Transcription Landscape of the Early Developmental Biology in Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. RNA Extraction, Library Preparation and Sequencing
2.3. RNA-Sequencing Data Analysis
2.4. Functional Analyses
3. Results
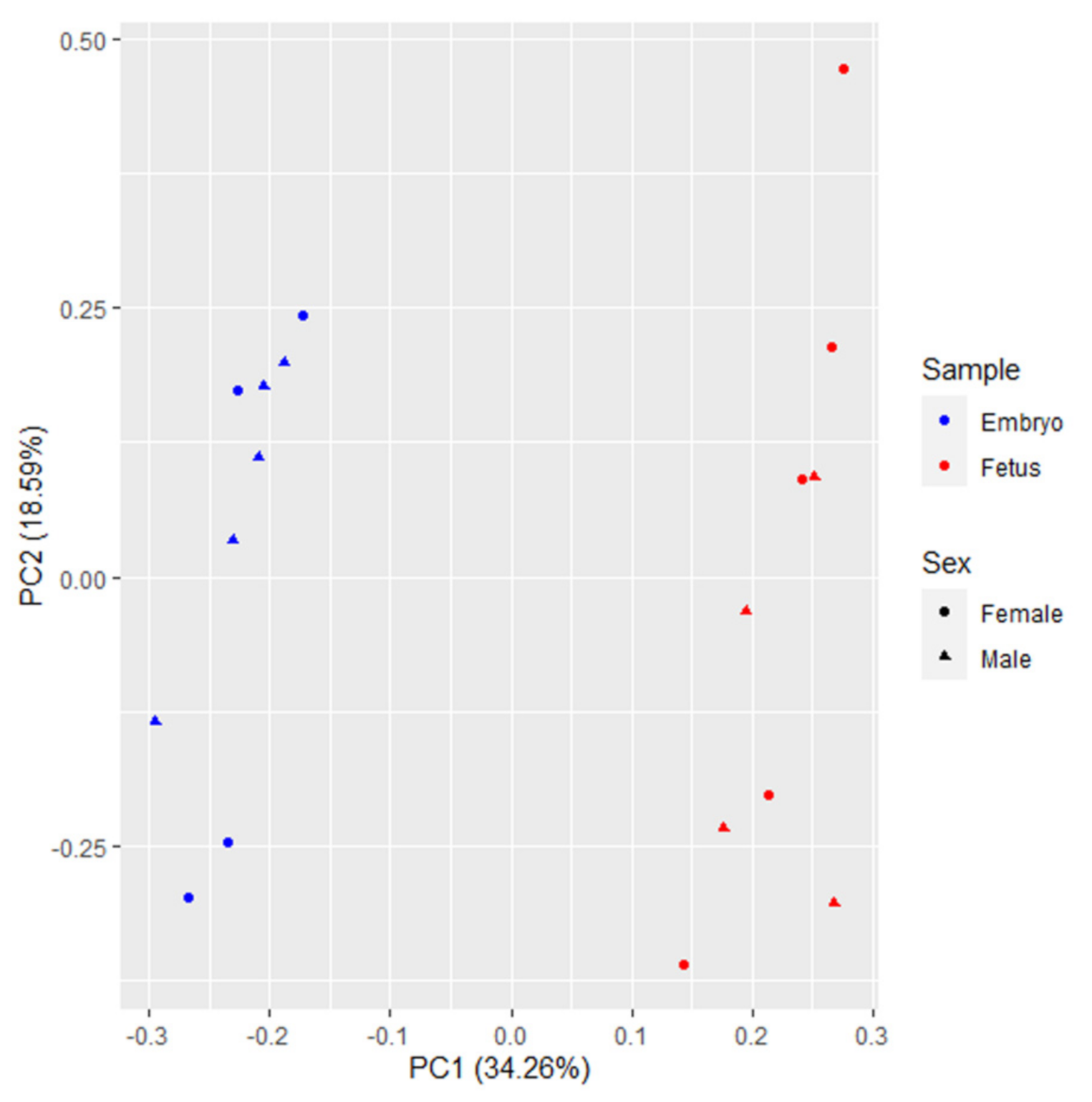
3.1. RNA-Sequencing Data
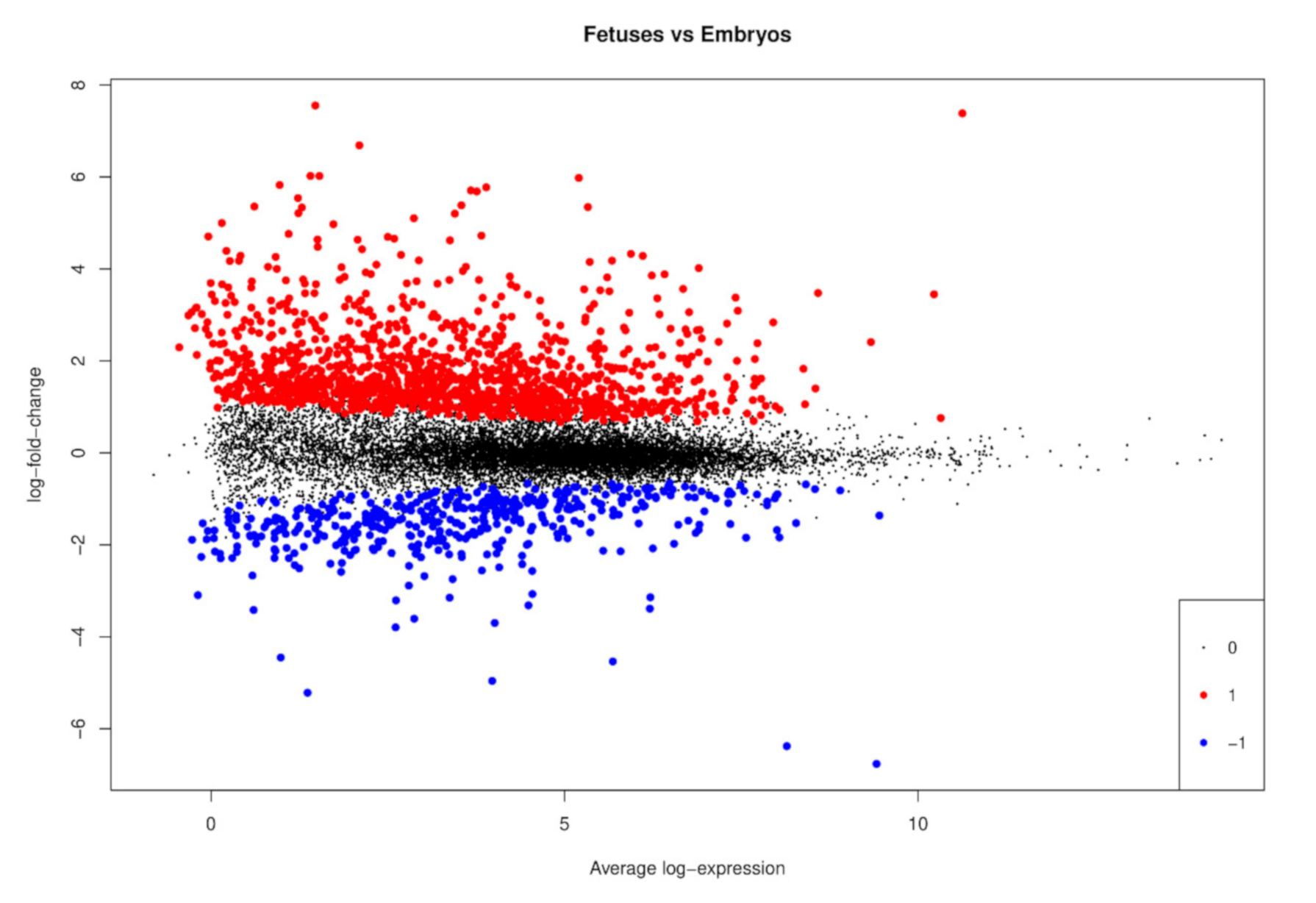
3.2. Differentially Expressed Genes between Fetuses and Embryos
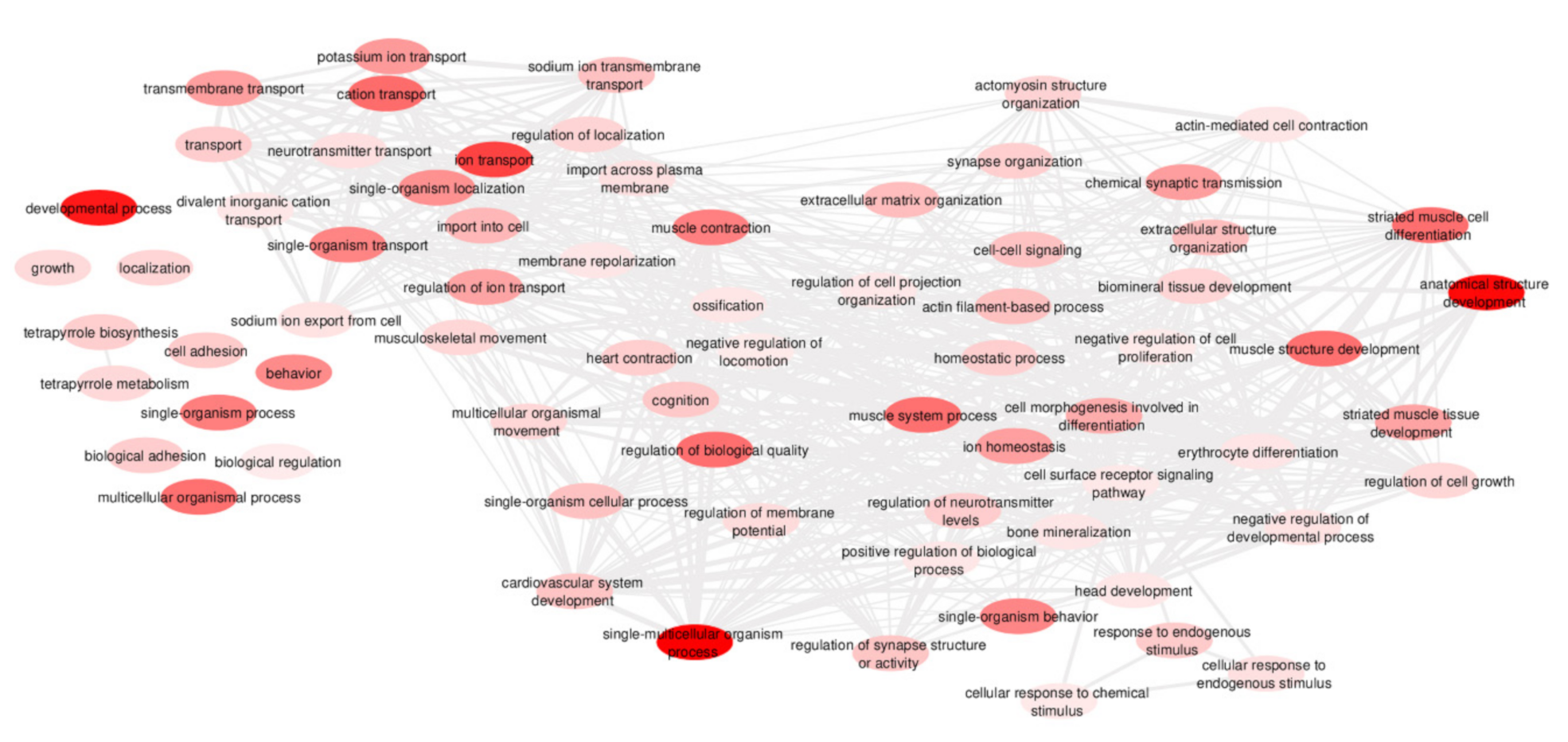
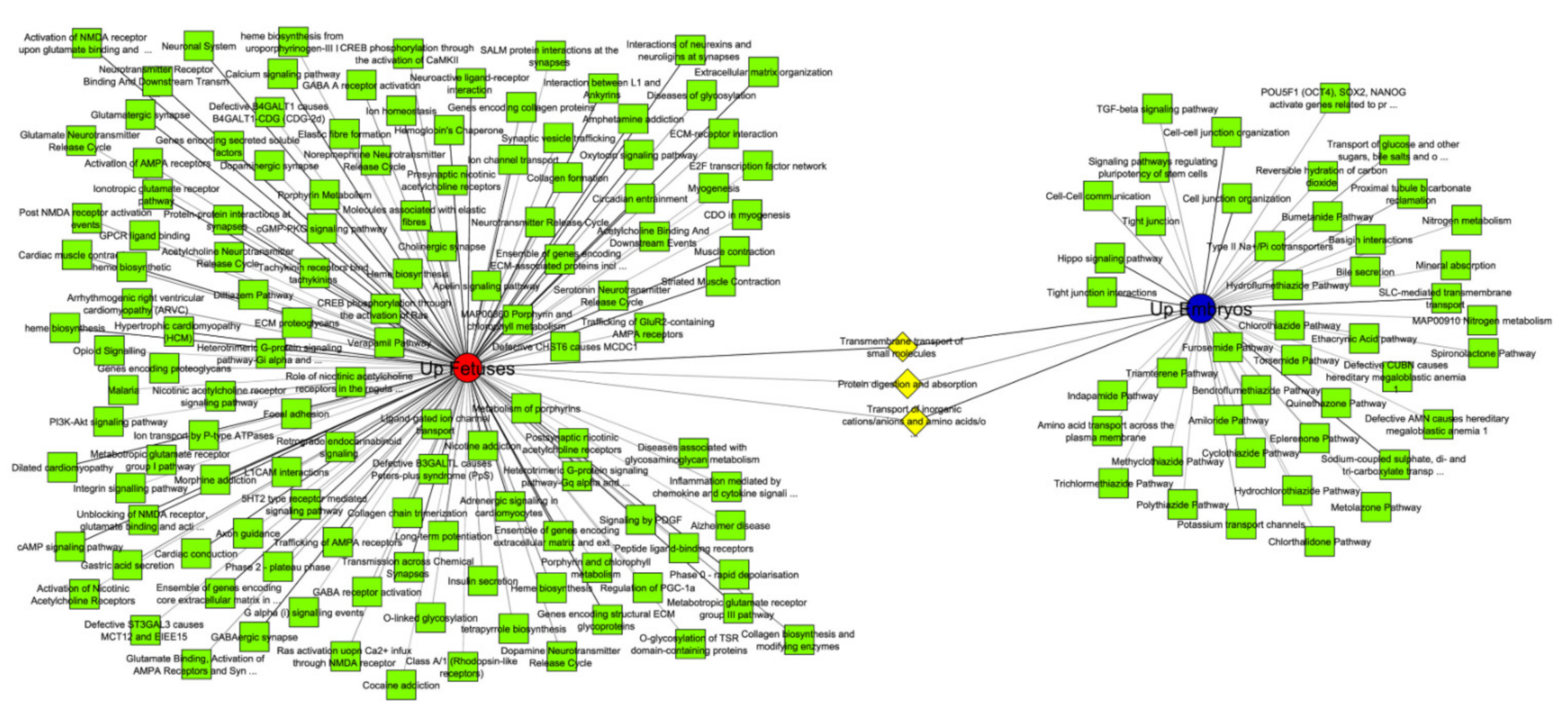
3.3. Functional Analyses
4. Discussion
4.1. General Pathways during Prenatal Development
4.2. Cardiovascular System
4.3. Kidney Development
4.4. Skeletal Muscle Development
4.5. Skeleton Development
4.6. Extracellular Matrix and Prenatal Development
4.7. Neuronal Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyttel, P.; Sinowatz, F.; Vejlsted, M.; Betteridge, K. Essentials of Domestic Animal Embriology; Edwards, R., Rodenhuis, J., Betteridge, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-7020-2899-1. [Google Scholar]
- Edwards, M.J.; Saunders, R.D.; Shiota, K. Effects of heat on embryos and foetuses. Int. J. Hyperth. 2003, 19, 295–324. [Google Scholar] [CrossRef]
- Pieri, N.C.G.; Souza, A.F.; Casals, J.B.; Roballo, K.C.S.; Ambrósio, C.E.; Martins, D.S. Comparative Development of Embryonic Age by Organogenesis in Domestic Dogs and Cats. Reprod. Domest. Anim. 2015, 50, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Danesh, S.M.; Villasenor, A.; Chong, D.; Soukup, C.; Cleaver, O. BMP and BMP receptor expression during murine organogenesis. Gene Expr. Patterns 2009, 9, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesolowski, S.R.; Raney, N.E.; Ernst, C.W. Developmental changes in the fetal pig transcriptome. Physiol. Genom. 2004, 16, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 2006, 84, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wu, Z.; Dai, Z.; Wang, X.; Li, J.; Wang, B.; Wu, G. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J. Anim. Sci. Biotechnol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Lin, H.; Wang, H.; Wang, Y.; Liu, C.; Wang, C.; Guo, J. Transcriptomic Analysis of the Porcine Endometrium during Embryo Implantation. Genes (Basel) 2015, 1330–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Ulbrich, S.E.; Bauersachs, S. Spatial organization of endometrial gene expression at the onset of embryo attachment in pigs. BMC Genom. 2019, 20, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Pas, M.F.W.; De Wit, A.A.W.; Priem, J.; Cagnazzo, M.; Davoli, R.; Russo, V.; Pool, M.H. Transcriptome expression profiles in prenatal pigs in relation to myogenesis. J. Muscle Res. Cell Motil. 2005, 26, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Sollero, B.P.; Guimarães, S.E.F.; Rilington, V.D.; Tempelman, R.J.; Raney, N.E.; Steibel, J.P.; Guimarães, J.D.; Lopes, P.S.; Lopes, M.S.; Ernst, C.W. Transcriptional profiling during foetal skeletal muscle development of Piau and Yorkshire-Landrace cross-bred pigs. Anim. Genet. 2011, 42, 600–612. [Google Scholar] [CrossRef] [Green Version]
- Costa, K.A.; Saraiva, A.; Guimaraes, J.D.; Marques, D.B.D.; Machado-neves, M.; Reis, L.M.B.; Alberto, F.; Veroneze, R.; Oliveira, L.F.D.; Garcia, I.S.; et al. Dietary L-arginine supplementation during early gestation of gilts affects conceptuses development. Theriogenology 2019, 140, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.S.; Teixeira, S.A.; Costa, K.A.; Marques, D.B.D.; Rodrigues, G.d.A.; Costa, T.C.; Guimarães, J.D.; Otto, P.I.; Saraiva, A.; Ibelli, A.M.G.; et al. l- Arginine supplementation of gilts during early gestation modulates energy sensitive pathways in pig conceptuses. Mol. Reprod. Dev. 2020, 1–16. [Google Scholar]
- Teixeira, S.A.; Ibelli, A.M.G.; Cantão, E.; Oliveira, H.C.D.; Ledur, M.C.; Peixoto, J.d.O.; Marques, D.B.D.; Costa, K.A.; Coutinho, L.L.; Guimarães, S.E.F. Sex Determination Using RNA-Sequencing Analyses in Early Prenatal Pig Development. Genes (Basel) 2019, 10, 1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- BAQCOM Bioinformatics Analysis for Quality Control and Mapping. Available online: https://github.com/hanielcedraz/BAQCOM (accessed on 15 February 2019).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, 1–13. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controllin the false discovery rate: A practical and powerful approch to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaimal, V.; Bardes, E.E.; Tabar, S.C.; Jegga, A.G.; Aronow, B.J. ToppCluster: A multiple gene list feature analyzer for comparative enrichment clustering and networkbased dissection of biological systems. Nucleic Acids Res. 2010, 38, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Dong, J.; Hu, Y.; Fan, X.; Wu, X.; Mao, Y.; Hu, B.; Guo, H.; Wen, L.; Tang, F. Single-cell RNA-seq analysis unveils a prevalent epithelial/mesenchymal hybrid state during mouse organogenesis. Genome Biol. 2018, 19, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [Green Version]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Fu, V.; Plouffe, S.W.; Guan, K. The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 2017, 99–107. [Google Scholar] [CrossRef]
- Lorthongpanich, C.; Issaragrisil, S. Emerging Role of the Hippo Signaling Pathway in Position Sensing and Lineage Specification in Mammalian Preimplantation Embryos. Biol. Reprod. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Sharma, J.; Madan, P. Characterisation of the Hippo signalling pathway during bovine preimplantation embryo development. Reprod. Fertil. Dev. 2019. [Google Scholar] [CrossRef]
- Karaulanov, E.; Knöchel, W.; Niehrs, C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J. 2004, 23, 844–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tramullas, M.; Lantero, A.; Díaz, Á.; Morchón, N.; Merino, D.; Villar, A.; Buscher, D.; Merino, R.; Hurlé, J.M.; Izpisúa-Belmonte, J.C.; et al. BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-β family in pain modulation. J. Neurosci. 2010, 30, 1502–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dituri, F.; Cossu, C.; Mancarella, S.; Giannelli, G. The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells 2019, 8, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotewold, L.; Plum, M.; Dildrop, R.; Peters, T.; Rüther, U. Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech. Dev. 2001, 100, 327–330. [Google Scholar] [CrossRef]
- Higashihori, N.; Song, Y.; Richman, J.M. Expression and regulation of the decoy bone morphogenetic protein receptor BAMBI in the developing avian face. Dev. Dyn. 2008, 237, 1500–1508. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hill, C.S. TGF-β Superfamily Signaling in Embryonic Development and Homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Dagnino, L.; Fry, C.J.; Bartley, S.M.; Farnham, P.; Gallie, B.L.; Phillips, R.A. Expression patterns of the E2F family of transcription factors during murine epithelial development. Cell Growth Differ. 1997, 8, 553–563. [Google Scholar]
- Sears, R.C.; Nevins, J.R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 2002, 277, 11617–11620. [Google Scholar] [CrossRef] [Green Version]
- White, J.; Stead, E.; Faast, R.; Conn, S.; Cartwright, P.; Dalton, S. Developmental Activation of the Rb–E2F Pathway and Establishment of Cell Cycle-regulated Cyclin-dependent Kinase Activity during Embryonic Stem Cell Differentiation. Mol. Biol. Cell 2004, 15, 5318–5328. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Z.; Tora, L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 2007, 26, 5341–5357. [Google Scholar] [CrossRef] [Green Version]
- Indovina, P.; Pentimalli, F.; Casini, N.; Vocca, I.; Giordano, A. RB1 dual role in proliferation and apoptosis: Cell fate control and implications for cancer therapy. Oncotarget 2015, 6, 17873–17890. [Google Scholar] [CrossRef]
- Sen, R.; Pezoa, S.A.; Shull, L.C.; Hernandez-Lagunas, L.; Niswander, L.A.; Artinger, K.B. Kat2a and Kat2b acetyltransferase activity regulates craniofacial cartilage and bone differentiation in Zebrafish and mice. J. Dev. Biol. 2018, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.A.; Aghajanian, H.; Singh, M.K. Semaphorin signaling in cardiovascular development. Cell Metab. 2015, 21, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Dabbagh, A.; Amini, A.; Mohammad-Amin Abdollahifar, M.A.S. Congenital Heart Disease in Pediatric and Adult Patients: Anesthetic and Perioperative Management. In Congenital Heart Disease in Pediatric and Adult Patients; Dabbagh, A., Conte, A.H., Lubin, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–64. ISBN 9783319446912. [Google Scholar]
- Hu, W.; Xin, Y.; Hu, J.; Sun, Y.; Zhao, Y. Inhibitor of DNA binding in heart development and cardiovascular diseases. Cell Commun. Signal. 2019, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Franciolli, A.L.R.; Cordeiro, B.M.; da Fonseca, E.T.; Rodrigues, M.N.; Sarmento, C.A.P.; Ambrosio, C.E.; de Carvalho, A.F.; Miglino, M.A.; Silva, L.A. Characteristics of the equine embryo and fetus from days 15 to 107 of pregnancy. Theriogenology 2011, 76, 819–832. [Google Scholar] [CrossRef]
- McFadden, D.G.; Barbosa, A.C.; Richardson, J.A.; Schneider, M.D.; Srivastava, D.; Olson, E.N. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development 2005, 132, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Valenti, O.; Prima, F.A.F.D.; Renda, E.; Faraci, M.; Hyseni, E.; Domenico, R.D.; Monte, S.; Giorgio, E. Fetal cardiac function during the first trimester of pregnancy. J. Prenat. Med. 2011, 5, 59–62. [Google Scholar]
- Tan, C.M.J.; Lewandowski, A.J. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2019. [Google Scholar] [CrossRef]
- Leiva, M.C.; Tolosa, J.E.; Binotto, C.N.; Weiner, S.; Huppert, L.; Denis, A.L.; Huhta, J.C. Fetal cardiac development and hemodynamics in the first trimester. Ultrasound Obstet. Gynecol. 1999, 14, 169–174. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Kelly, M.A.; Rehm, H.L.; Lakdawala, N.K.; Funke, B.H. Inherited cardiomyopathies: Molecular genetics and clinical genetic testing in the postgenomic era. J. Mol. Diagnostics 2013, 15, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Meadows, S.M.; Fletcher, P.J.; Moran, C.; Xu, K.; Neufeld, G.; Chauvet, S.; Mann, F.; Krieg, P.; Cleaver, O. Integration of Repulsive Guidance Cues Generates Avascular Zones that Shape Mammalian Blood Vessels. Circ. Res. 2012, 110, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M. Signaling required for blood vessel maintenance: Molecular basis and pathological manifestations. Int. J. Vasc. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolte, D.; McClung, J.A.; Aronow, W.S. Vasculogenesis and Angiogenesis. In Translational Research in Coronary Artery Disease: Pathophysiology to Treatment; Aronow, W.S., McClung, J.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 49–65. ISBN 9780128023853. [Google Scholar]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.E.; Frame, J.M.; Fromm, G.J.; Koniski, A.D.; Kingsley, P.D.; Little, J.; Bulger, M.; Palis, J. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood 2011, 117, 4600–4608. [Google Scholar] [CrossRef] [Green Version]
- Nandakumar, S.K.; Ulirsch, J.C.; Sankaran, V.G. Advances in Understanding Erythropoiesis: Evolving Perspectives. Br. J. Haematol. 2016, 173, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.; Kertesz, N.; Joseph, S.B.; Jegalian, A.; Wu, H. Erythropoietin (Epo) and EpoR expression and 2 waves of erythropoiesis. Blood 2001, 98, 1408–1415. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, V.G.; Xu, J.; Orkin, S.H. Advances in the understanding of haemoglobin switching. Br. J. Haematol. 2010, 149, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Seely, J.C. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: Juvenile animal relevancy. J. Toxicol. Pathol. 2017, 30, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Moritz, K.M.; Wintour, E.M. Functional development of the meso- and metanephros. Pediatr. Nephrol. 1999, 13, 171–178. [Google Scholar] [CrossRef]
- Georgas, K.M.; Chiu, H.S.; Lesieur, E.; Rumballe, B.A.; Little, M.H. Expression of metanephric nephron-patterning genes in differentiating mesonephric tubules. Dev. Dyn. 2011, 240, 1600–1612. [Google Scholar] [CrossRef]
- Little, M.H.; McMahon, A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012, 4, 3. [Google Scholar] [CrossRef]
- Schedl, A. Renal abnormalities and their developmental origin. Nat. Rev. Genet. 2007, 8, 791–802. [Google Scholar] [CrossRef]
- Raciti, D.; Reggiani, L.; Geffers, L.; Jiang, Q.; Bacchion, F.; Subrizi, A.E.; Clements, D.; Tindal, C.; Davidson, D.R.; Kaissling, B.; et al. Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol. 2008, 9. [Google Scholar] [CrossRef] [Green Version]
- Roselli, S.; Gribouval, O.; Boute, N.; Sich, M.; Benessy, F.; Attié, T.; Gubler, M.C.; Antignac, C. Podocin localizes in the kidney to the slit diaphragm area. Am. J. Pathol. 2002, 160, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chen, Y.; Yong, J.; Cui, Y.; Wang, R.; Wen, L.; Qiao, J.; Tang, F. Dissecting the Global Dynamic Molecular Profiles of Human Fetal Kidney Development by Single-Cell RNA Sequencing. Cell Rep. 2018, 24, 3554–3567.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalova, E.; Semerjyan, Z.; Manukyan, A.; Flora, I.; Panyan, N.; Karalyan, Z.; Tatoyan, M. The embryonic development of the pig excretory system. Porcine Res. 2018, 8, 1–12. [Google Scholar]
- Kuure, S.; Vuolteenaho, R.; Vainio, S. Kidney morphogenesis: Cellular and molecular regulation. Mech. Dev. 2000, 92, 31–45. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Fiedler, I.; Dietl, G.; Ender, K. Myogenesis and postnatal skeletal muscle cell growth as influenced by selection. Livest. Prod. Sci. 2000, 66, 177–188. [Google Scholar] [CrossRef]
- Maltin, C.A.; Delday, M.I.; Sinclair, K.D.; Steven, J.; Sneddon, A.A. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction 2001, 122, 359–374. [Google Scholar] [CrossRef]
- Chang, K.C. Key signalling factors and pathways in the molecular determination of skeletal muscle phenotype. Animal 2007, 1, 681–698. [Google Scholar] [CrossRef] [Green Version]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigmore, P.M.; Stickland, N.C. Muscle development in large and small pig fetuses. J. Anat. 1983, 137 Pt 2, 235–245. [Google Scholar]
- Tasleem Jan, A.; Ju Lee, E.; Ahmad, S.; Choi, I. Meeting the meat: Delineating the molecular machinery of muscle development. J. Anim. Sci. Technol. 2016, 1–10. [Google Scholar]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker: Amembrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Feng, C.; Liu, T.; Shi, M.; Wu, G.; Bazer, F.W. Physiological alterations associated with intrauterine growth restriction in fetal pigs: Causes and insights for nutritional optimization. Mol. Reprod. Dev. 2017, 84, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, J.B.; Duan, C. IGF-binding proteins: Why do they exist and why are there so many? Front. Endocrinol. (Lausanne). 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiGirolamo, D.J.; Kiel, D.P.; Esser, K.A. Bone and Skeletal Muscle: Neighbors With Close Ties. J. Bone Miner. Res. 2013, 28, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Olsen, B.R.; Reginato, A.M.; Wang, W. BONE DEVELOPMENT. Annu. Rev. Cell Dev. Biol. 2000, 191–220. [Google Scholar] [CrossRef]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.J.; Glass, D.A.; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- McKee, M.D.; Cole, W.G. Bone Matrix and Mineralization. In Pediatric Bone; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 9–37. ISBN 9780123820402. [Google Scholar]
- Qin, C.; Brunn, J.C.; Cook, R.G.; Orkiszewski, R.S.; Malone, J.P.; Veis, A.; Butler, W.T. Evidence for the proteolytic processing of dentin matrix protein 1. J. Biol. Chem. 2003, 278, 34700–34708. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, K.; Miyamoto, T.; Imai, J.I.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Yagi, M.; Watanabe, S.; Toyama, Y.; Suda, T. Osteoclastic activity induces osteomodulin expression in osteoblasts. Biochem. Biophys. Res. Commun. 2007, 362, 460–466. [Google Scholar] [CrossRef]
- Qin, C.; D’Souza, R.; Feng, J.Q. Dentin Matrix Protein 1 (DMP1): New and important roles for biomineralization and phosphate homeostasis. J. Dent. Res. 2007, 86, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Van Der Lende, T.; Van Rens, B.T.T.M. Critical periods for foetal mortality in gilts identified by analysing the length distribution of mummified foetuses and frequency of non-fresh stillborn piglets. Anim. Reprod. Sci. 2003, 75, 141–150. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens - structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, P.; Gualandi, F.; Gara, S.K.; Grumati, P.; Zamparelli, A.; Martoni, E.; Pellegrini, C.; Merlini, L.; Ferlini, A.; Bonaldo, P.; et al. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. 2012, 31, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Smits, P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. Part C Embryo Today Rev. 2005, 200–212. [Google Scholar] [CrossRef]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Liang, H.P.H.; Xu, J.; Xue, M.; Jackson, C. Matrix metalloproteinases in bone development and pathology: Current knowledge and potential clinical utility. Met. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Jin, X. The role of neurogenesis during development and in the adult brain. Eur. J. Neurosci. 2016, 44, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Martynoga, B.; Drechsel, D.; Guillemot, F. Molecular control of neurogenesis: A view from the mammalian cerebral cortex. Cold Spring Harb. Perspect. Biol. 2012, 4, a008359. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E. Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 1997, 7, 13–20. [Google Scholar] [CrossRef]
- Kim, E.J.; Hori, K.; Wyckoff, A.; Dickel, L.K.; Koundakjian, E.J.; Goodrich, L.V.; Johnson, J.E. Spatiotemporal Fate Map of Neurogenin1 (Neurog1) Lineages in the Mouse Central Nervous System. J. Comp. Neurol. 2011, 519, 1355–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.M.; Ku, R.Y.; Hashimoto-Torii, K. Prenatal Environment That Affects Neuronal Migration. Front. Cell Dev. Biol. 2019, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Luján, R.; Shigemoto, R.; López-Bendito, G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience 2005, 130, 567–580. [Google Scholar] [CrossRef]
- Cuzon, V.C.; Yeh, P.W.; Cheng, Q.; Yeh, H.H. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb. Cortex 2006, 16, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Manent, J.; Represa, A. Neurotransmitters and Brain Maturation: Early Paracrine Actions of GABA and Glutamate Modulate Neuronal Migration. Neuroscientist 2007, 13, 268–279. [Google Scholar] [CrossRef]
- Barber, M.; Di Meglio, T.; Andrews, W.D.; Hernández-Miranda, L.R.; Murakami, F.; Chédotal, A.; Parnavelas, J.G. The role of Robo3 in the development of cortical interneurons. Cereb. Cortex 2009, 19. [Google Scholar] [CrossRef] [Green Version]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248. [Google Scholar] [CrossRef] [Green Version]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Borsani, E.; Della Vedova, A.M.; Rezzani, R.; Rodella, L.F.; Cristini, C. Correlation between human nervous system development and acquisition of fetal skills: An overview. Brain Dev. 2018, 41, 225–233. [Google Scholar] [CrossRef]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol. Pathol. 2017, 45, 705–744. [Google Scholar] [CrossRef] [Green Version]
| Embryos | ||||
|---|---|---|---|---|
| Ensembl ID | logFC 1 | Gene Name | Gene Description | adj. p-Value 2 |
| ENSSSCG00000014726 | −6.764396328 | HBE1 | Hemoglobin subunit epsilon 1 | 1.38 × 10−8 |
| ENSSSCG00000007975 | −6.379504386 | HBZ | Hemoglobin subunit zeta | 8.50 × 10−10 |
| ENSSSCG00000031865 | −5.217417343 | AQP8 | Aquaporin 8 | 8.04 × 10−6 |
| ENSSSCG00000021902 | −4.96041836 | GABRP | Gamma-aminobutyric acid type A receptor subunit pi | 3.70 × 10−12 |
| ENSSSCG00000040513 | −4.537759202 | AQP3 | Aquaporin 3 | 1.18 × 10−9 |
| ENSSSCG00000006731 | −4.451641793 | VTCN1 | V-set domain containing T cell activation inhibitor 1 | 7.08 × 10−10 |
| ENSSSCG00000031080 | −3.795925653 | HAND1 | Heart and neural crest derivatives expressed 1 | 6.49 × 10−7 |
| ENSSSCG00000000418 | −3.699759861 | TAC3 | Tachykinin precursor 3 | 1.53 × 10−11 |
| ENSSSCG00000030461 | −3.607432588 | HEPHL1 | Hephaestin like 1 | 2.04 × 10−9 |
| ENSSSCG00000002432 | −3.418006247 | KCNK13 | Potassium two pore domain channelsubfamily K member 13 | 1.87 × 10−8 |
| Fetuses | ||||
| ENSSSCG00000009219 | 7.553512845 | IBSP | Integrin binding sialoprotein | 3.58 × 10−12 |
| ENSSSCG00000014725 | 7.383041924 | HBB | Hemoglobin, beta | 1.01 × 10−10 |
| ENSSSCG00000040098 | 6.688192867 | Uncharacterized | 1.21 × 10−11 | |
| ENSSSCG00000037430 | 6.022006128 | COL6A6 | Collagen type VI alpha 6 chain | 3.09 × 10−10 |
| ENSSSCG00000039501 | 6.020672325 | NEUROD6 | Neuronal differentiation 6 | 4.32 × 10−8 |
| ENSSSCG00000008072 | 5.978854537 | ASPN | Asporin | 1.58 × 10−14 |
| ENSSSCG00000009955 | 5.824620103 | CRYBB2 | Crystallin beta A2 | 9.52 × 10−8 |
| ENSSSCG00000035520 | 5.774356588 | Uncharacterized | 0.01103 | |
| ENSSSCG00000040641 | 5.70621141 | CRYBA1 | Crystallin beta A1 | 9.23 × 10−5 |
| ENSSSCG00000031903 | 5.684525698 | TNNT3 | Troponin T3, fast skeletal type | 2.33 × 10−12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, S.A.; Marques, D.B.D.; Costa, T.C.; Oliveira, H.C.; Costa, K.A.; Carrara, E.R.; Silva, W.d.; Guimarães, J.D.; Neves, M.M.; Ibelli, A.M.G.; et al. Transcription Landscape of the Early Developmental Biology in Pigs. Animals 2021, 11, 1443. https://doi.org/10.3390/ani11051443
Teixeira SA, Marques DBD, Costa TC, Oliveira HC, Costa KA, Carrara ER, Silva Wd, Guimarães JD, Neves MM, Ibelli AMG, et al. Transcription Landscape of the Early Developmental Biology in Pigs. Animals. 2021; 11(5):1443. https://doi.org/10.3390/ani11051443
Chicago/Turabian StyleTeixeira, Susana A., Daniele B. D. Marques, Thaís C. Costa, Haniel C. Oliveira, Karine A. Costa, Eula R. Carrara, Walmir da Silva, José D. Guimarães, Mariana M. Neves, Adriana M. G. Ibelli, and et al. 2021. "Transcription Landscape of the Early Developmental Biology in Pigs" Animals 11, no. 5: 1443. https://doi.org/10.3390/ani11051443
APA StyleTeixeira, S. A., Marques, D. B. D., Costa, T. C., Oliveira, H. C., Costa, K. A., Carrara, E. R., Silva, W. d., Guimarães, J. D., Neves, M. M., Ibelli, A. M. G., Cantão, M. E., Ledur, M. C., Peixoto, J. O., & Guimarães, S. E. F. (2021). Transcription Landscape of the Early Developmental Biology in Pigs. Animals, 11(5), 1443. https://doi.org/10.3390/ani11051443







