Effects of Dietary Inclusion of Dry Hydrastis canadensis on Laying Performance, Egg Quality, Serum Biochemical Parameters and Cecal Microbiota in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Goldenseal Source Materials
2.2. Laying Hens and Experimental Design
2.3. Proximate Analysis
2.4. DNA Extraction and Microbiota Analysis
2.5. Laying Performance and Egg Quality
2.6. Serum Biochemical Parameters
2.7. Statistical Analysis
3. Results
3.1. Effect of Hydrastis canadensis on Laying Performance, Egg Quality, and Blood Biochemical Parameters of Laying Hens
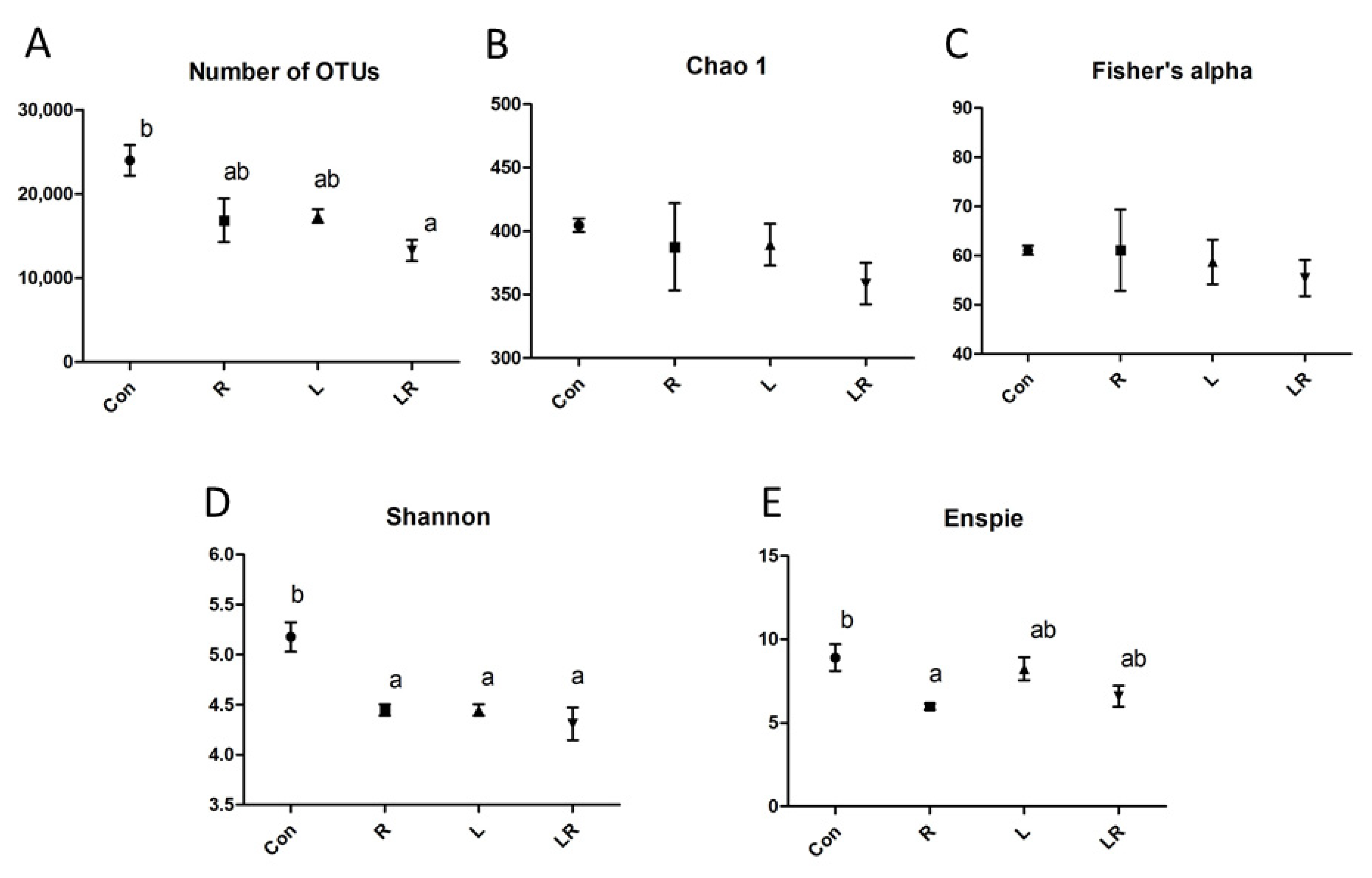
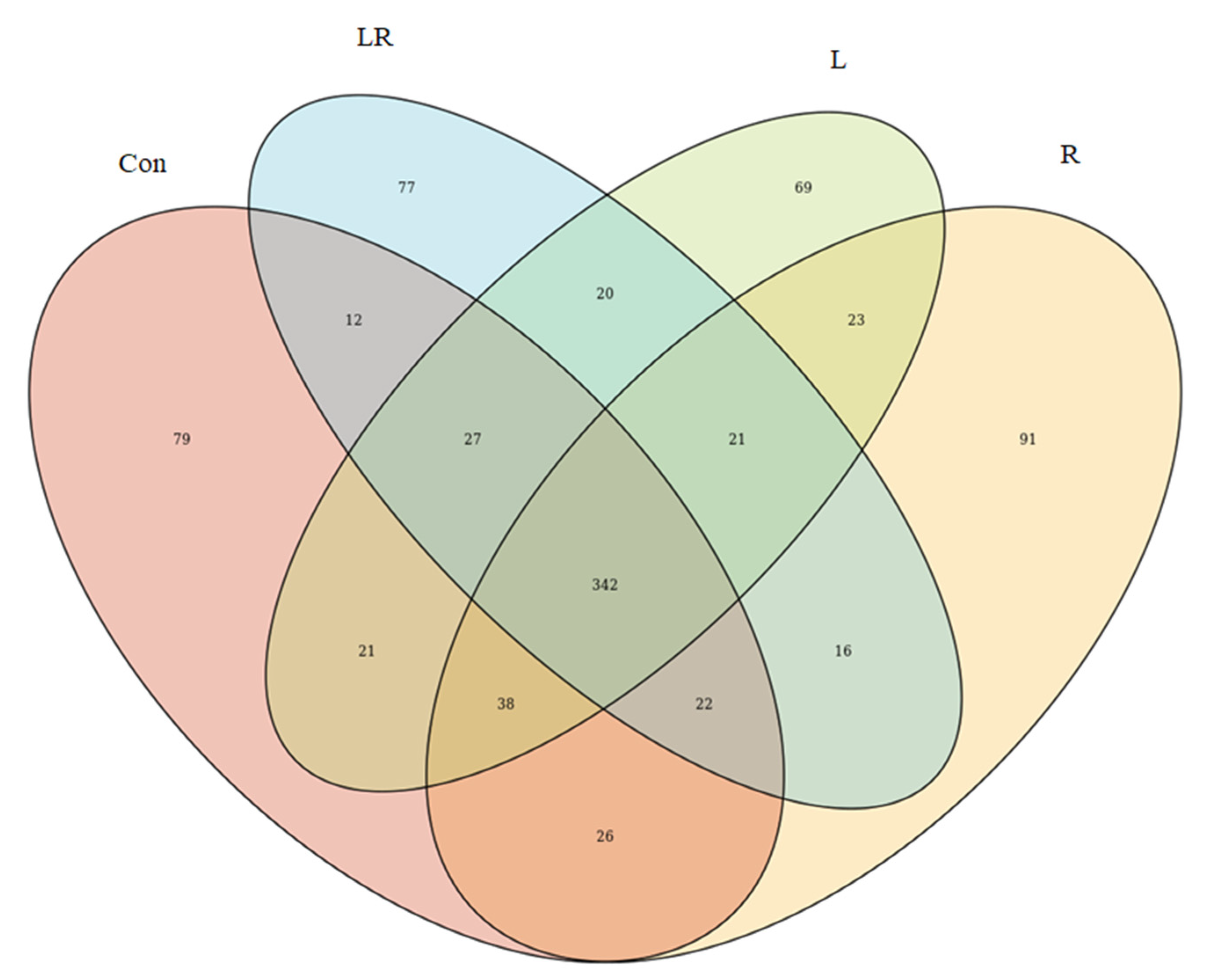
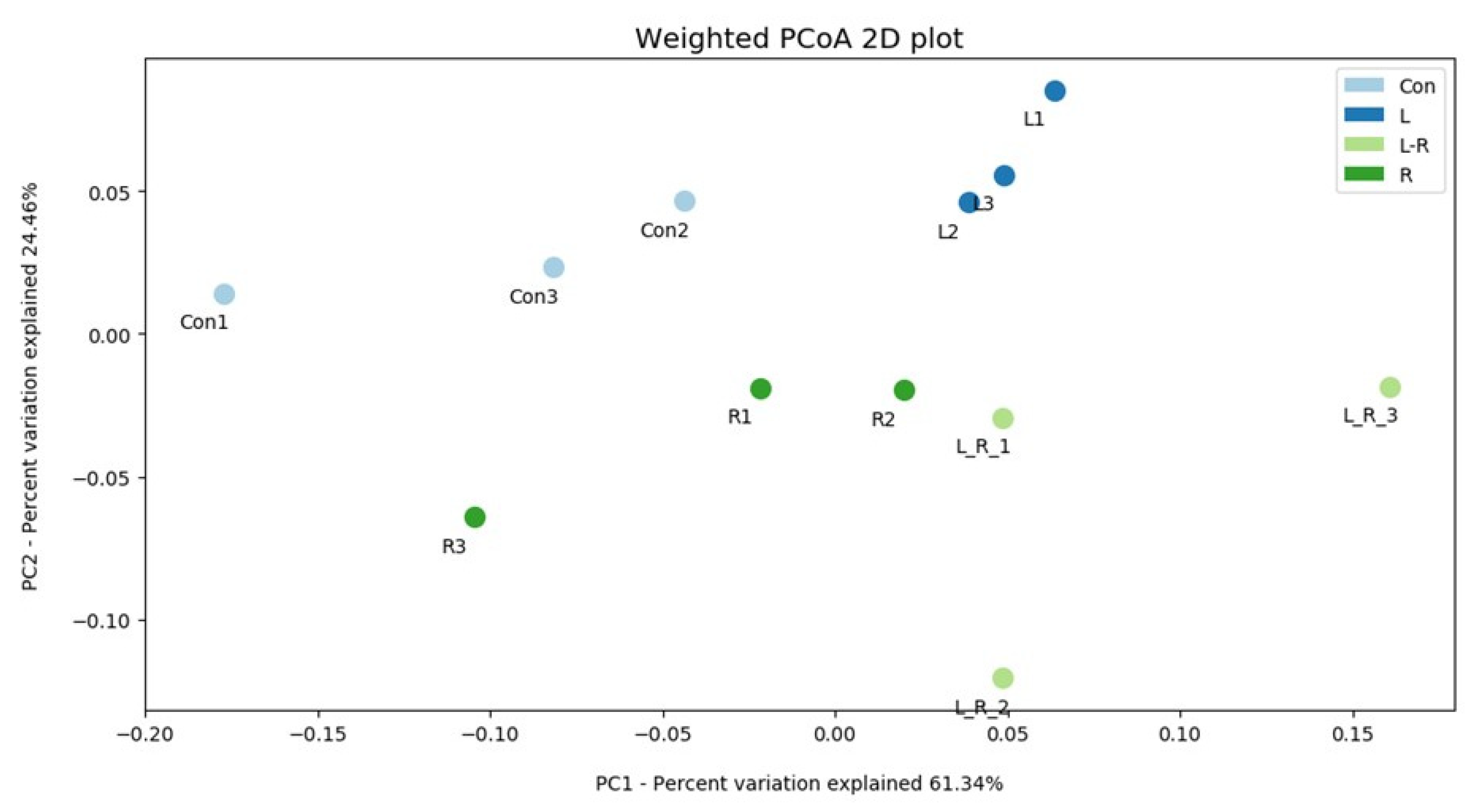
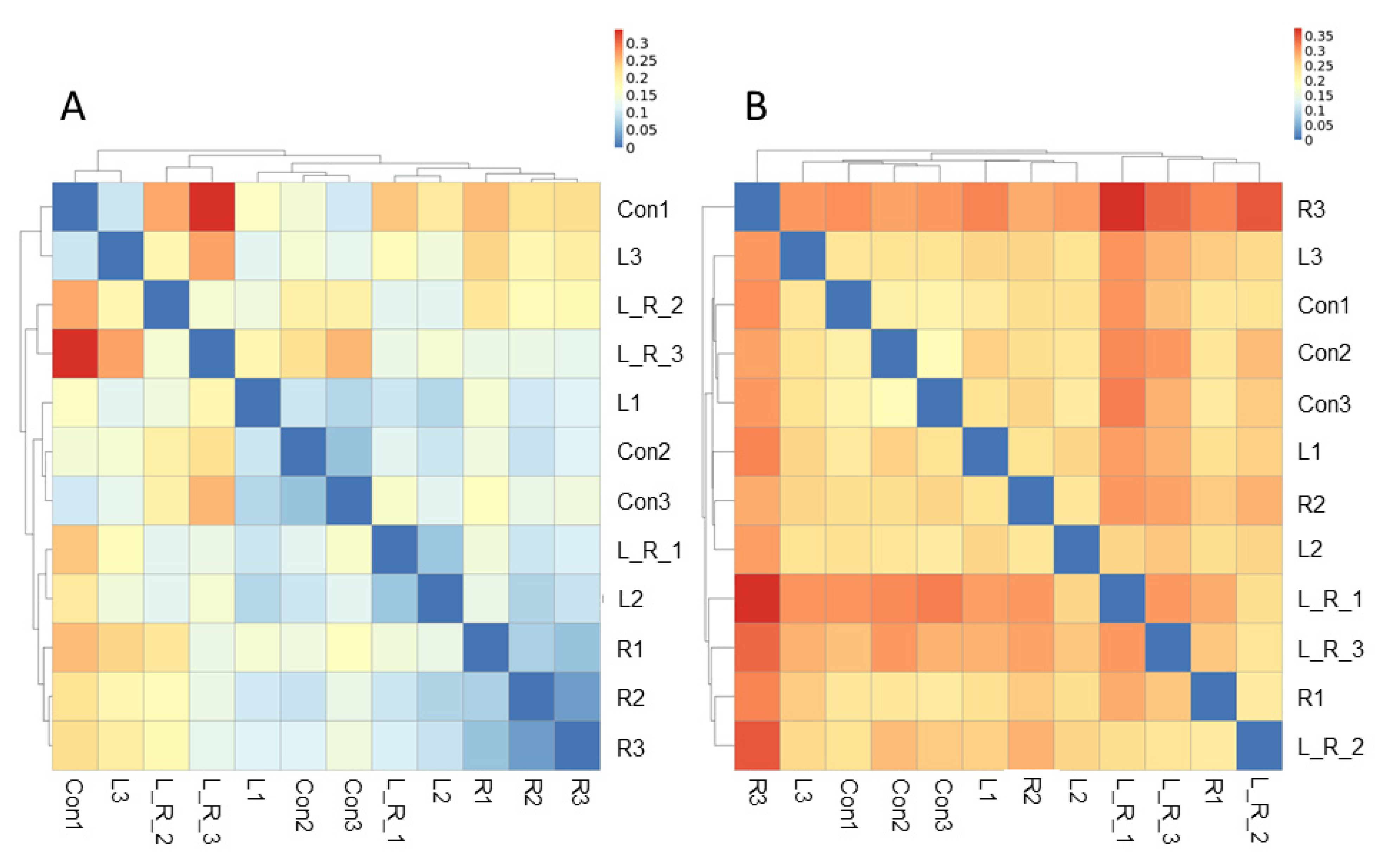
3.2. Effect of Hydrastis canadensis on Cecal Digesta Microbiota
3.3. Effect of Hydrastis canadensis on Bacterial Taxonomic Composition of Cecal Digesta
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, H.A.; Zart, M.K.; Hodges, A.E.; Molloy, H.M.; O’Brien, B.M.; Moody, L.A.; Clark, A.P.; Harris, R.; Overstreet, J.D.; Smith, C.S. Chemical comparison of goldenseal (Hydrastis canadensis L.) root powder from three commercial supploers. J. Agric. Food Chem. 2003, 51, 7352–7358. [Google Scholar] [CrossRef] [PubMed]
- Berkel, G.J.V.; Tomkins, B.A.; Kertesz, V. Thin-layer chromatography/desorption electrospray ionization mass spectrometry: Investigation of goldenseal alkaloids. Anal. Chem. 2007, 79, 2778–2789. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Draper, E.J. Variations in alkaloid content of herbal products containing goldenseal. J. Am. Pharm. Assoc. 2003, 43, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Inflammation and Native American medicine: The role of botanicals. Am. J. Clin. Nutr. 2000, 72, 339–347. [Google Scholar] [CrossRef]
- Brown, P.N.; Roman, M.C. Determination of hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements by high-performance liquid chromatography with UV: Collaborative study. J. AOAC Int. 2008, 91, 694–701. [Google Scholar] [CrossRef]
- Egan, J.M.; Kaur, A.; Raja, H.A.; Kellogg, J.J.; Oberlies, N.H.; Cech, N.B. Antimicrobial fungal endophytes from the botanical medicine goldenseal (Hydrastis canadensis). Phytochem. Lett. 2016, 17, 219–225. [Google Scholar] [CrossRef]
- Silva, A.R.D.; Neto, J.B.D.A.; Silva, C.R.D.; Campos, R.D.S.; Silva, R.A.C.; Freitas, D.D.; Nascimento, F.B.S.A.D.; Andrade, L.N.D.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in fluconazole-resistance pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef]
- Tims, M.C.; Batista, C. Effects of root isoquinoline alkaloids from Hydrastis canadensis on Fusarium oxysporum isolated from Hydrastis root Tissue. J. Chem. Ecol. 2007, 33, 1449–1455. [Google Scholar] [CrossRef]
- Saha, P.; Sen, R.; Hariharan, C.; Kumar, D.; Das, P.; Chatterjee, M. Berberine chloride causes a caspase-independent, apoptotic-like death in Leishmania donovani promastigotes. Free Radic. Res. 2009, 43, 1101–1110. [Google Scholar] [CrossRef]
- Cecil, C.E.; Davis, J.M.; Cech, N.B.; Laster, S.M. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). Int. Immunopharmacol. 2011, 11, 1706–1714. [Google Scholar] [CrossRef]
- Arjoon, A.V.; Saylor, C.V.; May, M. In Vitro efficacy of antimicrobial extracts against the atypical ruminant pathogen Mycoplasma mycoides subsp. capri. BMC Complement. Altern. Med. 2012, 12, 169. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Cunha, B.G.; Paulini, M.B.; Goiato, M.C.; Dos Santos, D.M.; Duque, C.; Caiaffa, K.S.; Brandini, D.A.; de Oliveira, D.T.N.; Brizzotti, N.S.; et al. Antimicrobial activity of conventional and plant-extract disinfectant solutions on microbial biofilms on a maxillofacial polymer surface. J. Prosthet. Dent. 2016, 116, 136–143. [Google Scholar] [CrossRef]
- Cech, N.B.; Junio, H.A.; Ackermann, L.W.; Kavanaugh, J.S.; Horswill, A.R. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2012, 78, 1556–1561. [Google Scholar] [CrossRef]
- Britton, E.R.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). J. Nat. Prod. 2018, 81, 484–493. [Google Scholar] [CrossRef]
- Ettefagh, K.A.; Burns, J.T.; Junio, H.A.; Kaatz, G.W.; Cech, N.B. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 2011, 77, 835–840. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Britton, E.R.; Foil, D.H.; Brown, A.R.; Todd, D.A.; Rivera-Chavez, J.; Oberlies, N.H.; Cech, N.B. Secondary Metabolites from the Leaves of the Medicinal Plant Goldenseal (Hydrastis canadensis). Phytochem. Lett. 2017, 20, 54–60. [Google Scholar] [CrossRef]
- Clement-Kruzel, S.; Hwang, S.A.; Kruzel, M.C.; Dasgupta, A.; Actor, J.K. Immune modulation of macrophage pro-inflammatory response by goldenseal and Astragalus extracts. J. Med. Food 2008, 11, 493–498. [Google Scholar] [CrossRef]
- Abidi, P.; Chen, W.; Kraemer, F.B.; Li, H.; Liu, J. The medicinal plant goldenseal is a natural LDL-lowering agent with multiple bioactive components and new action mechanisms. J. Lipid Res. 2006, 47, 2134–2147. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine and inflammatory bowel disease: A concise review. Pharmacol. Res. 2016, 113 Pt A, 592–599. [Google Scholar] [CrossRef]
- Karmakar, S.R.; Biswas, S.J.; Khuda-Bukhsh, A.R. Anti-carcinogenic potentials of a plant extract (Hydrastis canadensis): I. Evidence from In Vivo studies in mice (Mus musculus). Asian Pac. J. Cancer Prev. 2010, 11, 545–551. [Google Scholar] [PubMed]
- Kim, J.B.; Yu, J.H.; Ko, E.; Lee, K.W.; Song, A.K.; Park, S.Y.; Shin, I.; Han, W.; Noh, D.Y. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine 2010, 17, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, H.; Cometa, M.F.; Palmery, M.; Leone, M.G.; Silvestrini, B.; Saso, L. Relaxant effects of Hydrastis canadensis L. and its major alkaloids on guinea pig isolated trachea. Pharmacol. Toxicol. 2000, 87, 218–222. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Roberts, S.K.; Chadwick, Wu, C.D.; Kinghorn, A.D. Antimicrobial constituents from goldenseal (the Rhizomes of Hydrastis canadensis) against selected oral pathogens. Planta Med. 2003, 69, 623–627. [Google Scholar] [PubMed]
- Gentry, E.J.; Jampani, H.B.; Keshavarz-Shokri, A.; Morton, M.D.; Velde, D.V.; Telikepalli, H.; Mitscher, L.A.; Shawar, R.; Humble, D.; Baker, W. Antitubercular natural products: Berberine from the roots of commercial Hydrastis canadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, beta-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and -2. J. Nat. Prod. 1998, 61, 1187–1193. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.P.; Gaucher, M.L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Rajaian, H.; Jalaee, J.; Aghajani, A. Berberis vulgaris as growth promoter in broiler chickens. Int. J. Poult. Sci. 2006, 5, 395–397. [Google Scholar]
- Xiao, P.; He, J.; Pouton, C.; Xiao, Z.C. A large scale safety study to investigate the inclusion of phytogenic compounds in broiler chicken feed. Approaches Poult. Dairy Vet. Sci. 2018, 5, 417–423. [Google Scholar]
- Maehre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination-method Matters. Foods 1984, 7, 5. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.T.; Peng, X.; Deng, G.H.; Sheng, H.F.; Wang, Y.; Zhou, H.W.; Tam, N.F.Y. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S.; et al. Berberine improved experimental chronic colitis by regulating interferon-γand IL-17A-producing lamina propria CD4+ Tcells through AMPK activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef]
- Jin, F.; Xie, T.; Huang, X.; Zhao, X. Berberine inhibits angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway. Pharm. Biol. 2018, 56, 665–671. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M.; et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine Improves Lipid Dysregulation in Obesity by Controlling Central and Peripheral AMPK Activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, M.; Hu, Y.; Zhao, Y.; Teng, F.; Lv, X.; Li, J.; Zhang, Y.; Hatch, G.M.; Chen, L. Increased biovailable berberine protects against myocardial ischenia reperfusion injury through attenuation of NFκB and JNK signaling pathway. Int. Heart J. 2018, 59, 1378–1388. [Google Scholar] [CrossRef]
- Yu, D.X.; He, Z.; Pouton, C.; Hoerr, F.J.; Xiao, Z.C. Target animal safety and residual study for berberine and other phytogenic compounds in broiler chickens. Arch. Clin. Microbiol. 2017, 8, 69. [Google Scholar]
- Xu, J.H.; Liu, X.Z.; Pan, W.; Zou, D.J. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017, 15, 2765–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Liang, X.; Wang, M.; Zhu, X.; Luo, Y.; Shang, Y.; Yang, J.Q.; Zhou, P.; Gu, X.L. Effects of berberine on the growth performance, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Anim. Sci. J. 2019, 90, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Castillo, A.M.; Adánez, G.; Fernández-Rufete, A.; Pérez, B.G.; Castells, M.T. Hyperlipidemic chicken as a model of non-alcoholic steatohepatitis. Exp. Biol. Med. 2009, 234, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Zhou, H.; Zhou, X.; Xia, Y.; Dong., X.; Zhong, W.; Tang, S.; Wang, L.; Wen, S.; et al. Gut microbiome associates with lipid-lowering effect of rosuvastatin in vivo. Front. Microbiol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Robben, J.; Janssen, G.; Merckx, R.; Eyssen, H. Formation of delta 2- and delta 3-cholenoic acids from bile acid 3-sulfates by a human intestinal Fusobacterium strain. Appl. Environ. Microbiol. 1989, 55, 2954–2959. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; He, Z.; Ma, K.Y.; Chen, Z.Y. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male Syrian hamster. J. Agric. Food Chem. 2017, 65, 10984–10992. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Marcil, V.; Delvin, E.; Garofalo, C.; Levy, E. Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J. Nutr. 2003, 133, 2180–2183. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]

| Ingredient | Root (R) | Leave (L) |
|---|---|---|
| Dry matter (DM, %) | 96.40 | 96.81 |
| Crude protein (% DM) | 13.36 | 9.72 |
| Neutral detergent fiber (% DM) | 32.67 | 29.96 |
| Acid detergent fiber (% DM) | 29.20 | 27.47 |
| Acid detergent lignin (% DM) | 6.03 | 5.62 |
| Ether extract (% DM) | 0.94 | 1.98 |
| Ash (% DM) | 4.53 | 11.59 |
| Berberine (%) | 0.38 | 0.11 |
| Ingredient | Composition (%) | |||
|---|---|---|---|---|
| Basal Diet | R Diet | L Diet | LR Diet | |
| Corn | 52.20 | 51.89 | 51.89 | 51.89 |
| Soybean meal (CP 47%) | 30.30 | 30.12 | 30.12 | 30.12 |
| CaCO3 | 11.10 | 11.03 | 11.03 | 11.03 |
| monoCaP | 2.20 | 2.19 | 2.19 | 2.19 |
| Soybean oil | 3.00 | 2.98 | 2.98 | 2.98 |
| DL-Met | 0.30 | 0.30 | 0.30 | 0.30 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin premix | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix | 0.05 | 0.05 | 0.05 | 0.05 |
| NaHCO3 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated composition (%) | ||||
| 5.38 kcal/kg | 2953.17 | 2954.36 | 2954.36 | 2954.36 |
| Crude protein (%) | 18.05 | 18.02 | 18.00 | 18.01 |
| Crude fat (%) | 5.38 | 5.35 | 5.35 | 5.35 |
| Calcium (%) | 4.72 | 4.69 | 4.69 | 4.69 |
| Available phosphorus (%) | 0.57 | 0.57 | 0.57 | 0.57 |
| Methionine + cysteine (%) | 0.93 | 0.92 | 0.92 | 0.92 |
| Dry matter | 90.39 | 90.43 | 90.43 | 90.43 |
| Crude protein | 17.08 | 16.98 | 16.98 | 16.98 |
| Total fatty acid | 5.03 | 5.00 | 5.00 | 5.00 |
| Egg Production | Egg Weight | Eggshell Strength | Eggshell Thickness | Yolk Color Score | Haugh Unit | Albumen Height | Yolk Height | |
|---|---|---|---|---|---|---|---|---|
| % | g | N | mm | - | - | mm | mm | |
| Control | 91.03 | 66.34 | 54.63 | 0.37 | 6.33 ab | 82.52 | 6.84 a | 18.54 |
| R | 90.05 | 65.05 | 52.76 | 0.39 | 5.83 a | 84.03 | 7.33 ab | 18.70 |
| L | 91.94 | 64.21 | 54.11 | 0.40 | 7.20 b | 85.87 | 7.39 ab | 19.41 |
| LR | 90.44 | 64.77 | 51.93 | 0.37 | 6.50 ab | 84.90 | 8.27 b | 18.92 |
| SEM | 1.25 | 0.71 | 2.71 | 0.01 | 0.27 | 2.19 | 0.34 | 0.39 |
| p-Value | 0.74 | 0.25 | 0.88 | 0.22 | 0.05 | 0.77 | 0.05 | 0.49 |
| GLU | TG | CHOL | HDL | LDL | AST | ALT | CK | |
|---|---|---|---|---|---|---|---|---|
| mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | U/L | U/L | U/L | |
| Control | 275.40 | 2121.60 | 147.20 a | 25.40 | 84.40 a | 231.80 | 10.20 | 2077.40 a |
| R | 232.83 | 1289.83 | 58.33 b | 11.00 | 76.17 ab | 176.33 | 10.00 | 974.67 b |
| L | 277.33 | 1958.33 | 70.17 b | 23.33 | 74.83 b | 205.80 | 10.17 | 1246.50 ab |
| LR | 274.33 | 1914.5 | 52.00 b | 26.50 | 78.00 ab | 185.60 | 10.00 | 1189.20 ab |
| SEM | 23.48 | 218.58 | 14.09 | 5.97 | 2.03 | 30.02 | 0.09 | 246.22 |
| p-Value | 0.48 | 0.10 | <0.01 | 0.31 | 0.03 | 0.60 | 0.58 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, T.-R.J.; Liu, T.-Y.; Lin, C.-W.; Chang, P.-E.; Liao, P.-X.; Yang, W.-Y.; Cheng, C.-Y.; Liao, P.-C.; Chiang, W.-D.; Ding, S.-T.; et al. Effects of Dietary Inclusion of Dry Hydrastis canadensis on Laying Performance, Egg Quality, Serum Biochemical Parameters and Cecal Microbiota in Laying Hens. Animals 2021, 11, 1381. https://doi.org/10.3390/ani11051381
Tzeng T-RJ, Liu T-Y, Lin C-W, Chang P-E, Liao P-X, Yang W-Y, Cheng C-Y, Liao P-C, Chiang W-D, Ding S-T, et al. Effects of Dietary Inclusion of Dry Hydrastis canadensis on Laying Performance, Egg Quality, Serum Biochemical Parameters and Cecal Microbiota in Laying Hens. Animals. 2021; 11(5):1381. https://doi.org/10.3390/ani11051381
Chicago/Turabian StyleTzeng, Tzuen-Rong J, Tzu-Yu Liu, Chiao-Wei Lin, Pei-En Chang, Pei-Xin Liao, Wen-Yuan Yang, Chih-Yuan Cheng, Pei-Chun Liao, Wen-Dee Chiang, Shih-Torng Ding, and et al. 2021. "Effects of Dietary Inclusion of Dry Hydrastis canadensis on Laying Performance, Egg Quality, Serum Biochemical Parameters and Cecal Microbiota in Laying Hens" Animals 11, no. 5: 1381. https://doi.org/10.3390/ani11051381
APA StyleTzeng, T.-R. J., Liu, T.-Y., Lin, C.-W., Chang, P.-E., Liao, P.-X., Yang, W.-Y., Cheng, C.-Y., Liao, P.-C., Chiang, W.-D., Ding, S.-T., & Lin, Y.-Y. (2021). Effects of Dietary Inclusion of Dry Hydrastis canadensis on Laying Performance, Egg Quality, Serum Biochemical Parameters and Cecal Microbiota in Laying Hens. Animals, 11(5), 1381. https://doi.org/10.3390/ani11051381









