Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of PRP
2.3. Lyopholoized L-GFequina
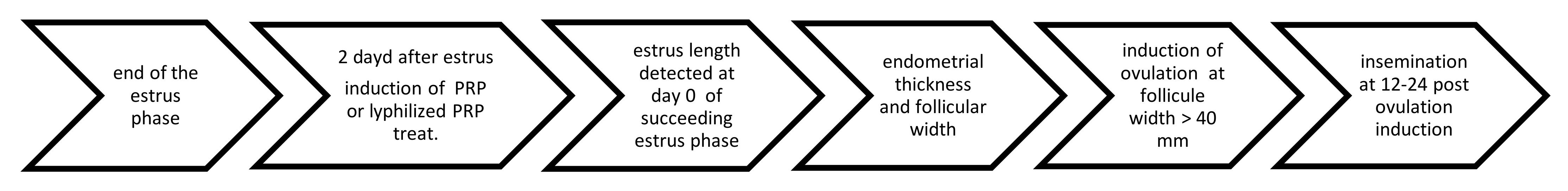
2.4. Assignment of Groups and Protocol of Administration
2.5. Mating and Pregnancy Diagnosis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arabian Horse Association. Horse of the Desert Bedouin. Arabian Horse History & Heritage; Arabian Horse Association: Aurora, CO, USA, 2019. [Google Scholar]
- Canisso, I.F.; Segabinazzi, L.G.; Fedorka, C.E. Review: Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T. Problems after breeding. J. Equine Vet. Sci. 2008, 28, 635–639. [Google Scholar] [CrossRef]
- Canisso, I.F.; Stewart, J.; Coutinho da Silva, M.A. Endometritis: Managing persistent post-breeding endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 465–480. [Google Scholar] [CrossRef]
- Scoggin, C.F. Endometritis: Nontraditional therapies. Vet. Clin. N. Am. Equine Pract. 2016, 32, 499–511. [Google Scholar] [CrossRef]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The use of dexamethasone administered to mares at breeding time in the modulation of persistent mating induced endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Rojer, H.; Aurich, C. Treatment of persistent mating-induced endometritis in mares with the non-steroid anti-inflammatory drug vedaprofen. Reprod. Domest. Anim. 2010, 45, 458–460. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L. The effect of uterine lavage performed four hours post L-GFequina insemination on pregnancy rate in mares. Theriogenology 1991, 35, 1111–1119. [Google Scholar] [CrossRef]
- Roh, Y.H.; Kim, W.; Park, K.U.; Oh, J.H. Cytokine-release kinetics of Platelets-rich plasma according to various activation protocols. Bone Joint Res. 2016, 5, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Doda, V.; Kotwal, U.; Dogra, M. Quantification of Platelets and Platelets derived growth factors from Platelets-rich-plasma (PRP) prepared at different centrifugal force (g) and time. Transfus. Apher. Sci. 2006, 54, 103–110. [Google Scholar] [CrossRef]
- Everts, P.A.; Brown Mahoney, C.; Hoffmann, J.J.; Schönberger, J.P.; Box, H.A.; Van Zundert, A.; Knape, J.T. Platelets-rich plasma preparation using three devices: Implications for Platelets activation and Platelets growth factor release. Growth Factors 2006, 24, 165–171. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous Platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Gentile, P.; Orlandi, A.; Scioli, M.G.; Di Pasquali, C.; Bocchini, I.; Cervelli, V. Concise review: Adipose-derived stromal vascular fraction cells and Platelets-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl. Med. 2012, 1, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Reghini, M.F.S.; Neto, C.R.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Dell’Aqua, C.F.; Bussiere, M.C.C.; Dell’Aqua, J.A., Jr.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with Platelets-rich plasma. Theriogenology 2016, 86, 516–522. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A., Jr.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine clinical findings, fertility rate, leucocyte migration, and COX-2 protein levels in the endometrial tissue of susceptible mares treated with Platelets-rich plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Kaipainen, A.; Ho, D.; Orser, C.; Pebley, W.; Rudolph, A.; Orgill, D.P. Trehalose lyophilized Platelets for wound healing. Wound Repair Regen. 2007, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Kaipainen, A.; Czeczuga, J.M.; Wagner, C.T.; Orgill, D.P. Freeze-dried Platelets-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006, 14, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sum, R.; Hager, S.; Pietramaggiori, G.; Orgill, D.P.; Dee, J.; Rudolph, A.; Orser, C.; Fitzpatrick, G.M.; Ho, D. Wound healing properties of trehalose-stabilized freeze-dried outdated Platelets. Transfusion 2007, 47, 672–679. [Google Scholar] [CrossRef]
- Arguelles, D.; Carmona, J.U.; Pastor, J.; Iborra, A.; Viñals, L.; Martínez, P.; Bach, E.; Prades, M. Evaluation of single and double centrifugation tube methods for concentrating equine Platelets. Res. Vet. Sci. 2006, 81, 237–245. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Carmona, J.U.; Samudio, I. Use of autologous concentrate plasma for the treatment of a scapular fracture and brachial plexus injury caused by shot in a horse. Arch. Med. Vet. 2010, 42, 209–214. [Google Scholar]
- Risvanli, A.; Kaygusuzoglu, E. Immunological infertility in domestic animals. Turk. Vet. Med. 2004, 3–4, 52–55. [Google Scholar]
- Garverick, H.A.; Zollers, W.G.; Smith, M.F. Mechanisms associated with corpus luteum lifespan in animals having normal or subnormal luteal function. Anim. Reprod. Sci. 1992, 28, 111–124. [Google Scholar] [CrossRef]
- Copelin, J.P.; Smith, M.F.; Garverick, H.A.; Youngquist, R.S. Effect of the uterus on subnormal luteal function in anestrous beef cows. J. Anim. Sci. 1987, 64, 1506–1511. [Google Scholar] [CrossRef]
- Lishman, A.W.; Inskeep, E.K. Deficiencies in luteal function during reinitiation of cyclic breeding activity in beef cows and in ewes. J. Anim. Sci. 1991, 21, 59–75. [Google Scholar]
- Rantala, M.H.; Mutikainen, M.; Schuler, G.; Katila, T.; Taponen, J. Endometrial expression of progesterone, estrogen, and oxytocin receptors and of 20a-hydroxysteroid dehydrogenase and cyclooxygenase II 2 and 5 days after ovulation in induced short and normal estrous cycles in dairy cows. Theriogenology 2014, 81, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Pazoki, H.; Eimani, H.; Farokhi, F.; Shahverdi, A.H.; Salman Yazdi, R.; Tahaei, L.S. Comparing the growth and the development of mouse pre-antral follicle in medium with PL (Platelets Layset) and with FBS. Middle East. Fertil. Soc. J. 2015, 20, 231–236. [Google Scholar] [CrossRef]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Lange-Consiglio, A. Platelets Rich Plasma for Regenerative Medicine Treatment of Bovine Ovarian Hypofunction. Front. Vet. Sci. 2020, 7, 517. [Google Scholar] [CrossRef]
- Hosseini, L.; Shirazi, A.; Naderi, M.M.; Shams-Esfandabadi, N.; Boroujeni, S.B.; Ali Sarvari, A.; Sadeghnia, S.; Behzadi, B.; Akhondi, M.M. Platelets-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Med. Online 2017, 35, 343–350. [Google Scholar] [CrossRef]
- Dehghani, F.; Aboutalebi, H.; Esmaeilpour, T.; Panjehshahin, M.R.; Bordbar, H. Effect of Platelets-rich plasma (PRP) on ovarian structures in cyclophosphamide-induced ovarian failure in female rats: A stereological study. Toxicol. Mech. Methods 2018, 28, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.S. The effect of Platelets-Rich Plasma (PRP) on intraluminal fluid and pregnancy rates in mares susceptible to Persistent Mating-Induced Endometritis (PMIE). J. Equine Vet. Sci. 2014, 34, 128. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Mou, S.; Zhao, H.; Fang, J.; Xiang, Y.; Zhao, T.; Sha, T.; Ding, J.; Hao, C. Investigation of Platelets-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J. Cell Biochem. 2019, 120, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, J.; Wei, L.N.; Pang, J.; Chen, J.; Liang, X. Autologous Platelets-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine 2019, 98, 14062. [Google Scholar] [CrossRef] [PubMed]
- Coksuer, H.; Akdemir, Y.; Barut, M.U. Autologous Platelets-rich plasma treatment in infertility patients with RIF improve IVF success. Gynecol. Endocrinol. 2019, 35, 815–818. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Peyvandi, S.; Gorji, H.H.; Moradi, S.; Jamal, J.; Aghmashhadi, F.Y.P.; Mohammadi, M.H. Effect of Platelets-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: A randomized controlled trial. Gynecol. Endocrinol. 2020, 2, 1–5. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Scoggin, K.; Troedsson, M.H.T. The effect of Platelets-rich plasma on endometrial pro-inflammatory cytokines in susceptible mares following semen deposition. J. Equine Vet. Sci. 2012, 32, 498. [Google Scholar] [CrossRef]
- Marini, M.G.; Perrini, C.; Esposti, P.; Brorradetti, B.; Bizzaro, D.; Riccaboni, P.; Fantinato, E.; Urbani, G.; Gelati, G.; Cremonesi, F.; et al. Effects of Platelets-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod. Biol. Endocrinol. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; El Kasmi, I.; Bourdiec, A.; Crespo, K.; Bissonnette, L.; Le Saint, C.; Bissonnette, F.; Kadoch, I.J. 15 years of transcriptomic analysis on endometrial receptivity: What have we learnt? Fertil. Res. Prac. 2019, 5, 9. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.E.; Koo, H.S.; Kwon, H.; Choi, D.H.; Kim, J.H. Effect of autologous Platelets-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: A pilot study. Front. Endocrinol. 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control | PRP | L-GFequina | SEM | p-Value | Sig. |
|---|---|---|---|---|---|---|
| Age | 10.13 | 8.69 | 10.33 | 0.41 | 0.20 | No |
| Parity | 3.99 | 2.80 | 3.64 | 0.22 | 0.82 | No |
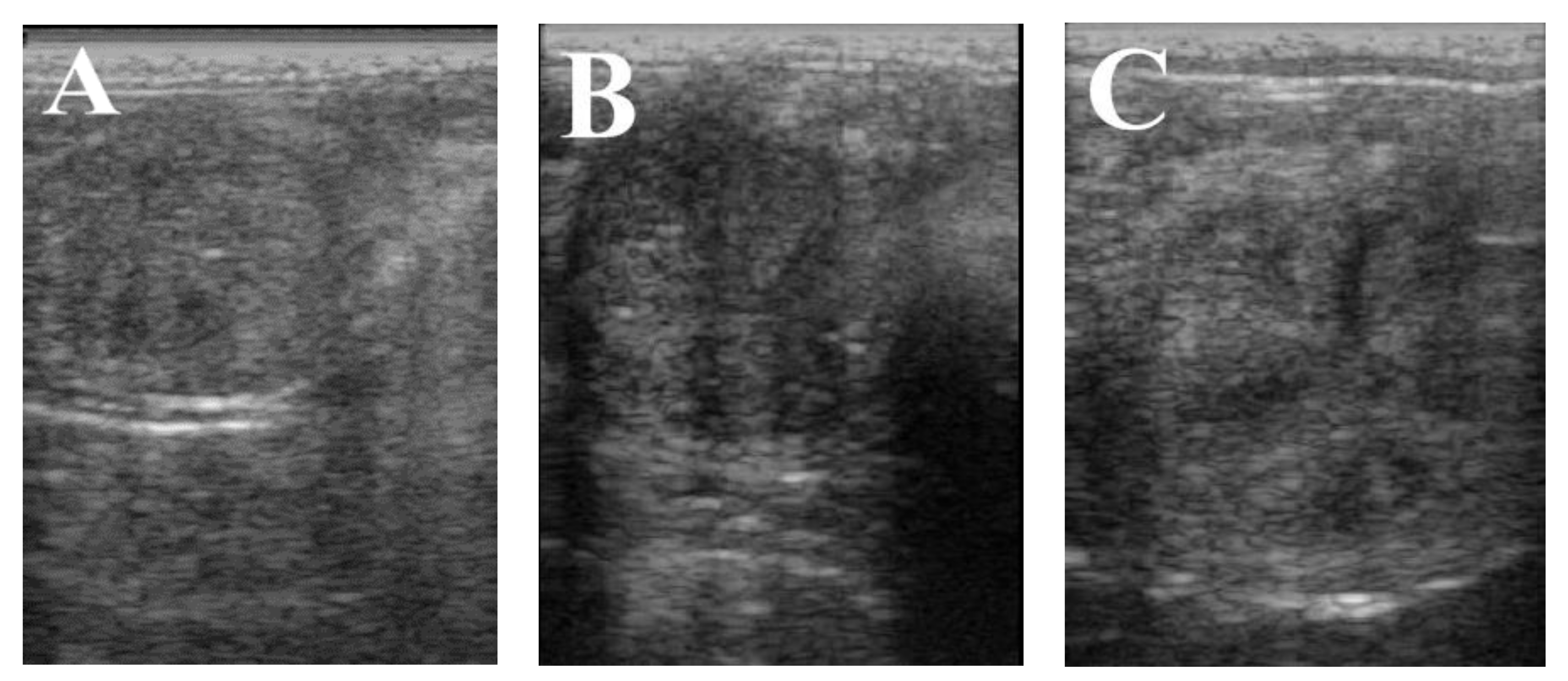
| Graafian follicular diameter (mm) | 41.68 | 42.21 | 43.30 | 0.04 | 0.06 | No |
| Endometrium thickness (mm) | 5.87 c | 7.33 a | 6.86 b | 0.31 | 0.00 | Yes |
| Estrous cycle length (day) | 21.06 a | 9.63 b | 7.56 c | 0.05 | 0.00 | Yes |
| Group | N | Pregnant Mares | Pregnancy Rate (%) | Odds Ratio | 95% CI 1 | p-Value |
|---|---|---|---|---|---|---|
| Control | 32 | 4 | 6.25 | 1 | 1 | 0.01 |
| PRP | 32 | 16 | 50 | 7.00 | 1.99–24.58 | |
| L-GFequina | 9 | 6 | 66.67 | 14.00 | 2.46–79.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawod, A.; Miro, J.; Elbaz, H.T.; Fahmy, H.; Abdoon, A.S. Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares. Animals 2021, 11, 1123. https://doi.org/10.3390/ani11041123
Dawod A, Miro J, Elbaz HT, Fahmy H, Abdoon AS. Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares. Animals. 2021; 11(4):1123. https://doi.org/10.3390/ani11041123
Chicago/Turabian StyleDawod, Ahmed, Jordi Miro, Hamed T. Elbaz, Hossam Fahmy, and Ahmed S. Abdoon. 2021. "Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares" Animals 11, no. 4: 1123. https://doi.org/10.3390/ani11041123
APA StyleDawod, A., Miro, J., Elbaz, H. T., Fahmy, H., & Abdoon, A. S. (2021). Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares. Animals, 11(4), 1123. https://doi.org/10.3390/ani11041123








