Habitat Characteristics Coincidence of Dead and Living Long-Tailed Gorals (Naemorhedus caudatus) According to Extreme Snowfall
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Survey and Carcass Survey
2.3. Environmental Factors Influencing the Habitats of Goral
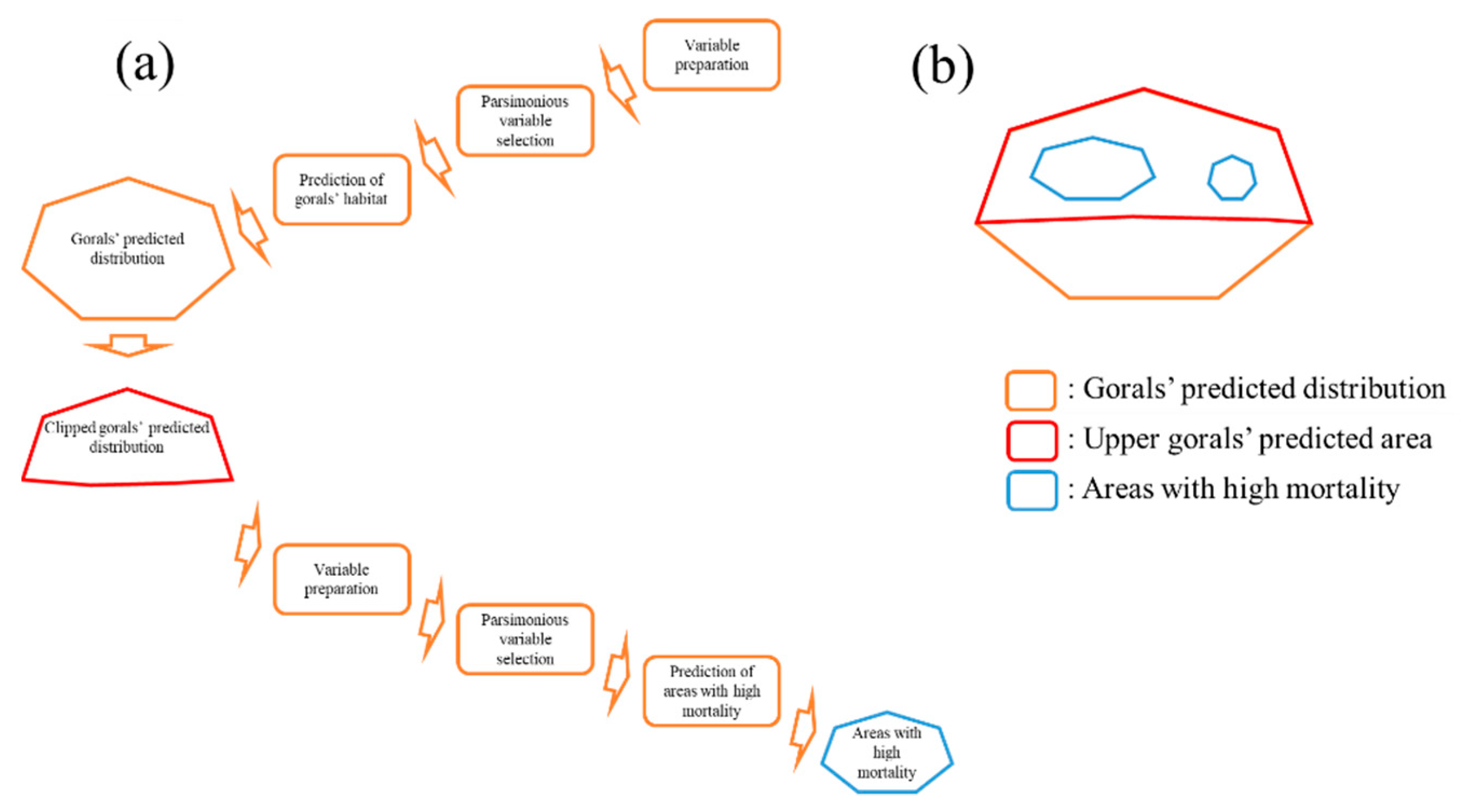
2.4. Definition of Habitat Characteristics of Gorals
2.5. Definition of Site Characteristics of Dead Gorals
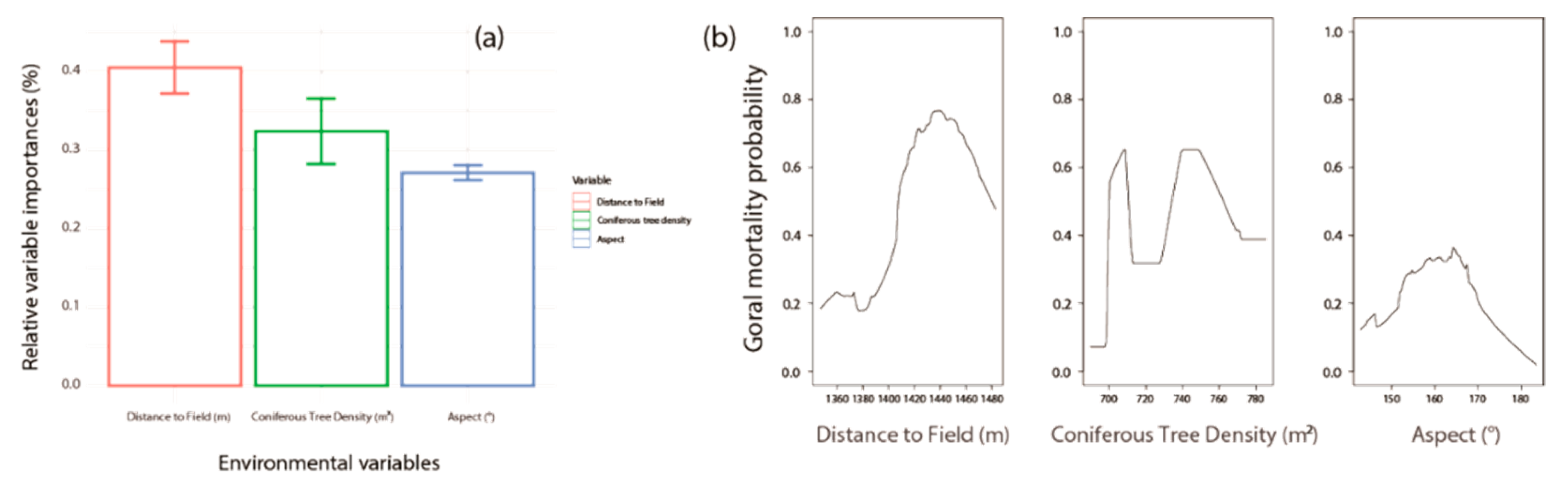
3. Results
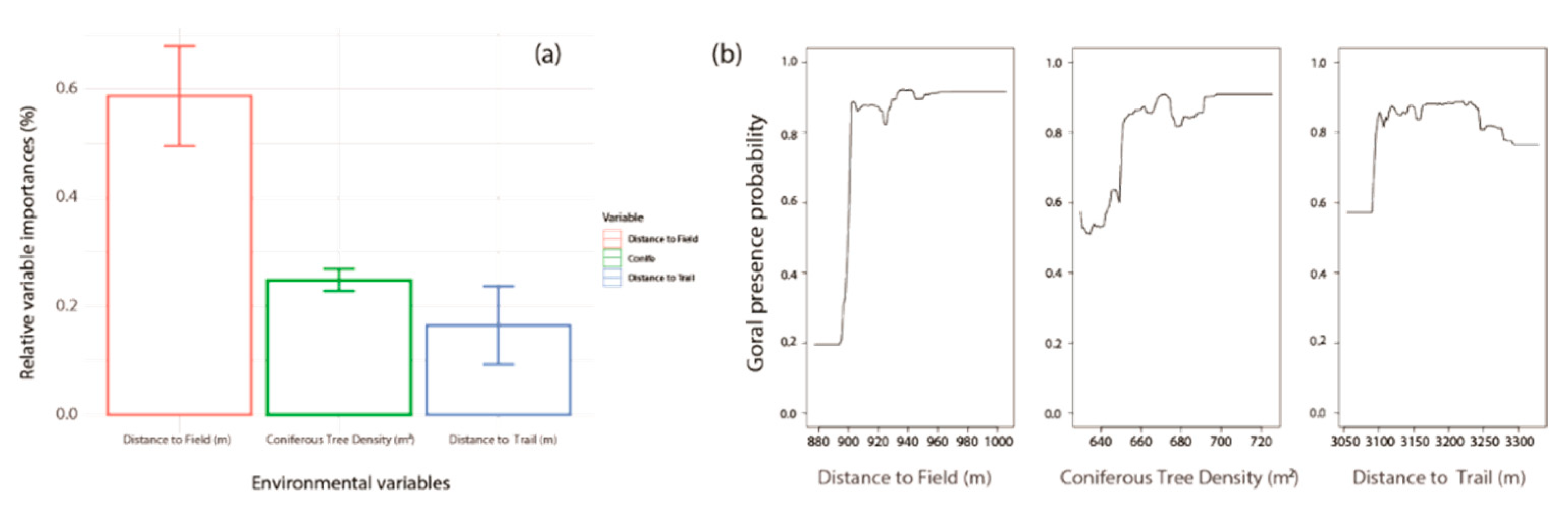
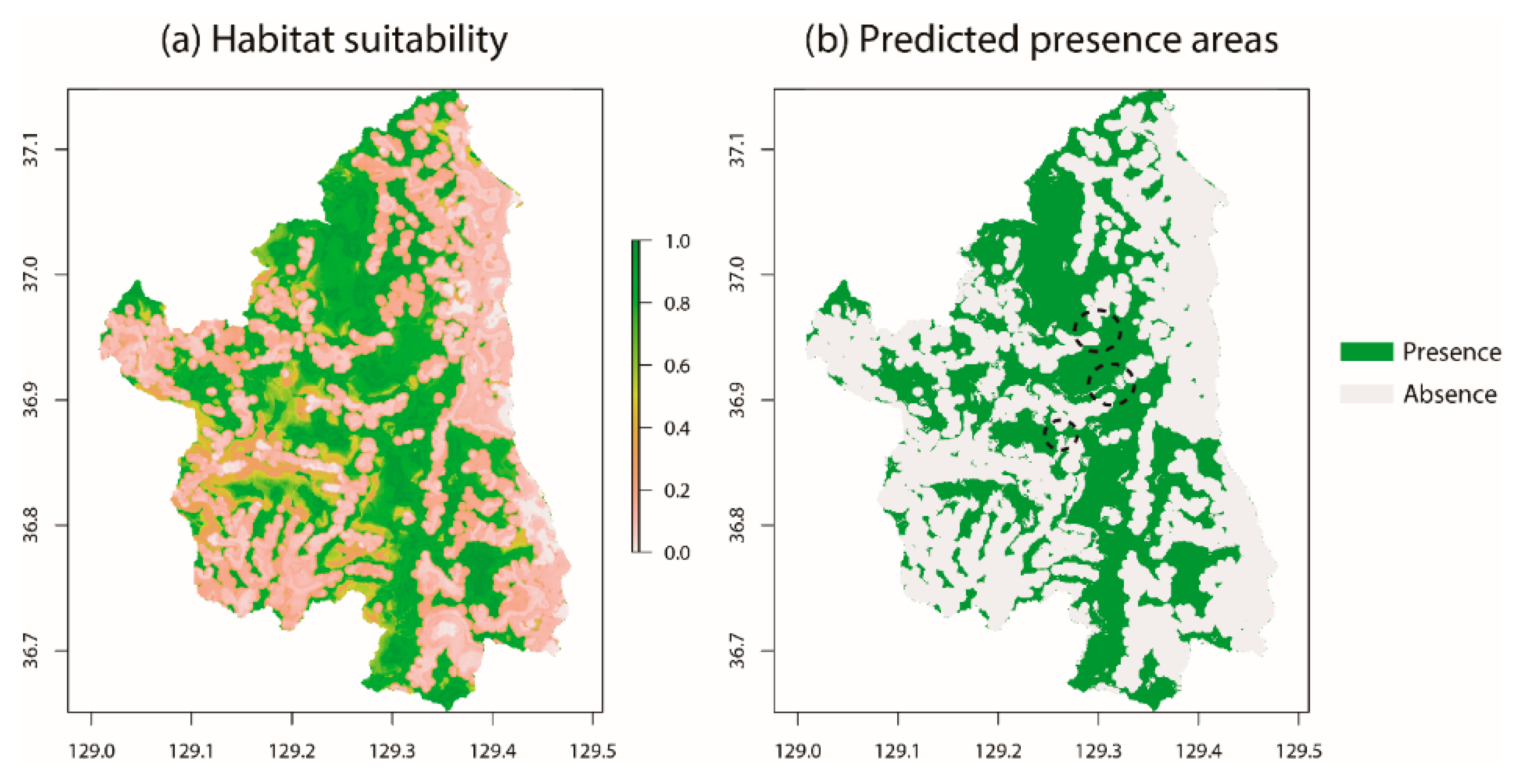
3.1. Habitat Characteristics of Gorals in Uljin
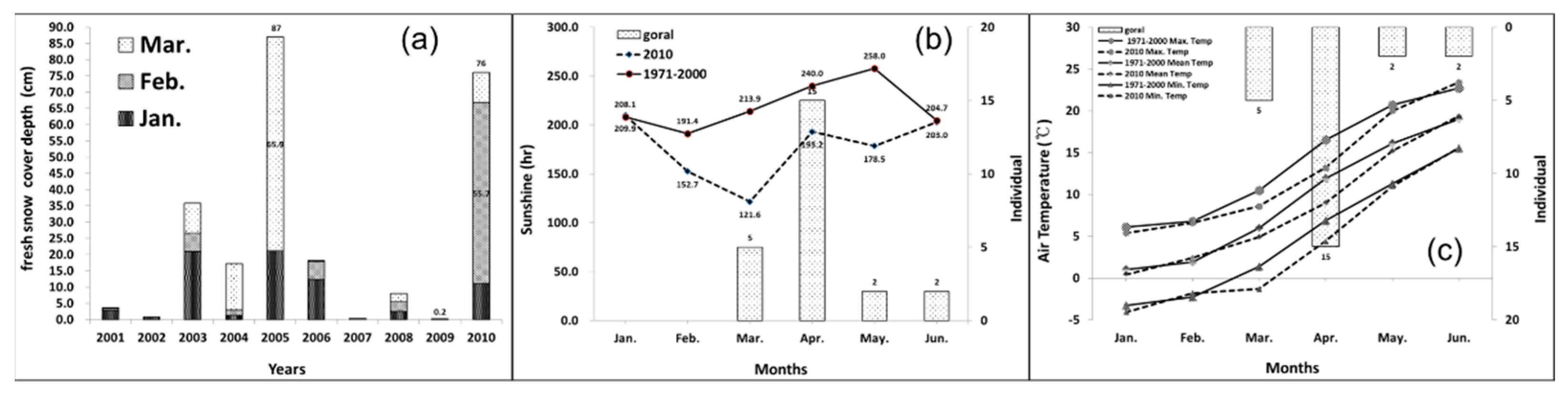
3.2. Site Characteristics of Dead Gorals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, J.A.; Messier, F. Habitat selection as a hierarchy: The spatial scales of winter foraging by muskoxen. Ecography 1995, 18, 333–344. [Google Scholar] [CrossRef]
- Tews, J.; Ferguson, M.A.D.; Fahrig, L. Modelling density dependence and climatic disturbances in caribou: A case study from the Bathurst Island complex, Canadian High Arctic. J. Zool. 2007, 272, 209–217. [Google Scholar] [CrossRef]
- Hong, S.; Cowan, P.; Do, Y.; Gim, J.S.; Joo, G.J. Seasonal feeding habits of coypu (Myocastor coypus) in South Korea. Hystrix 2016, 27, 123–128. [Google Scholar]
- Albon, S.D.; Irvine, R.J.; Halvorsen, O.; Langvatn, R.; Loe, L.E.; Ropstad, E.; Veiberg, V.; van der Wal, R.; Bjorkvoll, E.M.; Duff, E.I.; et al. Contrasting effects of summer and winter warming on body mass explain population dynamics in a food-limited Arctic herbivore. Glob. Chang. Biol. 2017, 23, 1374–1389. [Google Scholar] [CrossRef]
- Grubb, P. Order Artiodactyla. In Mammal Species of the World, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 637–722. [Google Scholar]
- Zaumyslova, O.Y.; Bondarchuk, S.N. The use of camera traps for monitoring the population of long-tailed gorals. Achiev. Life Sci. 2015, 9, 15–21. [Google Scholar] [CrossRef]
- Bragina, E.; Kim, S.; Zaumyslova, O.; Park, Y.S.; Lee, W. Naemorhedus caudatus. IUCN Red List Threat. Species 2020, 2020, e.T14295A22150540. Available online: https://iucnredlist.org/species/14295/22150540 (accessed on 1 April 2021).
- SRTI. Annual Report; Species Restoration Technology Institute (SRTI): Gurye, Korea, 2018. [Google Scholar]
- Park, H.B.; Park, H.C.; Cowan, P.E.; Hong, S. Comparison of hair-trapping methods for the long-tailed goral. Wildl. Soc. Bull. 2018, 42, 310–313. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, B.K.; Lee, H.; Jang, G.S. Considering threats to population viability of the endangered Korean long-tailed goral (Naemorhedus caudatus) using VORTEX. Anim. Cells Syst. 2016, 20, 52–59. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.J.; Bhang, K.J. Habitat analysis of endangered Korean long-tailed goral (Naemorhedus caudatus raddeanus) with weather forecasting model. Sustainability 2019, 11, 6086. [Google Scholar] [CrossRef]
- Won, B.H. Illustrated Encyclopedia of Fauna and Flora of Korean Mammals; Samhwa: Seoul, Korea, 1967. [Google Scholar]
- Yang, B.G. Systematics, Ecology and Current Population Status of the Goral, Naemorhedus caudatus, in Korea. Ph.D. Thesis, Chungbuk National University, Cheongju, Korea, 2002. [Google Scholar]
- Park, H.B. The Habitat Using Characteristics of Long-Tailed Goral (Naemorhedus caudatus) in the Northern Region of Gyeongbuk Province and the Effect of Climate Change. Master’s Thesis, Kyungpook National University, Dae-gu, Korea, 2011. [Google Scholar]
- Myslenkov, A.I.; Voloshina, I.V. Ecology and Behaviours of the Amur Goral; Nauka: Moscow, Russia, 1989. [Google Scholar]
- Chaiyarat, R.; Laohajinada, W.; Kutintara, U.; Nabhitabhata, J. Ecology of the Goral in Om Koi Wildlife Sanctuary, Thailand. Nat. Hist. Bull. Siam Soc. 1999, 47, 191–205. [Google Scholar]
- Park, H.B.; Han, C.W.; Hong, S. Camera trapping of long-tailed goral (Naemorhedus caudatus) in BaekAm and Geumjong Mountains, South Korea. J. For. Environ. Sci. 2018, 34, 71–76. [Google Scholar]
- Mishra, C.; Johnsingh, A.J.T. On habitat selection by the goral Nemorhaedus goral bedfordi (Bovidae, Artiodactyla). J. Zool. 1996, 240, 573–580. [Google Scholar] [CrossRef]
- Choi, S.K.; Chun, S.; An, J.; Lee, M.Y.; Kim, H.J.; Min, M.S.; Kwon, S.W.; Choi, T.Y.; Lee, H.; Kim, K.S. Genetic diversity and population structure of the long-tailed goral, Naemorhedus caudatus, in South Korea. Genes Genet. Syst. 2015, 90, 31–41. [Google Scholar] [CrossRef] [PubMed]
- CHA. Survey and Monitoring Long-Tailed Goral (Naemorhedus caudatus) in Uljin, Samcheok, Bonghwa Areas; Cultural Heritage Administration: Daejeon, Korea, 2007. [Google Scholar]
- CGRB. Autopsy Report of the Dead Long-Tailed Goral (Naemorhedus caudatus) in Uljin; Conservation Genome Resource Bank for Korean Wildlife: Seoul, Korea, 2010. [Google Scholar]
- Choi, T.Y.; Park, C.H. Korean goral potential habitat suitability model at Soraksan National Park using fuzzy set and multi-criteria evaluation. J. Korean Inst. Landsc. Archit. 2004, 32, 28–38. [Google Scholar]
- Seo, C.W.; Choi, T.Y.; Choi, Y.S.; Kim, D.Y. A study on wildlife habitat suitability modelling for goral (Nemorhaedus caudatus rddeanus) in Seoraksan National Park. J. Korea Soc. Environ. Restor. Technol. 2008, 11, 28–38. [Google Scholar]
- Kim, D.B.; Koo, K.A.; Kim, H.H.; Hwang, G.Y.; Kong, W.S. Reconstruction of the habitat range suitable for long-tailed goral (Naemorhedus caudatus) using fossils from the Paleolithic sites. Quat. Int. 2019, 519, 101–112. [Google Scholar] [CrossRef]
- Cho, C.; Kim, K.; Kwon, G. Habitat altitude and home range of the endangered long-tailed goral (Naemorhedus caudatus): Seasonal and monthly home range and altitude change. Mammalia 2015, 80, 481–489. [Google Scholar] [CrossRef]
- Cook, J.G.; Irwin, L.L.; Bryant, L.D.; Riggs, R.A.; Thomas, J.W. Relations of forest cover and condition of elk: A test of the thermal cover hypothesis in summer and winter. Wildl. Monogr. 1998, 141, 3–61. [Google Scholar]
- Hijmans, R.J. Package “Raster”: Geographic Data Analysis and Modeling. The Comprehensive R Archive Network, CRAN. 2014. Available online: https://CRAN.R-project.org/package=raster (accessed on 1 April 2021).
- Won, C.M.; Smith, K.G. History and current status of mammals of the Korean peninsula. Mamm Rev. 1999, 29, 3–36. [Google Scholar] [CrossRef]
- Chen, W.; Hu, J.C.; Lu, X. Habitat use and separation between the Chinese serow (Capricornis milneedwardsi) and the Chinese goral (Naemorhedus griseus) in winter. Mammalia 2009, 73, 249–252. [Google Scholar] [CrossRef]
- Araujo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, J.Y.; Kim, Y.M.; Kim, D.K.; Joo, G.J. Factors influencing initial population establishment and habitat expansion of introduced nutrias (Myocastor coypus) in South Korea. Ecol. Inform. 2020, 59, 101111. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling; R Package Version 1.1-4; 2017; Available online: https://cran.r-project.org/web/packages/dismo/index.html (accessed on 1 April 2021).
- Hastie, T. Gam: Generalized Additive Models; R Package Version 1.20; 2020; Available online: https://CRAN.R-project.org/package=gam (accessed on 1 April 2021).
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Tress; R Package Version 4.1-15; 2019; Available online: https://CRAN.R-project.org/package=rpart (accessed on 1 April 2021).
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Greenwell, B.; Boehmke, B.; Cunningham, J.; Developers, G.B.M. GBM: Generalized Boosted Regression Models; R Package Version 2.1.8; 2020; Available online: https://CRAN.R-project.org/package=gbm (accessed on 1 April 2021).
- Philips, S. Maxnet: Fitting ‘Maxent’ Species Distribution Models with ‘Glmnet’; R Package Version 0.1.2; 2017; Available online: https://CRAN.R-project.org/package=maxnet (accessed on 1 April 2021).
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- MacNally, R. Regression and model-building in conservation biology, biogeography and ecology: The distinction between—And reconciliation of—‘predictive’ and explanatory’ models. Biodivers. Conserv. 2000, 9, 655–671. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training; R Package Version 6.0-86; 2020; Available online: https://CRAN.R-project.org/package=caret (accessed on 1 April 2021).
- Biecek, P. DALEX: Explainers for complex predictive models in R. J. Mach. Learn. Res. 2018, 19, 1–5. [Google Scholar]
- Krebs, C. Ecology: The Experimental Analysis of Distribution and Abundance, 6th ed.; Pearson: San Francisco, CA, USA, 2009. [Google Scholar]
- Pepin, D.; Adrados, C.; Janeau, G.; Joachim, J.; Mann, C. Individual variation in migratory and exploratory movements and habitat use by adult red deer (Cervus elaphus L.) in a mountainous temperate forest. Ecol. Res. 2008, 23, 1005–1013. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Bashir, T.; Poudyal, K.; Sathyakumar, S.; Saha, G.K. Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and Serow (Capricornis thar) in Khangchendzonga Biosphere Reserve, Sikkim, India. Mamm. Study 2012, 37, 173–181. [Google Scholar] [CrossRef]
- Ilyas, O.; Khan, J.A. Food habits of barking deer (Muntiacus muntjak) and goral (Naemorhedus goral) in Binsar Wildlife Sanctuary, India. Mammalia 2003, 67, 521–532. [Google Scholar] [CrossRef]
- Ekman, J.B.; Askenmo, C.E.H. Social rank and habitat use in willow tit groups. Anim. Behav. 1984, 32, 508–514. [Google Scholar] [CrossRef]
- Barroso, F.G.; Alados, C.L.; Boza, J. Social hierarchy in the domestic goat: Effect on food habits and production. Appl. Anim. Behav. Sci. 2000, 69, 35–53. [Google Scholar] [CrossRef]
- Krause, J. Differential fitness returns in relation to spatial position in groups. Biol. Rev. 1994, 69, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Fritzell, E.K. Habitat use by prairie raccoons during the waterfowl breeding season. J. Wildl. Manag. 1978, 42, 118–127. [Google Scholar] [CrossRef]
- Malagnino, A.; Marchand, P.; Garel, M.; Cargnelutti, B.; Itty, C.; Chaval, Y.; Hewison, A.J.M.; Loison, A.; Morellet, N. Do reproductive constraints or experience drive age-dependent space use in two large herbivores? Anim. Behav. 2021, 172, 121–133. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Grondahl, C.; Evans, A.L.; Desforges, J.; Blake, J.; Hanse, L.H.; Beumer, L.T.; Mosbacher, J.B.; Stelvig, M.; Greunz, E.M.; et al. On the interplay between hypothermia and reproduction in a high arctic ungulate. Sci. Rep. 2020, 10, 1514. [Google Scholar] [CrossRef]
- Mitra, S. Gorals (Nemorhaedus goral hodgsoni) in Mahananda wildlife sanctuary, West Bengal, India. Zoos Print J. 2003, 18, 1199–1201. [Google Scholar] [CrossRef]
- Pendharkar, A.P.; Goyal, S.P. Group size and composition of the gray goral in Simbalbara Sanctuary and Darpur reserved forest, India. J. Mammal. 1995, 76, 906–911. [Google Scholar] [CrossRef]


| No | Classification | Factors | Description and Hypothesis from Literature | Reference | Source Map |
|---|---|---|---|---|---|
| 1 | Anthropo genic fac tors | Distance to factory (m) | Human activity can negatively affect the goral habitats. | [22,23,24,28] | EGIS (https://egis.me.go.kr (accessed on 1 April 2021), 30 × 30 m, 2007) |
| 2 | Distance to road (m) | ||||
| 3 | Road density (m2/1.46 km2) | ||||
| Human population density (n/1.46 km2) | |||||
| 4 | Distance to rice paddy (m) | ||||
| 5 | Distance to field (m) | ||||
| 6 | Field density (m2/1.46 km2) | ||||
| 7 | Distance to trail (m) | ||||
| 8 | Forest | Distance to deciduous forest (m) | Types of forest can influence goral’s feeding and marking sites. | [13,15,23] | National Spatial Data Infrastructure Portal (http://nsdi.go.kr (accessed on 1 April 2021), 5 × 5 m, 2018) |
| 9 | Density of deciduous forest (m2/1.46 km2) | ||||
| 10 | Density of coniferous forest (m2/1.46 km2) | ||||
| 11 | Density of forest (m2/1.46 km2) | ||||
| 12 | Age class (1 (young) to 9 (old)) | ||||
| 13 | Diameter class (0 (young) to 3 (large)) | ||||
| 14 | Crown density (1 (sparse) to 3 (dense)) | ||||
| 15 | Landscape | Distance to stream (m) | Gorals used to live near the waterway. | [14,15] | EGIS (https://egis.me.go.kr (accessed on 1 April 2021), 10 × 10 m, 2013) |
| 16 | Stream density (m2/1.46 km2) | ||||
| 17 | Lake density (m2/1.46 km2) | ||||
| 18 | Altitude (m) | Gorals used to live at high altitudes to avoid anthropogenic disturbance. | [17,24] | Biz-gis (www.biz-gis.com (accessed on 1 April 2021), 30 × 30 m) | |
| 19 | Aspect (1 to 360) | As snow can be more easily melt, the gorals prefer the habitats in southern regions. | [14,15,29] | Biz-gis (www.biz-gis.com (accessed on 1 April 2021), 30 × 30 m) | |
| 20 | Slope (°) | Gorals prefer to live in the deep valleys to avoid humans. | [22,28] | Biz-gis (www.biz-gis.com (accessed on 1 April 2021), 30 × 30 m) | |
| 21 | Soil map | Soil drainage (1 (bad) to 4 (very good)) | If water cannot be displaced, snow becomes stacked. The large quantity of snow can negatively affect the goral’s escape from the snow. | [14] | National Spatial Data Infrastructure Portal (http://nsdi.go.kr (accessed on 1 April 2021), 25 × 25 m, 2018) |
| 22 | Degree of rock exposure (1 (little) to 4 (a lot)) | Gorals prefer to live in front of large rocks to escape from predation. Besides, stacked snow melts more quickly than in other soil. | [13,15,26] | ||
| 23 | Soil type (1 (no) to 3 (many)) | Strongly eroded soil stacked by snowfall could make gorals fall easily. | |||
| 24 | Degree of wind exposure (1 (exposed) to 3 (closed)) | Exposure to wind can cause snow to remain for a long period. | [4,14] | ||
| 25 | Distance to wet soil (m) | Moist soil delays the rate of snow melting. | [14] | ||
| 26 | Wet soil density (m2/1.46 km2) | ||||
| 27 | Slant type (1 (rising slope) to 3 (falling slope)) | Gorals prefer to live in convex slopes to escape from predation. | [13] |
| No | Observed Day | Collected Day | Sex | Age Class | Estimated Ages | Pregnant |
|---|---|---|---|---|---|---|
| 1 | 2010.03.16 | 2010.03.19 | M | Juvenile | >1 | |
| 2 | 03.23 | 04.01 | F | Adult | >15 | Y |
| 3 | 03.25 | 03.26 | M | Yearling | <1 | |
| 4 | 03.28 | 04.01 | Adult | 5–6 | ||
| 5 | 03.31 | 04.02 | Juvenile | >1 | ||
| 6 | 04.07 | 04.07 | F | Adult | >10 | Y |
| 7 | 04.03 | 04.08 | F | Adult | >10 | Y |
| 8 | 04.09 | 04.09 | M | Juvenile | >1 | |
| 9 | 04.09 | 04.09 | Yearling | <1 | ||
| 10 | 04.10 | 04.10 | Juvenile | <1 | ||
| 11 | 04.10 | 04.10 | M | Juvenile | >1 | |
| 12 | 04.11 | 04.11 | F | Adult | 5–8 | Y |
| 13 | 04.10 | 04.10 | M | Juvenile | <1 | |
| 14 | 04.11 | 04.11 | F | Juvenile | >1 | |
| 15 | 04.11 | 04.11 | Juvenile | <1 | ||
| 16 | 04.20 | 04.21 | F | Adult | ||
| 17 | 04.20 | 04.21 | Juvenile | |||
| 18 | 04.21 | 04.29 | ||||
| 19 | 04.21 | 04.21 | Juvenile | |||
| 20 | 04.21 | 04.21 | Juvenile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-B.; Hong, S. Habitat Characteristics Coincidence of Dead and Living Long-Tailed Gorals (Naemorhedus caudatus) According to Extreme Snowfall. Animals 2021, 11, 997. https://doi.org/10.3390/ani11040997
Park H-B, Hong S. Habitat Characteristics Coincidence of Dead and Living Long-Tailed Gorals (Naemorhedus caudatus) According to Extreme Snowfall. Animals. 2021; 11(4):997. https://doi.org/10.3390/ani11040997
Chicago/Turabian StylePark, Hee-Bok, and Sungwon Hong. 2021. "Habitat Characteristics Coincidence of Dead and Living Long-Tailed Gorals (Naemorhedus caudatus) According to Extreme Snowfall" Animals 11, no. 4: 997. https://doi.org/10.3390/ani11040997
APA StylePark, H.-B., & Hong, S. (2021). Habitat Characteristics Coincidence of Dead and Living Long-Tailed Gorals (Naemorhedus caudatus) According to Extreme Snowfall. Animals, 11(4), 997. https://doi.org/10.3390/ani11040997






