiart, 1935 (Cestoda: Trypanorhyncha), with a Description of the Adult of Pintneriella Musculicola Yamaguti, 1934 and a Redescription of P. Gymnorhynchoides (Guiart, 1935) Comb. n. Folia Parasitol. 2003, 50, 61–71. [Google Scholar] [CrossRef]
- Rückert, S.; Klimpel, S.; Al-Quraishy, S.; Mehlhorn, H.; Palm, H.W. Transmission of fish parasites into grouper mariculture (Serranidae: Epinephelus coioides (Hamilton, 1822)) in Lampung Bay, Indonesia. Parasitol. Res. 2008, 104, 523–532. [Google Scholar] [CrossRef] [PubMed]
- European MSP Platform. Spain. Available online: https://www.msp-platform.eu/countries/spain (accessed on 17 April 2021).
- FAO. FAO Major Fishing Areas. ATLANTIC, EASTERN CENTRAL (Major Fishing Area 34). Available online: http://www.fao.org/fishery/area/Area34/en#FAO-fishing-area-34.1.1 (accessed on 17 April 2021).
- Esch, G.W.; Gardiner, C.H.; Fayer, R.; Dubey, J.P.; Poynton, S.L. An Atlas of Metazoan Parasites in Animal Tissues. J. Parasitol. 2001, 87, 961. [Google Scholar] [CrossRef]
- Abdou, N.E.-S.; Palm, H.W. New record of two genera of Trypanorhynch cestodes infecting Red Sea fishes in Egypt. J. Egypt. Soc. Parasitol. 2008, 38, 281–292. [Google Scholar]
- Al-Zubaidy, A.B.; Mhaisen, F.T. Larval Tapeworms (Cestoda: Trypanorhyncha) from Some Red Sea Fishes, Yemen. Mesoport. J. Sci. 2011, 26, 1–14. [Google Scholar]
- Kleinertz, S.; Palm, H. Parasites of the grouper fishEpinephelus coioides(Serranidae) as potential environmental indicators in Indonesian coastal ecosystems. J. Helminthol. 2013, 89, 86–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleinertz, S.; Damriyasa, I.; Hagen, W.; Theisen, S.; Palm, H. An environmental assessment of the parasite fauna of the reef-associated grouperEpinephelus areolatusfrom Indonesian waters. J. Helminthol. 2012, 88, 50–63. [Google Scholar] [CrossRef]
- Ragan, J.G.; Aldrich, D.V. Infection of Brown Shrimp, Penaeus Aztecus Ives by Prochristianella Penaei Kruse (Cestoda: Trypa-norhyncha) in Southeastern Louisiana Bays. Trans. Am. Fish. Soc. 1972, 101, 226–238. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Grover, J.J.; Lipcius, R.N. Ontogenetic Diet Shifts in Nassau Grouper: Trophic Linkages and Predatory Im-pact. Bull. Mar. Sci. 1998, 63, 111–126. [Google Scholar]
- John, J.S. Ontogenetic changes in the diet of the coral reef grouper Plectropomus leopardus (Serranidae): Patterns in taxa, size and habitat of prey. Mar. Ecol. Prog. Ser. 1999, 180, 233–246. [Google Scholar] [CrossRef]
- Overstreet, R.M. Poecilancistrium Caryophyllum and Other Trypanorhynch Cestode Plerocercoids from the Musculature of Cynoscion Nebulosus and Other Sciaenid Fishes in the Gulf of Mexico. J. Parasitol. 1977, 63, 780–789. [Google Scholar] [CrossRef]
- Beveridge, I.; Chauvet, C.; Justine, J.-L. Redescription of Pseudogilquinia pillersi (Southwell, 1929) (Cestoda, Trypanorhyncha) from serranid and lethrinid fishes from New Caledonia and Australia. Acta Parasitol. 2007, 52, 213–218. [Google Scholar] [CrossRef]
- Özer, A.; Ozturk, T.; Kornyushin, V.; Kornyychuk, Y.; Yurakhno, V. Grillotia erinaceus (van Beneden, 1858) (Cestoda:Trypanorhyncha) from whiting in the Black Sea, with observations on seasonality and host-parasite interrelationship. Acta Parasitol. 2014, 59, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Arme, C.; Owen, R.W. Occurrence and Pathology of Ligula intestinalis Infections in British Fishes. J. Parasitol. 1968, 54, 272. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.J.E.; Pike, A.W.; Secombes, C.J. The immune response of wild rainbow trout, Salmo gairdneri Richardson, to naturally acquired plerocercoid infections of Diphyllobothrium dendriticum (Nitzsch, 1824) and D. ditremum (Creplin, 1825). J. Fish Biol. 1989, 35, 781–794. [Google Scholar] [CrossRef]
- Sharp, G.J.E.; Pike, A.W.; Secombes, C.J. Sequential development of the immune response in rainbow trout [Oncorhynchus mykiss (Walbaum, 1792)] to experimental plerocercoid infections of Diphyllobothrium dendriticum (Nitzsch, 1824). Parasitology 1992, 104, 169–178. [Google Scholar] [CrossRef]
- Williams, C.; Reading, A.; Scholz, T.; Shinn, A. Larval gryporhynchid tapeworms (Cestoda: Cyclophyllidea) of British freshwater fish, with a description of the pathology caused by Paradilepis scolecina. J. Helminthol. 2011, 86, 1–9. [Google Scholar] [CrossRef]
- Williams, H.; Jones, A. Parasitic Worms of Fish; Taylor & Francis: London, UK, 1994; pp. 331–361. [Google Scholar]
- Arme, C.; Owen, R.W. Observations on a tissue response within the body cavity of fish infected with the plerocercoid larvae of Ligula intestinalis (L.) (Cestoda: Pseudophyllidea). J. Fish Biol. 1970, 2, 35–37. [Google Scholar] [CrossRef]
- Gause, W.C.; Wynn, T.A.; Allen, J.E. Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 2013, 13, 607–614. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins and Cotran–Pathological Basis of Disease, 9th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 93–109. [Google Scholar]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Lumsden, J.S. Gastrointestinal tract, swimbladder, pancreas and peritoneum. In Systemic Pathology of Fish, 2nd ed.; Ferguson, H.W., Ed.; Scotian Press: London, UK, 2006; pp. 169–196. [Google Scholar]
- Ackermann, M.R. Inflammation and Injury. In Pathologic Basis of Veterinary Disease, 5th ed.; Zachary, J.F., McGavin, M.D., Eds.; Elsevier: Maryland Heights, MO, USA, 2012; pp. 119–146. [Google Scholar]
- Ferguson, H.W. Systemic Pathology of Fish–A Text and Atlas of Comparative Tissue Responses in Diseases of Teleosts; Iova State University Press/AMES: Ames, IA, USA, 1989; pp. 6–9. [Google Scholar]
- Noga, E.J. Spleen, Thymus, Reticulo-Endothelial System, Blood. In Systemic Pathology of Fish, 2nd ed.; Ferguson, H.W., Ed.; Scotian Press: London, UK, 2006; pp. 121–139. [Google Scholar]
- Overstreet, R.; Thulin, J. Response by Plectropomus-Leopardus and Other Serranid Fishes to Pearsonellum-Corventum (Digenea, Sanguinicolidae), Including Melanomacrophage Centers in the Heart. Aust. J. Zoöl. 1989, 37, 129–142. [Google Scholar] [CrossRef]
- Wolke, R. Piscine macrophage aggregates: A review. Annu. Rev. Fish Dis. 1992, 2, 91–108. [Google Scholar] [CrossRef]
- McAdam, A.J.; Milner, D.A.; Sharpe, A.H. Infectious Diseases. In Robbins and Cotran–Pathological Basis of Disease, 9th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; p. 396. [Google Scholar]
- Sweeting, R.A. Studies on Ligula intestinalis Some aspects of the pathology in the second intermediate host. J. Fish Biol. 1977, 10, 43–50. [Google Scholar] [CrossRef]
- Myers, R.K.; McGavin, M.D.; Zachary, J.F. Cellular Adaptations, Injury, and Death: Morphologic, Biochemical, and Genetic Bases. In Pathologic Basis of Veterinary Disease, 5th ed.; Zachary, J.F., McGavin, M.D., Eds.; Elsevier: Maryland Heights, MO, USA, 2012; p. 39. [Google Scholar]
- MacKenzie, K. Some aspects of the biology of the plerocercoid of Gilquinia squali Fabricius 1794 (Cestoda: Trypanorhyncha). J. Fish Biol. 1975, 7, 321–327. [Google Scholar] [CrossRef]
- Rigby, M.C.; Dufour, V. Parasites of Coral Reef Fish Recruits, Epinephelus merra (Serranidae), in French Polynesia. J. Parasitol. 1996, 82, 405. [Google Scholar] [CrossRef]
- Palm, H.W.; Overstreet, R.M. New Records of Trypanorhynch Cestodes from the Gulf of Mexico, Including Kotorella Pronosoma (Stossich, 1901) and Heteronybelinia Palliata (Linton, 1924) Comb. N. Folia Parasitol. 2000, 47, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Linton, E. A Cestode Parasite in the Flesh of the Butterfish. Bur. Fish. 1906, 611, 111–134. [Google Scholar]
- Adjei, E.L.; Barnes, A.; Lester, R.J.G. A method for estimating possible parasite-related host mortality, illustrated using data from Callitetrarhynchus gracilis (Cestoda: Trypanorhyncha) in lizardfish (Saurida spp.). Parasitology 1986, 92, 227–243. [Google Scholar] [CrossRef]
- Lubieniecki, B. Aspects of the biology of the plerocercoid of Grillotia erinaceus (van Beneden, 1858) (Cestoda: Trypanorhyncha) in haddock Melanogrammus aeglefinus (L.). J. Fish Biol. 1976, 8, 431–439. [Google Scholar] [CrossRef]
- Rodero, M.; Cuéllar, C. Humoral immune responses induced by Gymnorhynchus gigas extracts in BALB/c mice. J. Helminthol. 1999, 73, 239–243. [Google Scholar] [CrossRef]
- Gòmez-Morales, M.A.; Ludovisi, A.; Giuffra, E.; Manfredi, M.T.; Piccolo, G.; Pozio, E. Allergenic activity of Molicola horridus (Cestoda, Trypanorhyncha), a cosmopolitan fish parasite, in a mouse model. Vet. Parasitol. 2008, 157, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Mattos, D.; Verícimo, M.; Lopes, L.; Clemente, S.S. Immunogenic activity of the fish tapewormPterobothrium heteracanthum(Trypanorhyncha: Pterobothriidae) in BALB/c mice. J. Helminthol. 2013, 89, 203–207. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sales-Ribeiro, C.; Rivero, M.A.; Fernández, A.; García-Álvarez, N.; González, J.F.; Quesada-Canales, O.; Caballero, M.J. A Study on the Pathological Effects of Trypanorhyncha Cestodes in Dusky Groupers Epinephelus marginatus from the Canary Islands. Animals 2021, 11, 1471. https://doi.org/10.3390/ani11051471
de Sales-Ribeiro C, Rivero MA, Fernández A, García-Álvarez N, González JF, Quesada-Canales O, Caballero MJ. A Study on the Pathological Effects of Trypanorhyncha Cestodes in Dusky Groupers Epinephelus marginatus from the Canary Islands. Animals. 2021; 11(5):1471. https://doi.org/10.3390/ani11051471
Chicago/Turabian Stylede Sales-Ribeiro, Carolina, Miguel A. Rivero, Antonio Fernández, Natalia García-Álvarez, Jorge Francisco González, Oscar Quesada-Canales, and María José Caballero. 2021. "A Study on the Pathological Effects of Trypanorhyncha Cestodes in Dusky Groupers Epinephelus marginatus from the Canary Islands" Animals 11, no. 5: 1471. https://doi.org/10.3390/ani11051471
APA Stylede Sales-Ribeiro, C., Rivero, M. A., Fernández, A., García-Álvarez, N., González, J. F., Quesada-Canales, O., & Caballero, M. J. (2021). A Study on the Pathological Effects of Trypanorhyncha Cestodes in Dusky Groupers Epinephelus marginatus from the Canary Islands. Animals, 11(5), 1471. https://doi.org/10.3390/ani11051471





