Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation
Simple Summary
Abstract
1. Introduction
2. Consciousness
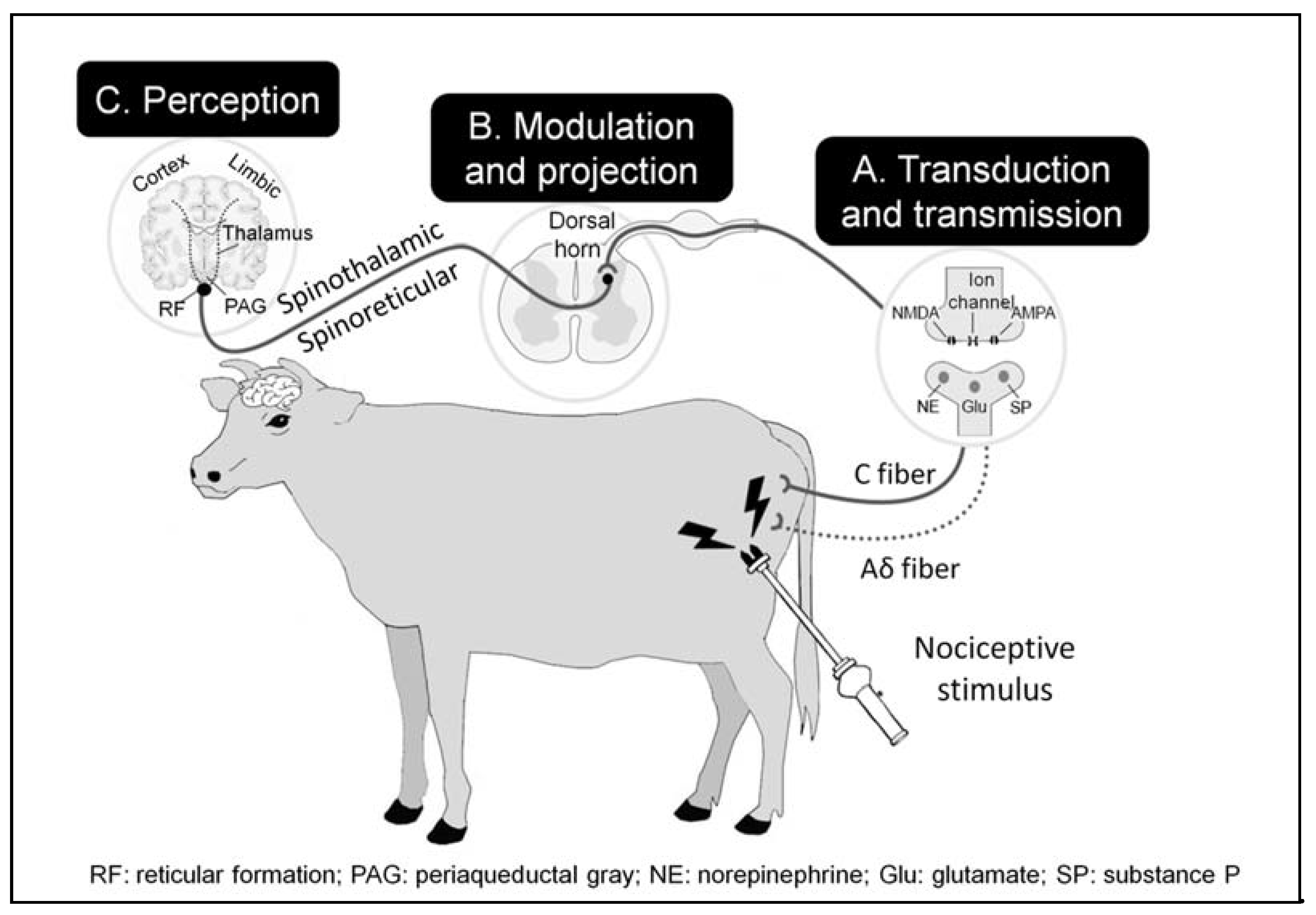
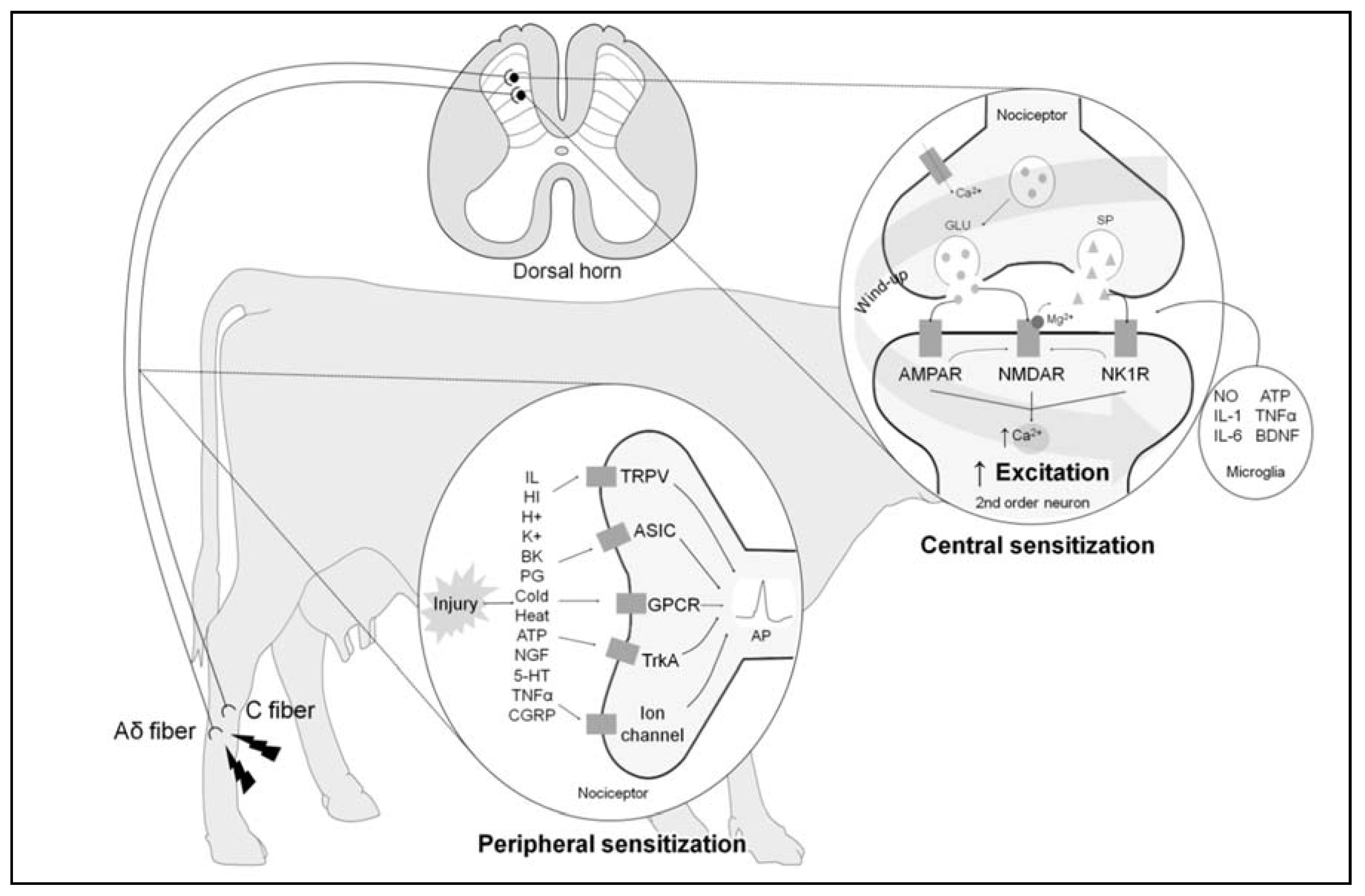
3. The Neurobiology of Pain
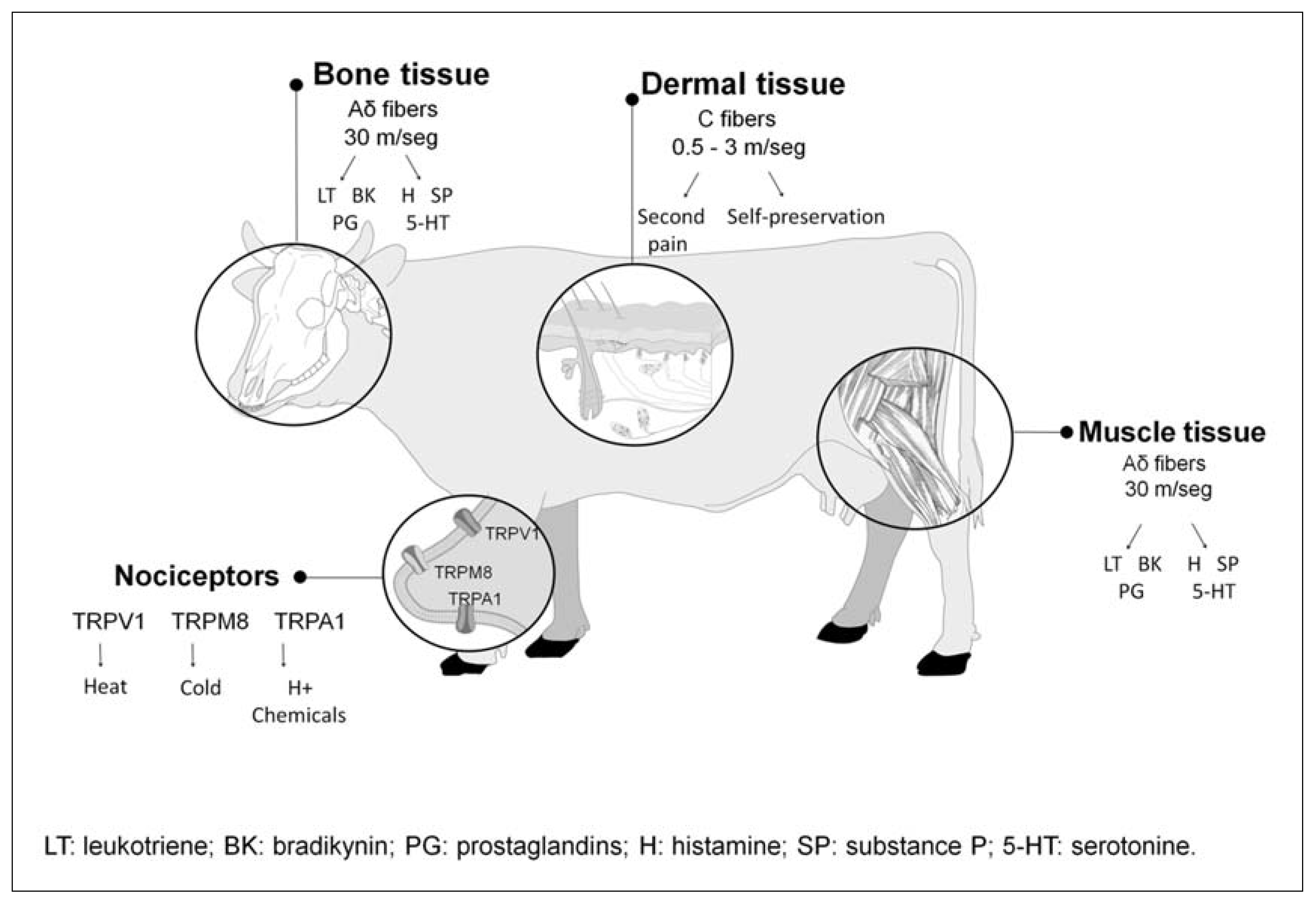
3.1. Transduction and Transmission
3.2. Hyperalgesia
3.3. Modulation and Projection
3.4. Perception
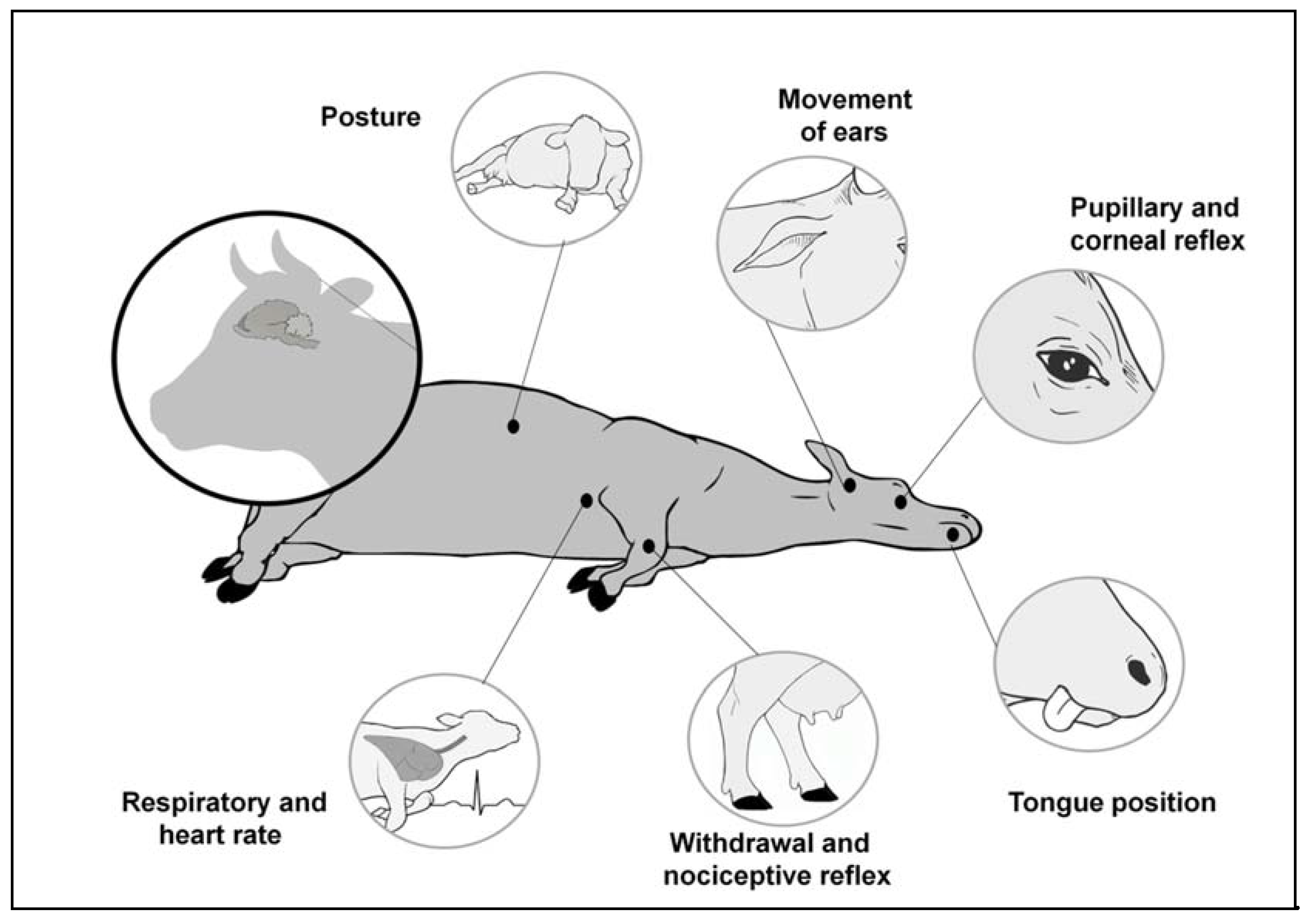
4. Hyperalgesia and Events at Slaughter
4.1. Hyperalgesia and Transport and Handling
4.2. Hyperalgesia and Stunning
4.3. Hyperalgesia and its Relationship with Slaughter Performed without Prior Stunning
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations/Nomenclature
References
- International Association for the Study of Pain (IASP). IASP Announces Revised Definition of Pain. Available online: https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=10475#:~:text=Jul%2016%2C%202020&text=The%20definition%20is%3A%20%E2%80%9CAn%20unpleasant,pain%20for%20further%20valuable%20context (accessed on 13 November 2020).
- Imlan, J.C.; Kaka, U.; Goh, Y.-M.; Idrus, Z.; Awad, E.A.; Abubakar, A.A.; Ahmad, T.; Nizamuddin, H.N.Q.; Sazili, A.Q. Effects of slaughter knife sharpness on blood biochemical and electroencephalogram changes in cattle. Animals 2020, 10, 579. [Google Scholar] [CrossRef]
- Gregory, N.G.; Fielding, H.R.; von Wenzlawowicz, M.; von Holleben, K. Time to collapse following slaughter without stunning in cattle. Meat Sci. 2010, 85, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.J.; Johnson, C.B.; Murrell, J.C.; Hulls, C.M.; Mitchinson, S.L.; Stafford, K.J.; Johnstone, A.C.; Mellor, D.J. Electroencephalographic responses of halothane-anaesthetised calves to slaughter by ventral-neck incision without prior stunning. N. Z. Vet. J. 2009, 57, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. The neurobiology of acute pain. Vet. J. 2018, 237, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.L. Physiology of pain. Crit. Care Nurs. Clin. N. Am. 2017, 29, 397–406. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I.; Napolitano, F.; Cruz-Monterrosa, R.; Mota-Rojas, D.; Mora-Medina, P.; Ramírez-Bribiesca, E.; Bertoni, A.; Berdugo-Gutiérrez, J.; Braghieri, A. River buffalo meat production and quality: Sustainability, productivity, nutritional information, and sensory properties. J. Buffalo Sci. 2020, 9, 159–169. [Google Scholar] [CrossRef]
- Terlouw, C.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part i. Neurobiological mechanisms underlying stunning and killing. Meat Sci. 2016, 118, 133–146. [Google Scholar] [CrossRef]
- Shimshony, A.; Chaudry, M.M. Slaughter of animals for human consumption. Rev. Sci. Tech. 2005, 24, 693–710. [Google Scholar] [CrossRef]
- Gibson, T.J.; Dadios, N.; Gregory, N.G. Effect of neck cut position on time to collapse in halal slaughtered cattle without stunning. Meat Sci. 2015, 110, 310–314. [Google Scholar] [CrossRef]
- Young, G.B.; Pigott, S.E. Neurobiological basis of consciousness. Arch. Neurol. 1999, 56, 153–157. [Google Scholar] [CrossRef]
- Finnie, J. Animal models of traumatic brain injury: A review. Aust. Vet. J. 2001, 79, 628–633. [Google Scholar] [CrossRef]
- Finnie, J.W.; Manavis, J.; Blumbergs, P.C.; Summersides, G.E. Brain damage in sheep from penetrating captive bolt stunning. Aust. Vet. J. 2002, 80, 67–69. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Bastuji, H. Pain and consciousness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chatelle, C.; Thibaut, A.; Whyte, J.; De Val, M.D.; Laureys, S.; Schnakers, C. Pain issues in disorders of consciousness. Brain Injury 2014, 28, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Ortega, M.E.T.; Guerrero-Legarreta, I.; Ramírez-Necoechea, R.; Roldan-Santiago, P.; Pérez-Sato, M.; Soní-Guillermo, E.; Mota-Rojas, D. Changes in blood constituents of swine transported for 8 or 16h to an abattoir. Meat Sci. 2010, 86, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Becerril-Herrera, M.; Roldan-Santiago, P.; Alonso-Spilsbury, M.; Flores-Peinado, S.; Ramírez-Necoechea, R.; Ramírez-Telles, J.A.; Mora-Medina, P.; Pérez, M.; Molina, E.; et al. Effects of long distance transportation and co2 stunning on critical blood values in pigs. Meat Sci. 2012, 90, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Wigham, E.E.; Butterworth, A.; Wotton, S. Assessing cattle welfare at slaughter—why is it important and what challenges are faced? Meat Sci. 2018, 145, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, P.; Stillman, A.M. Pathogenesis of pain. Semin. Pediatr. Neuro. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Meyer, R.A.; Ringkamp, M.; Campbell, J.N.; Raja, S.N. Peripheral mechanisms of cutaneous nociception. In Wall and Melzack’s Textbook of Pain; McMahon, S.B., Koltzenburg, M., Eds.; Elsevier: London, UK, 2006; pp. 3–34. [Google Scholar]
- Gibson, T.J.; Mason, C.W.; Spence, J.Y.; Barker, H.; Gregory, N.G. Factors affecting penetrating captive bolt gun performance. J. Appl. Anim. Welf. Sci. 2014, 18, 222–238. [Google Scholar] [CrossRef]
- Glardon, M.; Schwenk, B.K.; Riva, F.; von Holzen, A.; Ross, S.G.; Kneubuehl, B.P.; Stoffel, M.H. Energy loss and impact of various stunning devices used for the slaughtering of water buffaloes. Meat Sci. 2018, 135, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.R.; Tuckett, R.P.; Song, C.W. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J. Pain 2008, 9, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Eller-Smith, O.C.; Nicol, A.L.; Christianson, J.A. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front. Cell Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Grubb, B.D. Inflammatory nociceptor sensitization, prostaglandins and leukotrienes. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 1624–1627. [Google Scholar]
- Momin, A.; McNaughton, P.A. Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators. Exp. Brain Res. 2009, 196, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Dissecting out mechanisms responsible for peripheral neuropathic pain: Implications for diagnosis and therapy. Life Sci. 2004, 74, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Galhardo, V.; Maione, S.; Mackey, S.C. Forebrain pain mechanisms. Brain Res. Rev. 2009, 60, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Prim. Care 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef]
- Reid, K.; Rogers, C.W.; Gronqvist, G.; Gee, E.K.; Bolwell, C.F. Anxiety and pain in horses measured by heart rate variability and behavior. J. Vet. Behav. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.M.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.S.S.; Campos, A.C.P.; Fonoff, E.T.; Britto, L.R.G.; Pagano, R.L. Motor cortex and pain control: Exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav. Brain Funct. 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Callaway, L.N.; Walker, J.; Tucker, C.B. Culling decisions and dairy cattle welfare during transport to slaughter in the united states. Front. Vet. Sci. 2019, 5, 343. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, D.; Strobel, P.; Ramirez-Reveco, A.; Werner, M.; Bustamante, H. Chronic inflammatory lameness increases cytokine concentration in the spinal cord of dairy cows. Front. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, J.; von Keyserlingk, M.A.G.; Marchant-Forde, J.N.; Weary, D.M. Assessment of visceral pain associated with metritis in dairy cows. J. Dairy Sci. 2015, 98, 5352–5361. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Rosenfeld, C.; Gallo, C. Associations between pre-slaughter and post-slaughter indicators of animal welfare in cull cows. Animals 2019, 9, 642. [Google Scholar] [CrossRef]
- Tarrant, P.V. Transportation of cattle by road. Appl. Anim. Behav. Sci. 1990, 28, 153–170. [Google Scholar] [CrossRef]
- Chandra, B.S.; Das, N. The handling and short-haul road transportation of spent buffaloes in relation to bruising and animal welfare. Trop. Anim. Health Prod. 2001, 33, 155–163. [Google Scholar] [CrossRef]
- Ahsan, M.; Hasan, B.; Algotsson, M.; Sarenbo, S. Handling and welfare of bovine livestock at local abattoirs in Bangladesh. J. Appl. Anim. Welf. Sci. 2014, 17, 340–353. [Google Scholar] [CrossRef]
- Paton, R. Observations on rib fractures in slaughter cattle. Vet. Rec. 2014, 175, 123–124. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of human-animal relationship on animal productivity and welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Hultgren, J.; Wiberg, S.; Berg, C.; Cvek, K.; Lunner Kolstrup, C. Cattle behaviours and stockperson actions related to impaired animal welfare at swedish slaughter plants. Appl. Anim. Behav. Sci. 2014, 152, 23–37. [Google Scholar] [CrossRef]
- Villarroel, M.; Maria, G.A.; Sierra, I.; Saitudo, C.; Garcia-Belenguer, S.; Gebresenbet, G. Critical points in the transport of cattle to slaughter in spain that may compromise the animals’ welfare. Vet. Rec. 2001, 149, 173–176. [Google Scholar] [CrossRef]
- Muñoz, D.; Strappini, A.; Gallo, C. Indicadores de bienestar animal para detectar problemas en el cajón de insensibilización de bovinos. Arch. Med. Vet. 2012, 44, 297–302. [Google Scholar] [CrossRef]
- Probst, J.K.; Spengler Neff, A.; Leiber, F.; Kreuzer, M.; Hillmann, E. Gentle touching in early life reduces avoidance distance and slaughter stress in beef cattle. Appl. Anim. Behav. Sci. 2012, 139, 42–49. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.A.G.; Rushen, J.; de Passillé, A.M.; Weary, D.M. Invited review: The welfare of dairy cattle—key concepts and the role of science. J. Dairy Sci. 2009, 92, 4101–4111. [Google Scholar] [CrossRef]
- Grandin, T. Cattle vocalizations are associated with handling and equipment problems at beef slaughter plants. Appl. Anim. Behav. Sci. 2001, 71, 191–201. [Google Scholar] [CrossRef]
- Manteuffel, G.; Puppe, B.; Schön, P.C. Vocalization of farm animals as a measure of welfare. Appl. Anim. Behav. Sci. 2004, 88, 163–182. [Google Scholar] [CrossRef]
- Bourguet, C.; Deiss, V.; Tannugi, C.C.; Terlouw, E.M.C. Behavioural and physiological reactions of cattle in a commercial abattoir: Relationships with organisational aspects of the abattoir and animal characteristics. Meat Sci. 2011, 88, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Peters, M.; Baumgärtner, W.; Wohlsein, P. Electrical injuries in animals. Vet. Pathol. 2016, 53, 1018–1029. [Google Scholar] [CrossRef]
- Johnson, C.B.; Gibson, T.J.; Stafford, K.J.; Mellor, D.J. Pain perception at slaughter. Anim. Welf. 2012, 21, 113–122. [Google Scholar] [CrossRef]
- Nakyinsige, K.; Che Man, Y.B.; Aghwan, Z.A.; Zulkifli, I.; Goh, Y.M.; Abu Bakar, F.; Al-Kahtani, H.A.; Sazili, A.Q. Stunning and animal welfare from islamic and scientific perspectives. Meat Sci. 2013, 95, 352–361. [Google Scholar] [CrossRef]
- Loyer, J.; Whittaker, A.L.; Buddle, E.A.; Ankeny, R.A. A review of legal regulation of religious slaughter in Australia: Failure to regulate or a regulatory fail? Animals 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, A.; Knowles, T.G.; Lines, J.; Hadley, P.; Wotton, S. The stunning and slaughter of cattle within the eu: A review of the current situation with regard to the halal market. Anim. Welf. 2016, 25, 365–376. [Google Scholar] [CrossRef]
- Mota-Rojas, D.S.; Strappini, A.C.; Ghezzi, D.M.; Hernández-Ávalos, I.; Rosmini, M.R.; Miranda-Cortés, A.E.; Casas, A.; Lezama, K.; Guerrero, I.; Ciocca, J.R.; et al. Quality of death in buffalo and cattle. In The River Buffalo in the Americas, 2nd ed.; Guerrero-Legarreta, I., Napolitano, F., Mota-Rojas, D., Orihuela, A., Eds.; BM Editores Press: México City, México, 2019; pp. 1–889. [Google Scholar]
- Mota-Rojas, D.; Ghezzi, M.D.; Rosmini, M.; Thielo de la Vega, L.; Hernández-Ávalos, I.; Cajiao, M.N.; Ciocca, J.R.; Lezama, K.; Lemus, C.; Guerrero, I. Signs of sensitivity during death: Assessment of beef and buffalo meat quality. In The River Buffalo in the Americas, 2nd ed.; Guerrero-Legarreta, I., Napolitano, F., Mota-Rojas, D., Orihuela, A., Eds.; BM Editores Press: México City, México, 2019; pp. 1–889. [Google Scholar]
- Johnson, C.B.; Mellor, D.J.; Hemsworth, P.H.; Fisher, A.D. A scientific comment on the welfare of domesticated ruminants slaughtered without stunning. N. Z. Vet. J. 2015, 63, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Gibson, T.J.; Johnson, C.B. A re-evaluation of the need to stun calves prior to slaughter by ventral-neck incision: An introductory review. N. Z. Vet. J. 2009, 57, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T.; Cockram, M. The Slaughter of Farmed Animals: Practical Ways of Enhancing Animal Welfare; CABI Publishing: Wallingford, UK, 2020. [Google Scholar]
- Gregory, N.G.; Lee, C.J.; Widdicombe, J.P. Depth of concussion in cattle shot by penetrating captive bolt. Meat Sci. 2007, 77, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Caldwell, M.; Hecht, S.; Whitlock, B.K. Comparison of penetrating and nonpenetrating captive bolt methods in horned goats. Am. J. Vet. Res. 2017, 78, 151–157. [Google Scholar] [CrossRef]
- Schwenk, B.K.; Lechner, I.; Ross, S.G.; Gascho, D.; Kneubuehl, B.P.; Glardon, M.; Stoffel, M.H. Magnetic resonance imaging and computer tomography of brain lesions in water buffaloes and cattle stunned with handguns or captive bolts. Meat Sci. 2016, 113, 35–40. [Google Scholar] [CrossRef]
- Vecerek, V.; Kamenik, J.; Voslarova, E.; Volfova, M.; Machovcova, Z.; Konvalinova, J.; Vecerkova, L. The impact of deviation of the stun shot from the ideal point on motor paralysis in cattle. Animals 2020, 10, 280. [Google Scholar] [CrossRef]
- Atkinson, S.; Velarde, A.; Algers, B. Assessment of stun quality at commercial slaughter in cattle shot with captive bolt. Anim. Welf. 2013, 22, 473–481. [Google Scholar] [CrossRef]
- Gouveia, K.; Ferreira, P.G.; Costa, J.C.; Vaz-Pires, P.; Costa, P. Assessment of the efficiency of captive-bolt stunning in cattle and feasibility of associated behavioural signs. Anim. Welf. 2009, 18. [Google Scholar]
- Blumenfeld, H. Cellular and network mechanisms of spike-wave seizures. Epilepsia 2005, 46, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N. Profiles of currents during electrical stunning. Aust. Vet. J. 2001, 79, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Warrington, R. Electrical stunning: A review of the literature. Vet. Bull. 1974, 44, 617–635. [Google Scholar]
- Sabow, A.B.; Nakyinsige, K.; Adeyemi, K.D.; Sazili, A.Q.; Johnson, C.B.; Webster, J.; Farouk, M.M. High frequency pre-slaughter electrical stunning in ruminants and poultry for halal meat production: A review. Livest. Sci. 2017, 202, 124–134. [Google Scholar] [CrossRef]
- Simmons, N.J. The Use of High Frequency Currents for the Electrical Stunning of Pigs. Master’s Thesis, University of Bristol, Langford, UK, 1995. [Google Scholar]
- Velarde, A.; Dalmau, A. 12-slaughter without stunning. In Advances in Agricultural Animal Welfare; Mench, J.A., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 221–240. [Google Scholar]
- Gregory, N.G.; von Wenzlawowicz, M.; von Holleben, K.; Fielding, H.R.; Gibson, T.J.; Mirabito, L.; Kolesar, R. Complications during shechita and halal slaughter without stunning in cattle. Anim. Welf. 2012, 21, 81–86. [Google Scholar] [CrossRef]
- Sabow, A.B.; Goh, Y.; Idrus, Z.; Kaka, U.; Kadir, Z.; Ebrahimi, M.; Nakyinsige, K.; Adeyemi, K. Blood parameters and electroencephalographic responses of goats to slaughter without stunning. Meat Sci. 2016, 121. [Google Scholar] [CrossRef]
- Gibson, T.J.; Johnson, C.B.; Murrell, J.C.; Mitchinson, S.L.; Stafford, K.J.; Mellor, D.J. Amelioration of electroencephalographic responses to slaughter by non-penetrative captive-bolt stunning after ventral-neck incision in halothane—anaesthetised calves. N. Z. Vet. J. 2009, 57, 96–101. [Google Scholar] [CrossRef]
- Nakyinsige, K.; Sazili, A.Q.; Zulkifli, I.; Goh, Y.M.; Abu Bakar, F.; Sabow, A.B. Influence of gas stunning and halal slaughter (no stunning) on rabbits welfare indicators and meat quality. Meat Sci. 2014, 98, 701–708. [Google Scholar] [CrossRef]
- Bozzo, G.; Barrasso, R.; Marchetti, P.; Roma, R.; Samoilis, G.; Tantillo, G.; Ceci, E. Analysis of stress indicators for evaluation of animal welfare and meat quality in traditional and jewish slaughtering. Animals 2018, 8, 43. [Google Scholar] [CrossRef]
- Disanto, C.; Celano, G.; Varvara, M.; Fusiello, N.; Fransvea, A.; Bozzo, G.; Celano, G.V. Stress factors during cattle slaughter. Ital. J. Food Saf. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.G.; Wenzlawowicz, M.v.; Holleben, K.v. Blood in the respiratory tract during slaughter with and without stunning in cattle. Meat Sci. 2009, 82, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Von Wenzlawowicz, M. “Reversible (head-only) electrical stunning” Method and experience. In Animal Welfare at Religious Slaughter—The Ethics Workshops of the DIALREL Project; Caspar, J., et Luy, J., Eds.; NOMOS: Baden-Baden, Germany, 2010; Volume 6, pp. 243–245. ISBN 978-3-8329-4898-6. [Google Scholar]
- Canning, B.J. Encoding of the cough reflex. Pulm. Pharmacol. Ther. 2007, 20, 396–401. [Google Scholar] [CrossRef]
- Zulkifli, I.; Goh, Y.M.; Norbaiyah, B.; Sazili, A.Q.; Lotfi, M.; Small, A. Changes in blood parameters and electroencephalogram as affected by different stunning methods in cattle. Anim. Prod. Sci. 2014, 54, 187–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Ghezzi, M.D.; Hernández-Ávalos, I.; Mora-Medina, P.; Whittaker, A.L. Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals 2021, 11, 1085. https://doi.org/10.3390/ani11041085
Mota-Rojas D, Napolitano F, Strappini A, Orihuela A, Ghezzi MD, Hernández-Ávalos I, Mora-Medina P, Whittaker AL. Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals. 2021; 11(4):1085. https://doi.org/10.3390/ani11041085
Chicago/Turabian StyleMota-Rojas, Daniel, Fabio Napolitano, Ana Strappini, Agustín Orihuela, Marcelo Daniel Ghezzi, Ismael Hernández-Ávalos, Patricia Mora-Medina, and Alexandra L. Whittaker. 2021. "Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation" Animals 11, no. 4: 1085. https://doi.org/10.3390/ani11041085
APA StyleMota-Rojas, D., Napolitano, F., Strappini, A., Orihuela, A., Ghezzi, M. D., Hernández-Ávalos, I., Mora-Medina, P., & Whittaker, A. L. (2021). Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals, 11(4), 1085. https://doi.org/10.3390/ani11041085










