Dietary Natural Plant Extracts Can Promote Growth and Modulate Oxidative Status of Senegalese Sole Postlarvae under Standard/Challenge Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.1.1. Preparation of Diet Extracts for Antioxidant Capacity Assessment
2.1.2. Radical Scavenging Activity
2.1.3. Total Phenolic (TPC) and Flavonoids (TFC) Content
2.2. Senegalese Sole Husbandry and Experimental Set-Up
2.3. Thermal Stress Test
2.4. Key Performance Indicators
2.5. Preparation of Fish Sample for Physiologycal Biomarkers Response
2.5.1. Oxidative Status Assessment
2.5.2. Cellular Stress Response
2.6. Reverse Transcription–Quantitative Real-Time PCR (qPCR) Analyses
2.7. Data Analysis
3. Results
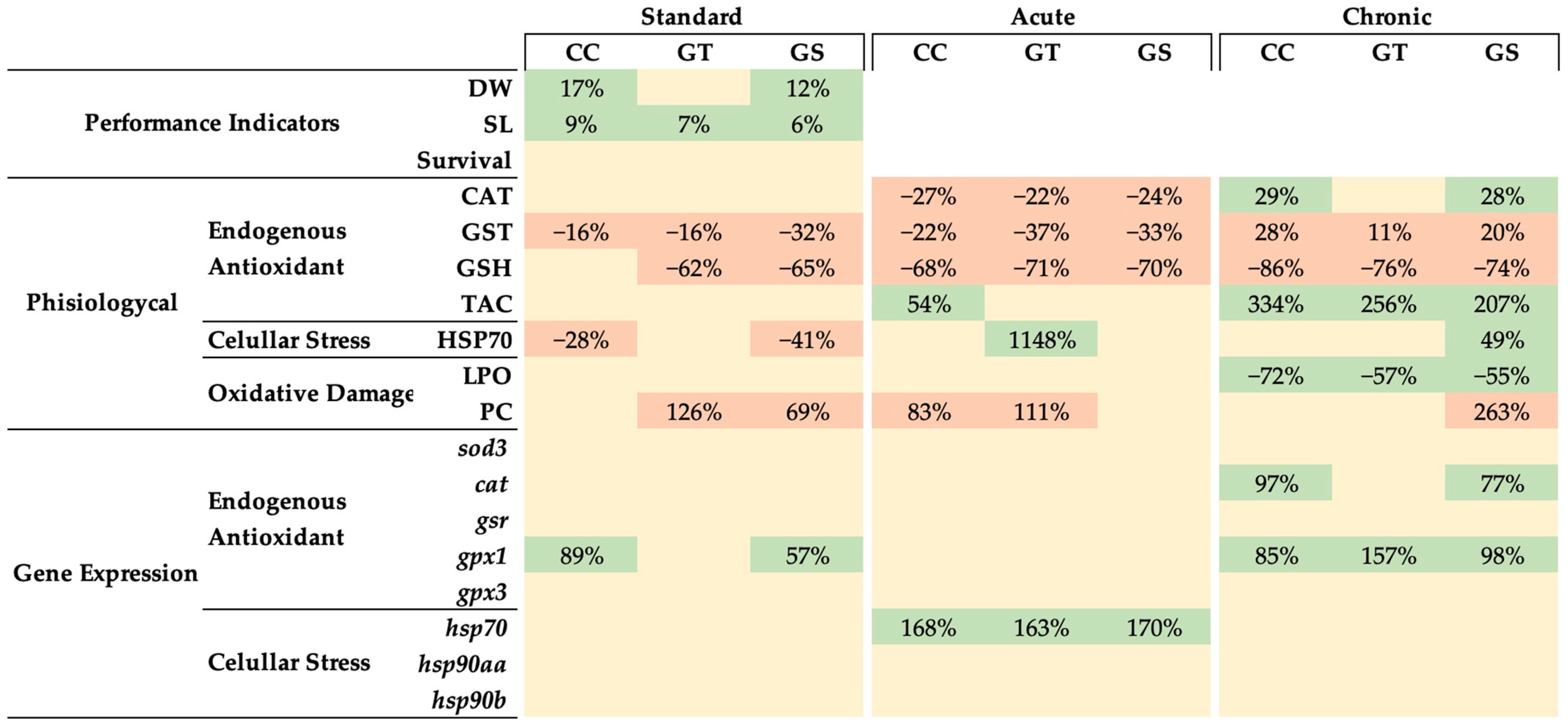
3.1. Dietary Antioxidant Properties
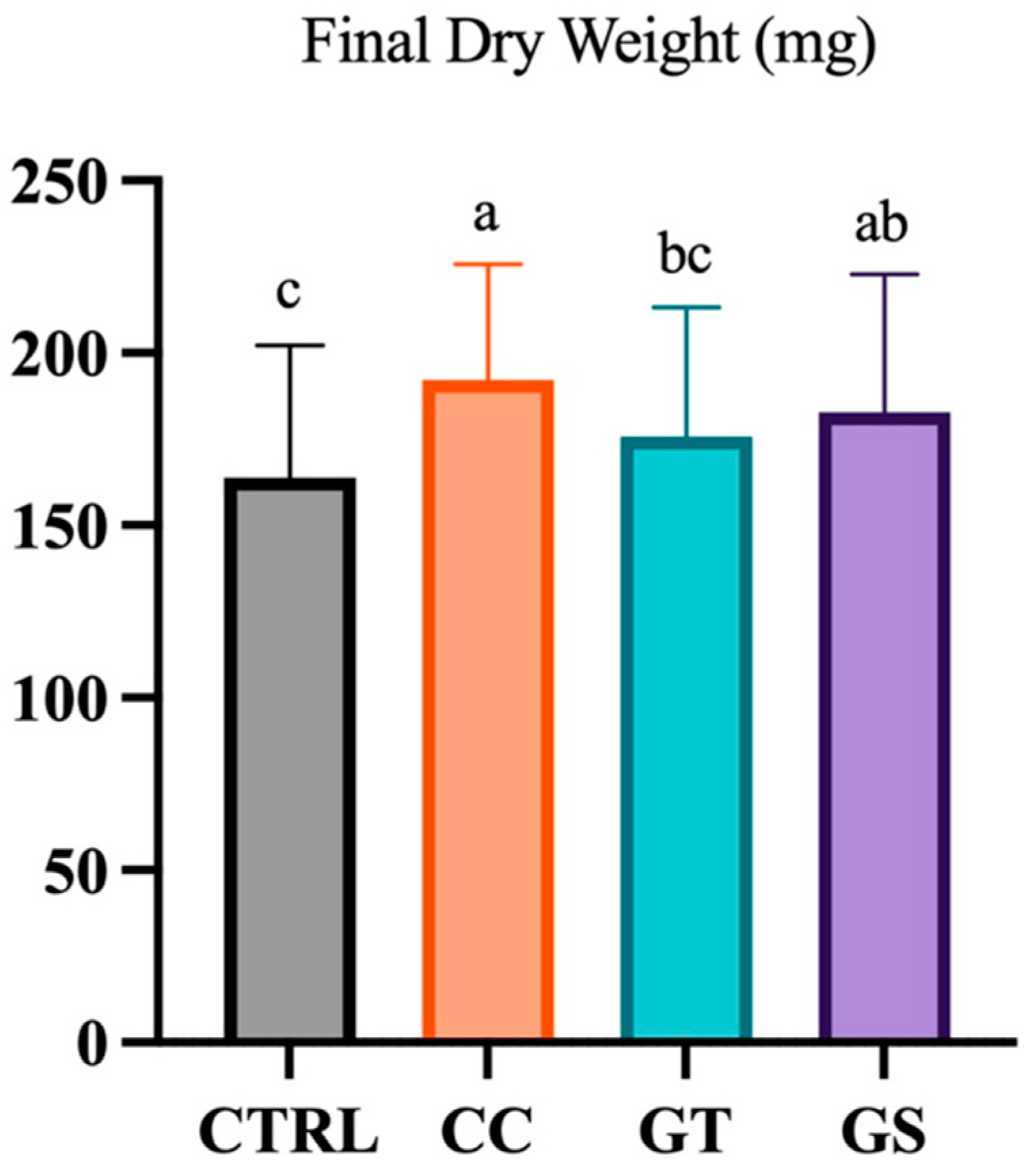
3.2. Key Performance Indicators
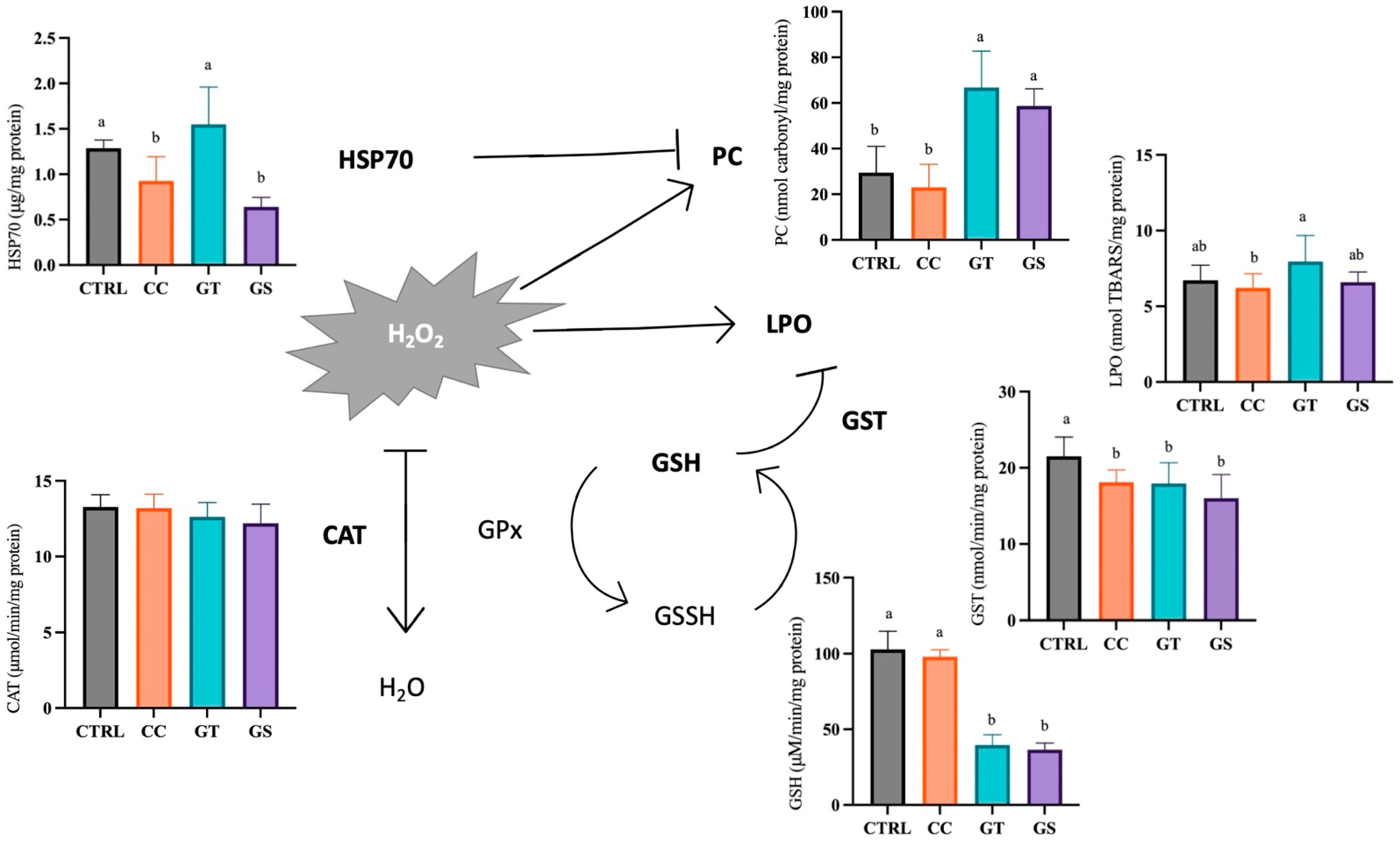
3.3. Oxidative Status and Cellular Stress Indicators
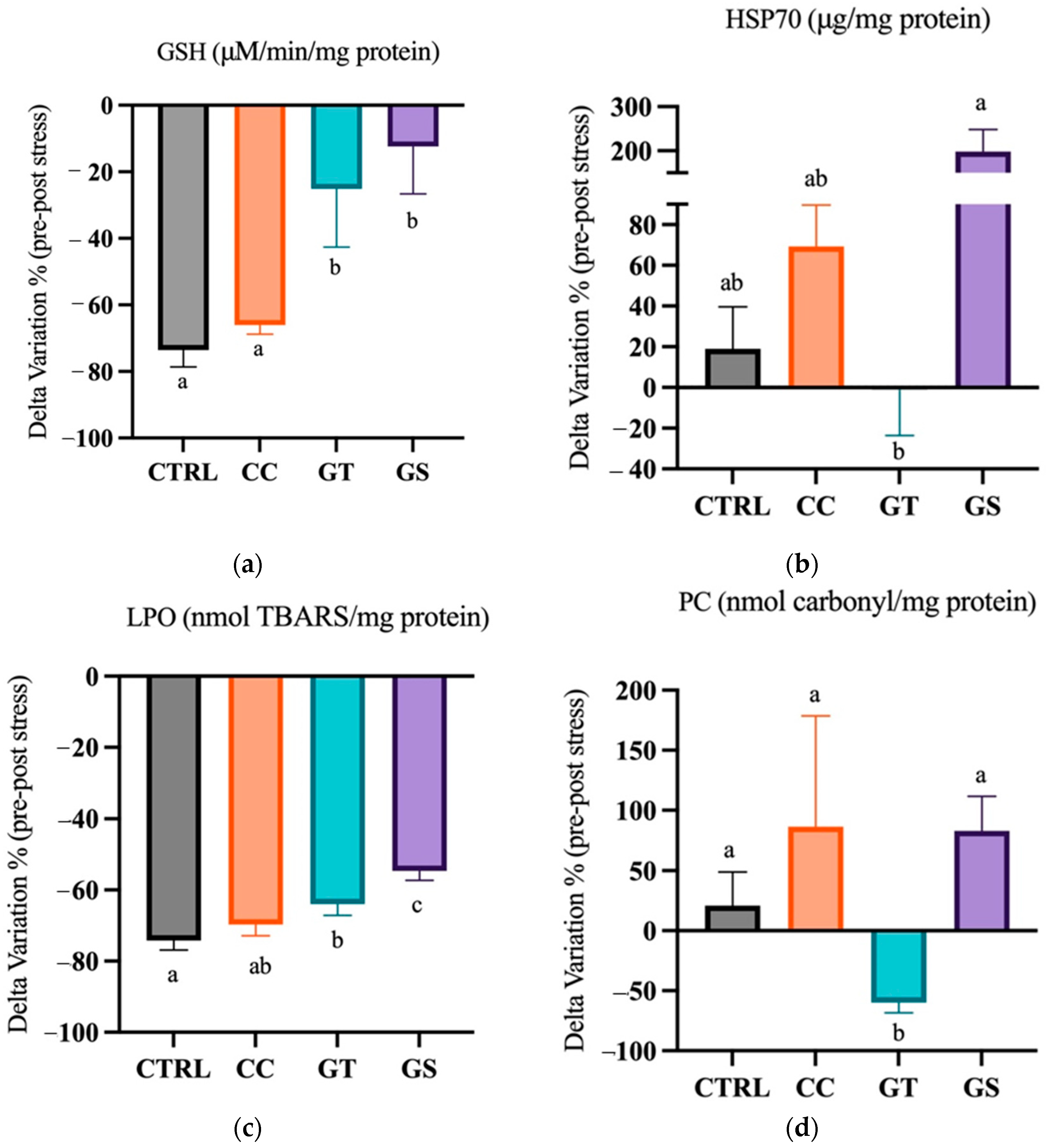
3.3.1. Thermal Stress—Acute Exposure
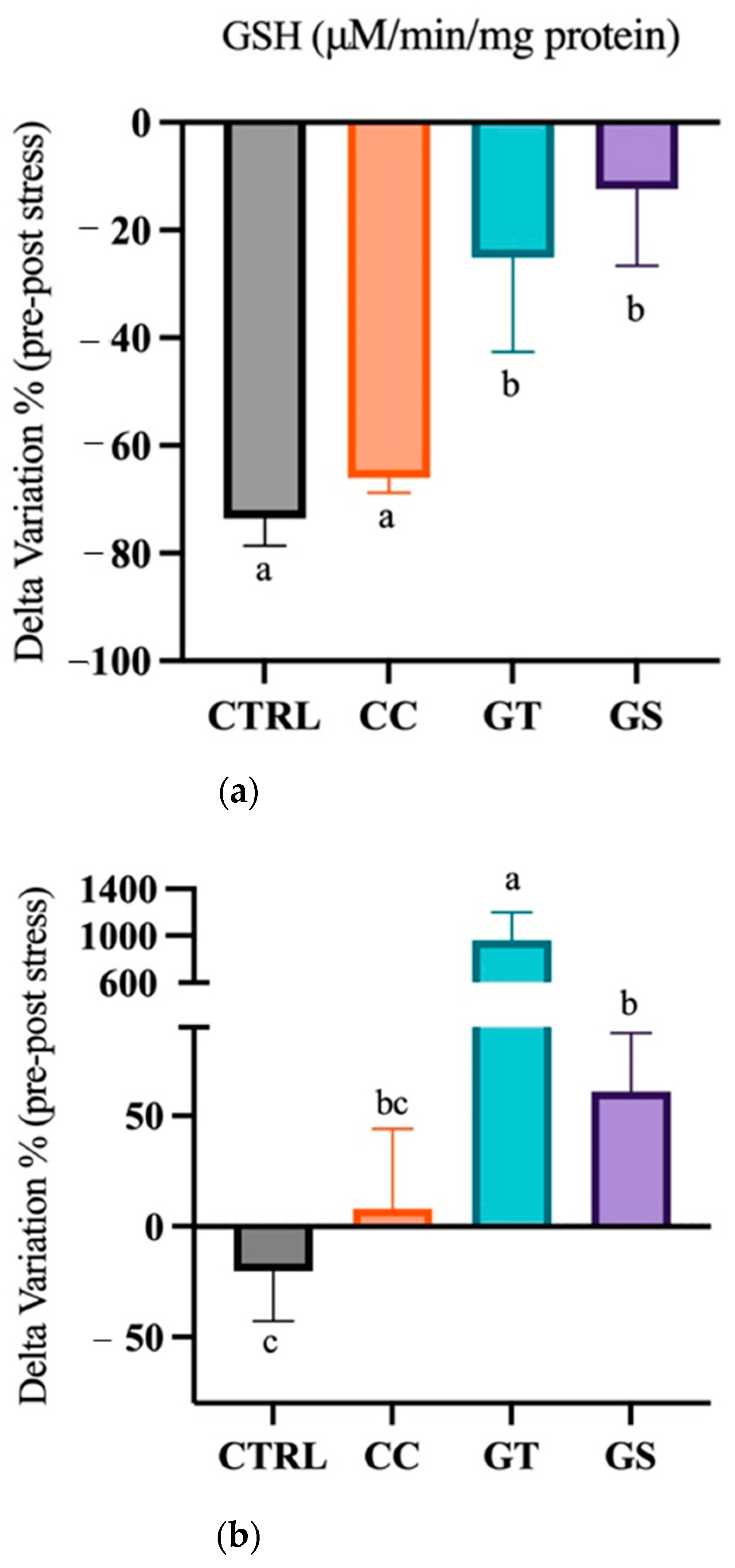
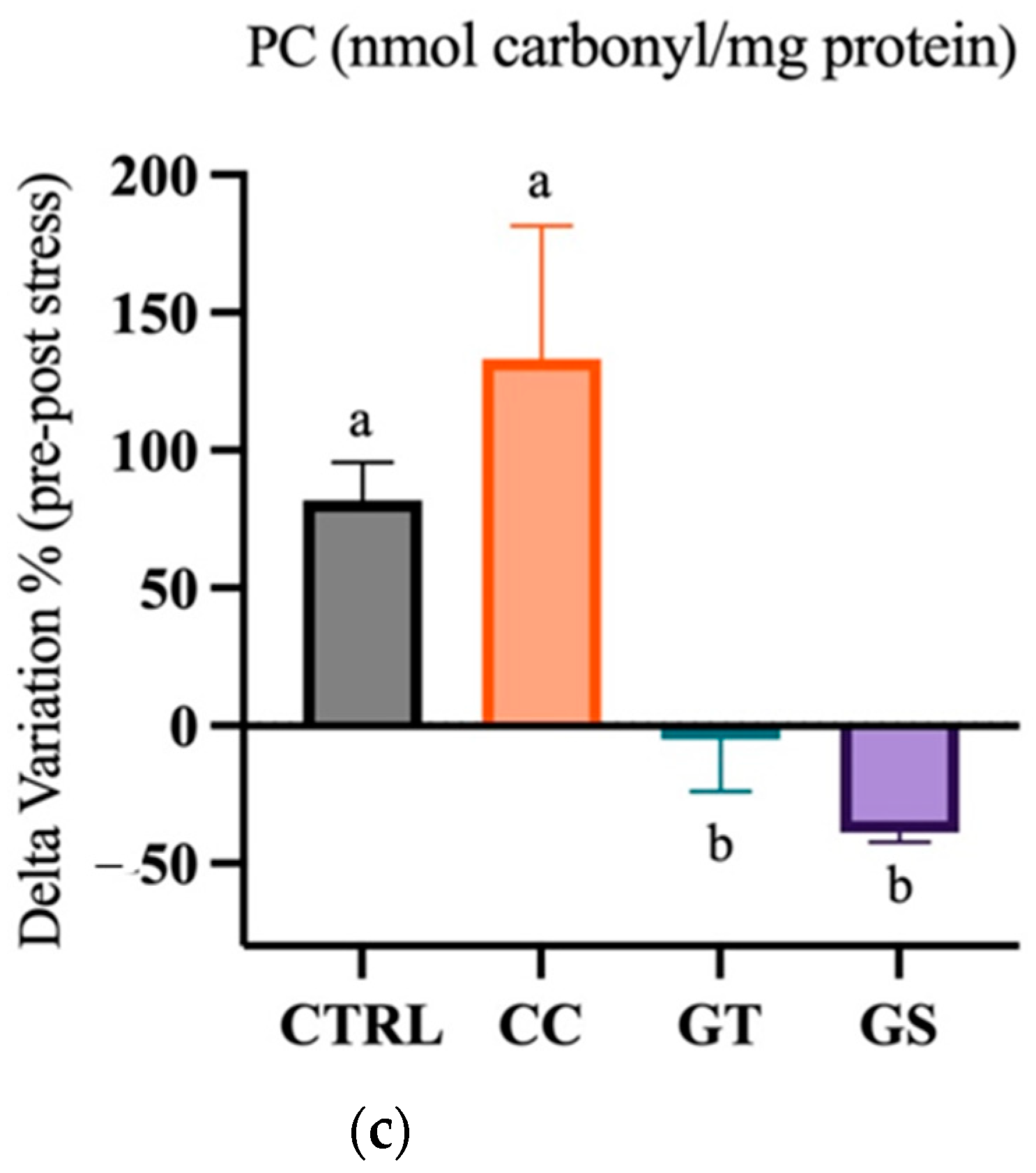
3.3.2. Thermal Stress—Chronic Exposure
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2016, 20, 1689–1699. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A.; Mohamadin, A.M.; Abdel-Naim, A.B. In Vitro Evaluation of the Antioxidant Activities of Grape Seed (Vitis vinifera) Extract, Blackseed (Nigella sativa) Extract and Curcumin. J. Taibah Univ. Med. Sci. 2009, 4, 23–35. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. 2009, 14, 226–246. [Google Scholar]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Orhan, I.E.; Bawazeer, S. Health perspectives of a bioactive compound curcumin: A review. Trends Food Sci. Technol. 2018, 74, 33–45. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Park, S.C. Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2019, 92, 612–620. [Google Scholar] [CrossRef]
- Xia, S.-L.; Ge, X.-P.; Liu, B.; Xie, J.; Miao, L.-H.; Zhou, Q.-L.; Zhang, W.-X.; Jiang, X.-J.; Chen, R.-L.; Pan, L.-K. Effects of supplemented dietary curcumin on growth and non-specific immune responses in juvenile wuchang bream (Megalobrama amblycephala). Isr. J. Aquacult. Bamidgeh 2015. [Google Scholar] [CrossRef]
- Midhun, S.J.; Arun, D.; Edatt, L.; Sruthi, M.V.; Thushara, V.V.; Oommen, O.V.; Sameer Kumar, V.B.; Divya, L. Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, Tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac. Int. 2016, 24, 1277–1286. [Google Scholar] [CrossRef]
- Akdemir, F.; Orhan, C.; Tuzcu, M.; Sahin, N.; Juturu, V.; Sahin, K. The efficacy of dietary curcumin on growth performance, lipid peroxidation and hepatic transcription factors in rainbow trout Oncorhynchus Mykiss (Walbaum) reared under different stocking densities. Aquac. Res. 2017, 48, 4012–4021. [Google Scholar] [CrossRef]
- Cui, H.; Liu, B.; Ge, X.-P.; XiE, J.; Xu, P.; Miao, L.-H.; Sun, S.; Liao, Y.; Chen, R.; Ren, M. Effects of dietary curcumin on growth performance, biochemical parameters, HSP70 gene expression and resistance to Streptococcus iniae of juvenile Gift Tilapia, Oreochromis niloticus. Isr. J. Aquac. 2013, 66, 986–996. [Google Scholar]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Y.; Ji, H.; Yu, H. The Effect of Green Tea Waste on Growth and Health of Grass Carp (Ctenopharyngodon idellus). Turk. J. Fish. Aquat. Sci. 2016, 16, 679–689. [Google Scholar] [CrossRef]
- Nootash, S.; Sheikhzadeh, N.; Baradaran, B.; Oushani, A.K.; Maleki Moghadam, M.R.; Nofouzi, K.; Monfaredan, A.; Aghebati, L.; Zare, F.; Shabanzadeh, S. Green tea (Camellia sinensis) administration induces expression of immune relevant genes and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 1916–1923. [Google Scholar] [CrossRef]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—Biochemistry and functionality. J. Med. Food 2003, 6, 91–299. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Yigit, M. Structural and chemical changes of grape seed extract after thermal processing and its use in rainbow trout (Oncorhynchus mykiss) diets as an organic feed supplement. Aquaculture 2019, 503, 275–281. [Google Scholar] [CrossRef]
- Xavier, M.J.; Engrola, S.; Conceição, L.E.C.; Manchado, M.; Carballo, C.; Gonçalves, R.; Colen, R.; Figueiredo, V.; Valente, L.M.P. Dietary Antioxidant Supplementation Promotes Growth in Senegalese Sole Postlarvae. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Husain, H.; Latief, U.; Ahmad, R. Pomegranate action in curbing the incidence of liver injury triggered by Diethylnitrosamine by declining oxidative stress via Nrf2 and NFκB regulation. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Lau, A.T.; Wang, Y.; Chiu, J.F. Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem. 2008, 104, 657–667. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, F.D. Fish antioxidant defenses—A comparative approach. Braz. J. Med. Biol. Res. 1996, 29, 1735. [Google Scholar]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crops Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Engrola, S.; Conceição, L.; Gavaia, P.; Cancela, M.L.; Dinis, M. Effects of pre-weaning feeding frequency on growth, survival, and deformation of Senegalese sole, Solea senegalensis (Kaup, 1858). Isr. J. Aquacult. Bamidgeh 2005, 57, 10–18. [Google Scholar]
- Ricker, W.E. Handbook of Computations for Biological Statistics of Fish Populations; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1958; pp. 1–300. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In Handbook Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.; Pestana, J.L. Exposure to chlorantraniliprole affects the energy metabolism of the caddisfly Sericostoma vittatum. Environ. Toxicol. Chem. 2017, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2, 4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Rosa, R.; Trübenbach, K.; Pimentel, M.S.; Boavida-Portugal, J.; Faleiro, F.; Baptista, M.; Dionísio, G.; Calado, R.; Pörtner, H.O.; Repolho, T. Differential impacts of ocean acidification and warming on winter and summer progeny of a coastal squid (Loligo vulgaris). J. Exp. Biol. 2014, 217, 518–525. [Google Scholar] [CrossRef]
- Firmino, J.; Carballo, C.; Armesto, P.; Campinho, M.A.; Power, D.M.; Manchado, M. Phylogeny, expression patterns and regulation of DNA Methyltransferases in early development of the flatfish, Solea senegalensis. BMC Dev. Biol. 2017, 17, 11. [Google Scholar] [CrossRef]
- Manchado, M.; Salas-Leiton, E.; Infante, C.; Ponce, M.; Asensio, E.; Crespo, A.; Zuasti, E.; Cañavate, J.P. Molecular characterization, gene expression and transcriptional regulation of cytosolic HSP90 genes in the flatfish Senegalese sole (Solea senegalensis Kaup). Gene 2008, 416, 77–84. [Google Scholar] [CrossRef]
- Infante, C.; Matsuoka, M.P.; Asensio, E.; Cañavate, J.P.; Reith, M.; Manchado, M. Selection of housekeeping genes for gene expression studies in larvae from flatfish using real-time PCR. BMC Mol. Biol. 2008, 9, 28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ennos, A.R. Statistical and Data Handling Skills in Biology, 2nd ed.; Pearson Prentice Hall: Harlow, UK, 2007; pp. 55–60. [Google Scholar]
- Beaulieu, M.; Costantini, D. Biomarkers of oxidative status: Missing tools in conservation physiology. Conserv. Physiol. 2014, 2. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Reeg, S.; Jung, T.; Castro, J.P.; Davies, K.J.; Henze, A.; Grune, T. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic. Biol. Med. 2016, 99, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Moustafa, A.A.; Rahman Mohamed, A.A. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef]
- Manju, M.; Akbarsha, M.A.; Oommen, O.V. In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol. Biochem. 2012, 38, 309–318. [Google Scholar] [CrossRef]
- Yonar, M.E.; Mise Yonar, S.; Ispir, U.; Ural, M.S. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Li, G.; Zhou, X.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Shi, H.; Feng, L. Dietary curcumin supplementation enhanced growth performance, intestinal digestion, and absorption and amino acid transportation abilities in on-growing grass carp (Ctenopharyngodon idella). Aquac. Res. 2020, 51, 4863–4873. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Galati, G.; O’brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Sönmez, A.Y.; Yank, T. Effects of grape Vitis vinifera seed oil supplementation on growth, survival, fatty acid profiles, antioxidant contents and blood parameters in rainbow trout Oncorhynchus mykiss. Aquac. Res. 2018, 49, 2256–2266. [Google Scholar] [CrossRef]
- Thawonsuwan, J.; Kiron, V.; Satoh, S.; Panigrahi, A.; Verlhac, V. Epigallocatechin-3-gallate (EGCG) affects the antioxidant and immune defense of the rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 2010, 36, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef]
- Iftikar, F.I.; Hickey, A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 2013, 8, e64120. [Google Scholar] [CrossRef] [PubMed]
- Lygren, B.; Hamre, K.; Waagbø, R. Effects of dietary pro-and antioxidants on some protective mechanisms and health parameters in Atlantic salmon. J. Aquat. Anim. Health 1999, 11, 211–221. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Verdi, C.M.; Zeppenfeld, C.C.; de Lima Silva, L.; Gindri, A.L.; Cunha, M.A.; Santos, R.C.V.; Baldisserotto, B.; et al. Effects of dietary grape pomace flour on the purinergic signaling and inflammatory response of grass carp experimentally infected with Pseudomonas aeruginosa. Aquaculture 2019, 503, 217–224. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.E.H.; Soliman, H.A.M. Modulatory effects of green tea extract against the hepatotoxic effects of 4-nonylphenol in catfish (Clarias gariepinus). Ecotoxicol. Environ. Saf. 2018, 149, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, S.; Salati, A.P.; Falahatkar, B.; Azarm, H.M. Effects of dietary green tea (Camellia sinensis L.) supplementation on growth performance, lipid metabolism, and antioxidant status in a sturgeon hybrid of Sterlet (Huso huso ♂ x Acipenser ruthenus ♀) fed oxidized fish oil. Fish Physiol. Biochem. 2017, 43, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
| Assay/Parameter | Experimental Diets | 1-Way Anova | |||
|---|---|---|---|---|---|
| CTRL | CC | GT | GS | p Value | |
| ABTS (% of activity) | 19.4 ± 2.4 b | 18.5 ± 0.9 b | 17.3 ± 1.4 b | 28.6 ± 1.3 a | <0.001 |
| DPPH (% of activity) | 8.9 ± 3.2 c | 50.2 ± 9.9 a | 34.1 ± 2.0 b | 39.9 ± 4.1 b | <0.001 |
| TPC (mg GAE/g diet) | 1.3 ± 0.6 b | 6.4 ± 1.9 a | 5.0 ± 2.1 a | 5.4 ± 0.9 a | <0.001 |
| TFC (mg QE/g diet) | 0.7 ± 0.3 c | 2.9 ± 0.7 ab | 1.5 ± 0.2 bc | 4.4 ± 3.7 a | <0.001 |
| Gene Expression | Standard Condition | 1-Way Anova | |||
|---|---|---|---|---|---|
| CTRL | CC | GT | GS | p Value | |
| sod3 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.446 |
| cat | 1.0 ± 0.1 ab | 1.1 ± 0.1 ab | 0.9 ± 0.1 b | 1.4 ± 0.1 a | 0.046 |
| gsr | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.0 | 1.4 ± 0.2 | 0.266 |
| gpx1 | 1.0 ± 0.1 b | 1.9 ± 0.5 a | 1.1 ± 0.1 b | 1.6 ± 0.2 a | 0.047 |
| gpx3 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 | 0.431 |
| hsp70 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.1 | 1.6 ± 0.3 | 0.309 |
| hsp90aa | 1.0 ± 0.1 | 3.3 ± 0.6 | 1.5 ± 0.5 | 1.8 ± 0.4 | 0.051 |
| hsp90b | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 | 1.0 ± 0.1 | 0.453 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, M.J.; Conceição, L.E.C.; Valente, L.M.P.; Colen, R.; Rodrigues, A.C.M.; Rocha, R.J.M.; Custódio, L.; Carballo, C.; Manchado, M.; Engrola, S. Dietary Natural Plant Extracts Can Promote Growth and Modulate Oxidative Status of Senegalese Sole Postlarvae under Standard/Challenge Conditions. Animals 2021, 11, 1398. https://doi.org/10.3390/ani11051398
Xavier MJ, Conceição LEC, Valente LMP, Colen R, Rodrigues ACM, Rocha RJM, Custódio L, Carballo C, Manchado M, Engrola S. Dietary Natural Plant Extracts Can Promote Growth and Modulate Oxidative Status of Senegalese Sole Postlarvae under Standard/Challenge Conditions. Animals. 2021; 11(5):1398. https://doi.org/10.3390/ani11051398
Chicago/Turabian StyleXavier, Maria J., Luís E. C. Conceição, Luisa M. P. Valente, Rita Colen, Andreia C. M. Rodrigues, Rui J. M. Rocha, Luísa Custódio, Carlos Carballo, Manuel Manchado, and Sofia Engrola. 2021. "Dietary Natural Plant Extracts Can Promote Growth and Modulate Oxidative Status of Senegalese Sole Postlarvae under Standard/Challenge Conditions" Animals 11, no. 5: 1398. https://doi.org/10.3390/ani11051398
APA StyleXavier, M. J., Conceição, L. E. C., Valente, L. M. P., Colen, R., Rodrigues, A. C. M., Rocha, R. J. M., Custódio, L., Carballo, C., Manchado, M., & Engrola, S. (2021). Dietary Natural Plant Extracts Can Promote Growth and Modulate Oxidative Status of Senegalese Sole Postlarvae under Standard/Challenge Conditions. Animals, 11(5), 1398. https://doi.org/10.3390/ani11051398









