Muscle Fibre Architecture of Thoracic and Lumbar Longissimus Dorsi Muscle in the Horse
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Specimen Preparation
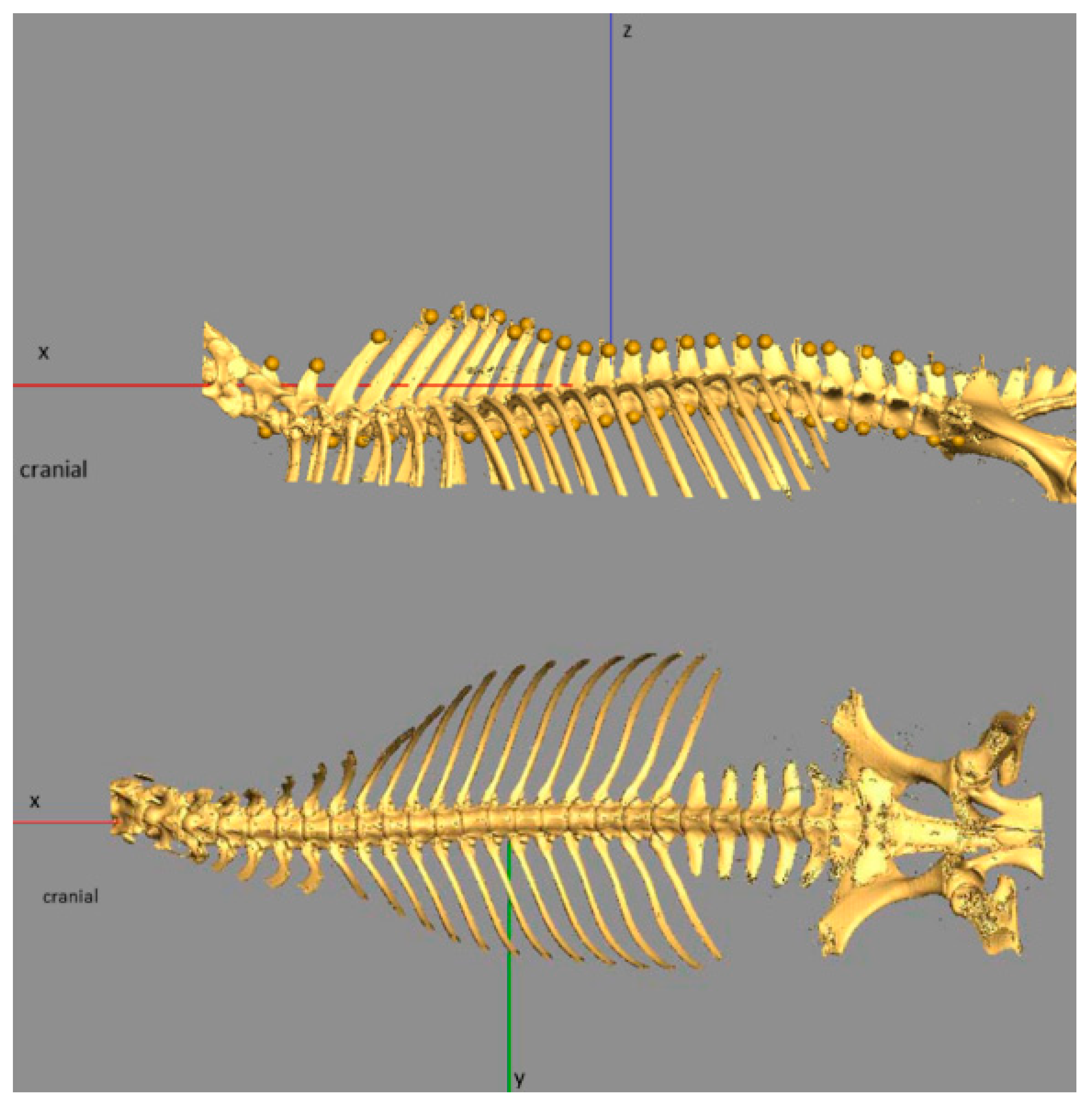
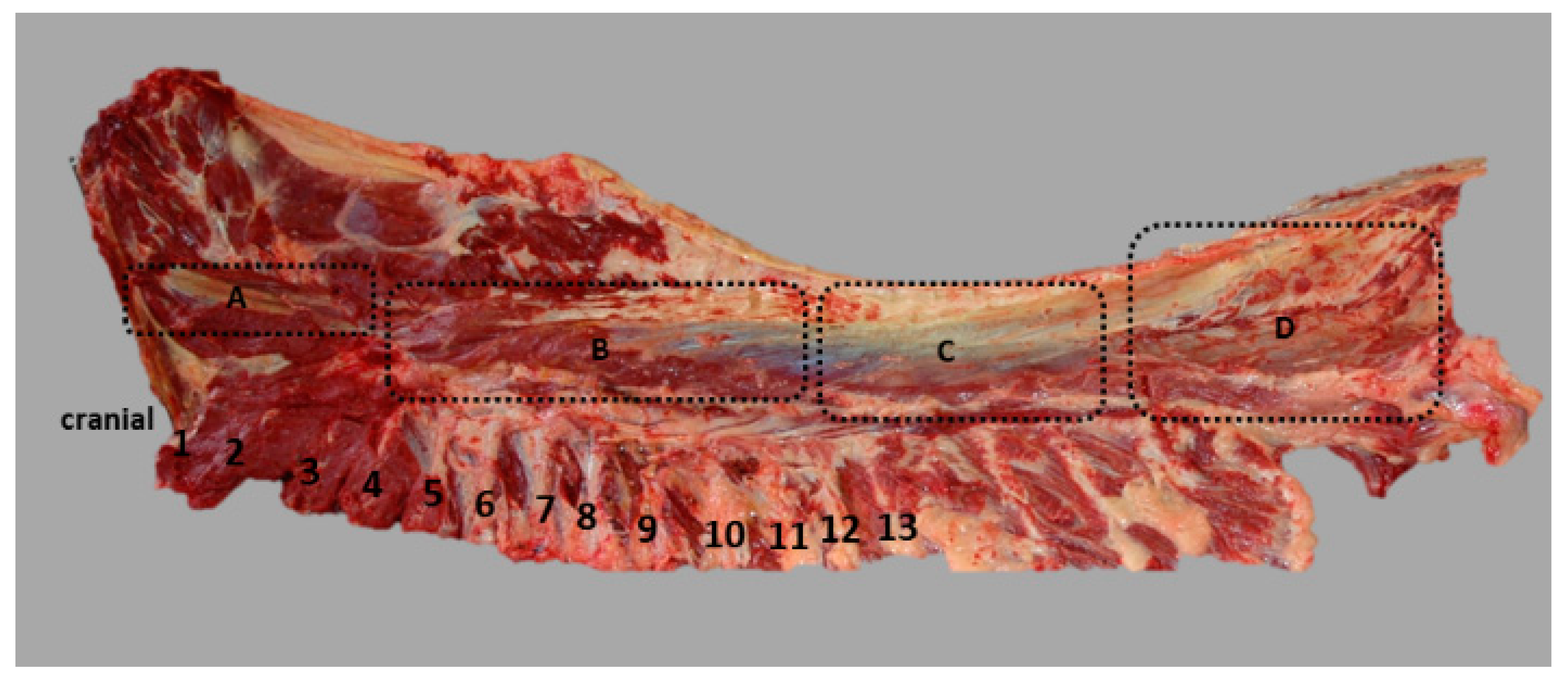
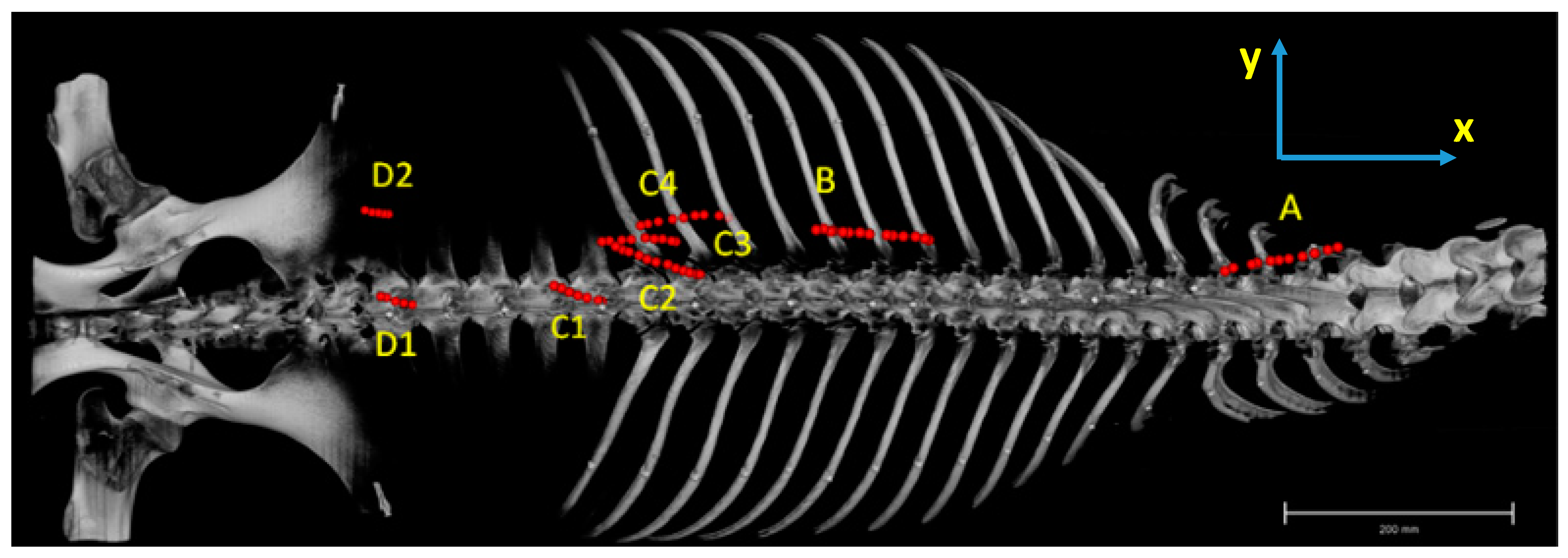
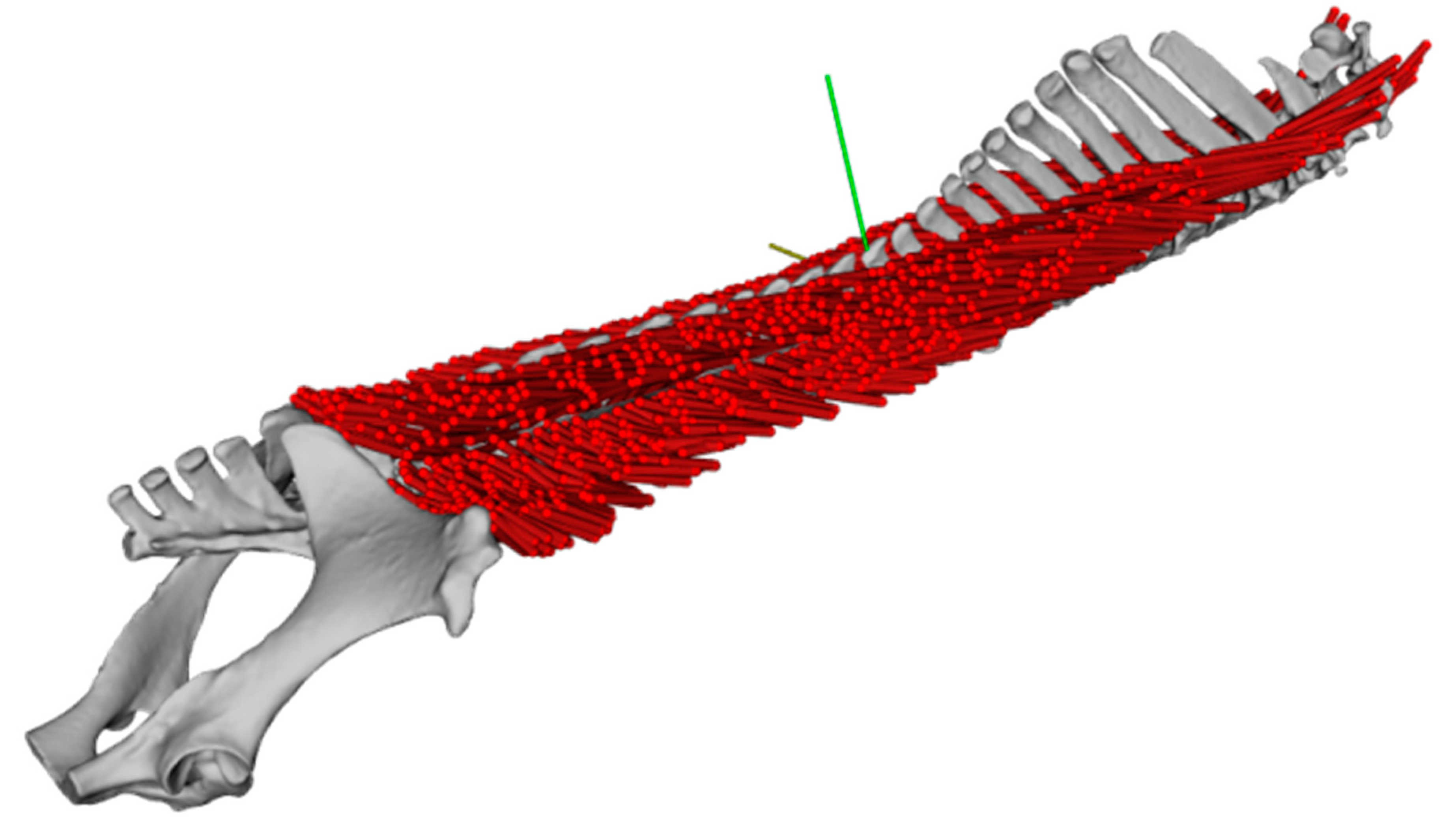
2.2. Dissection, Digitisation, Three-Dimensional Modelling
2.3. Data Analysis
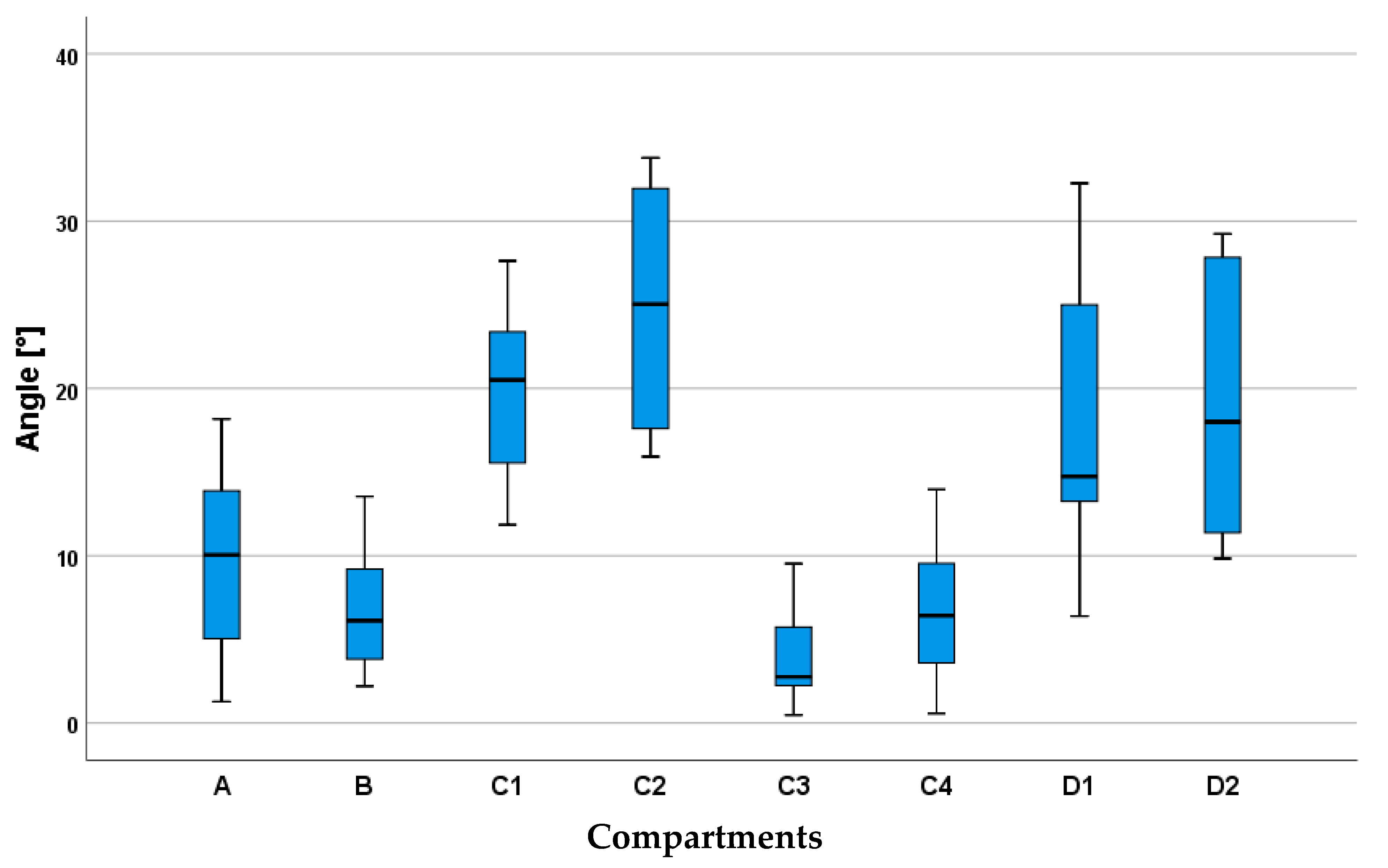
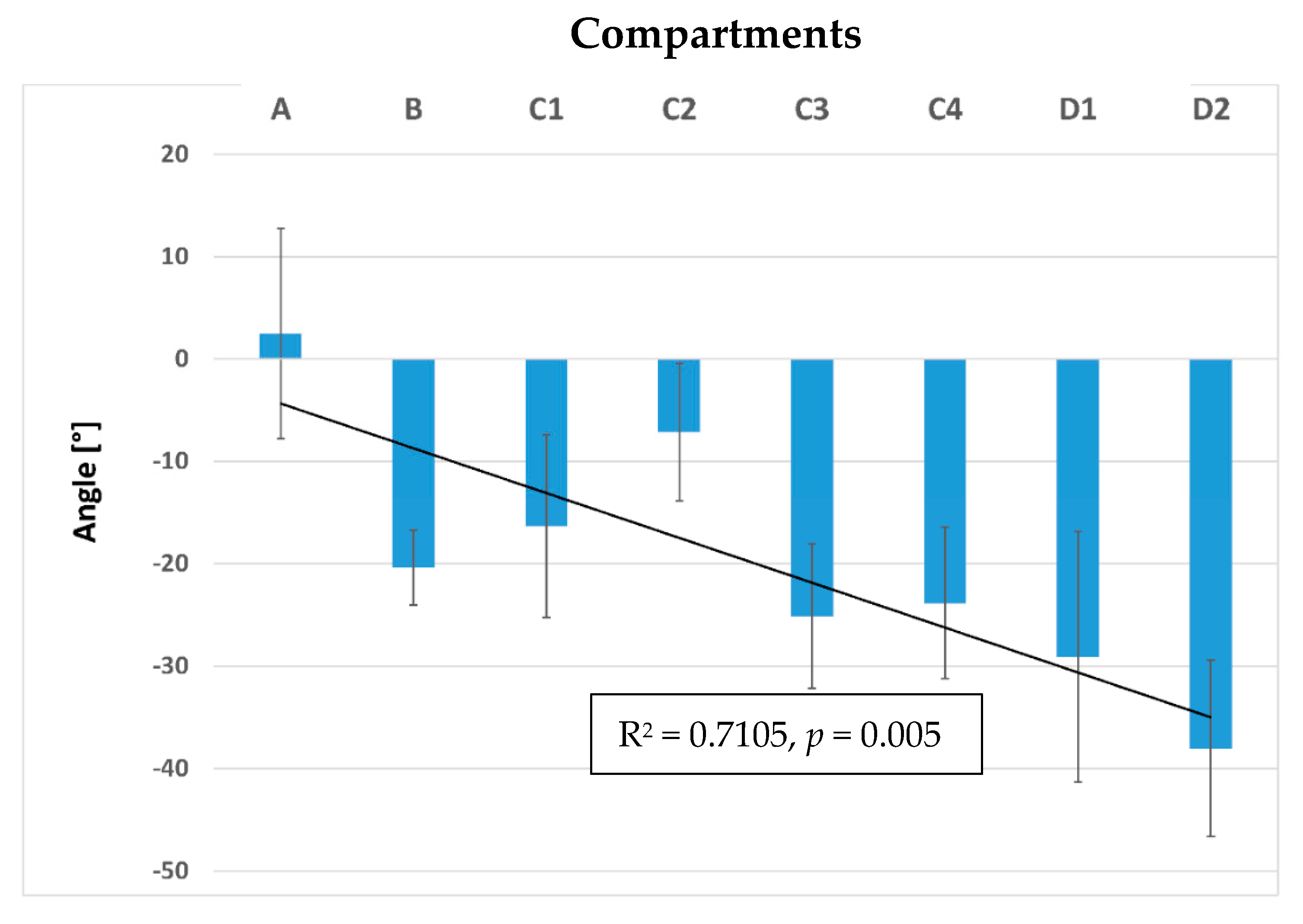
3. Results
3.1. Anatomical Description
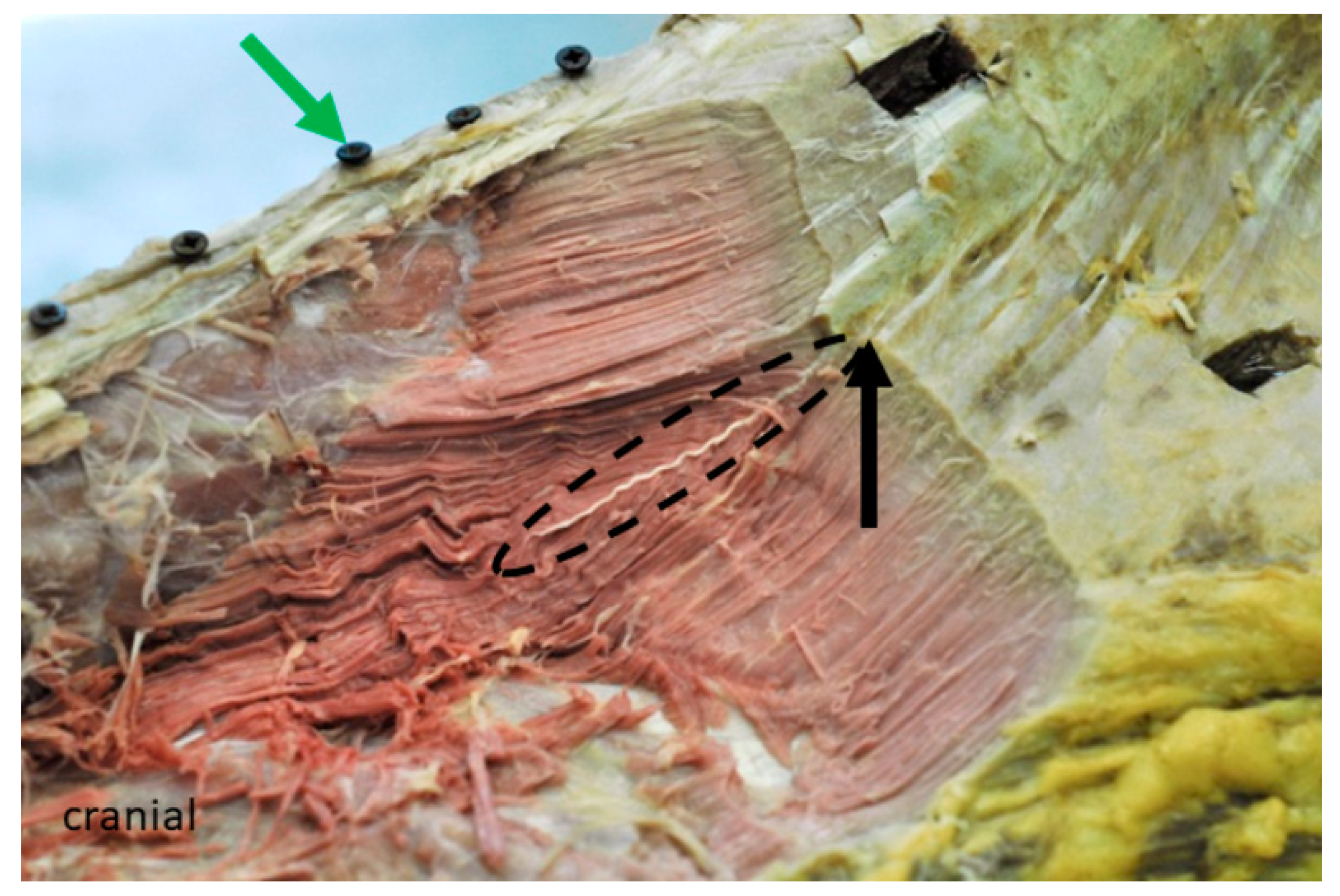
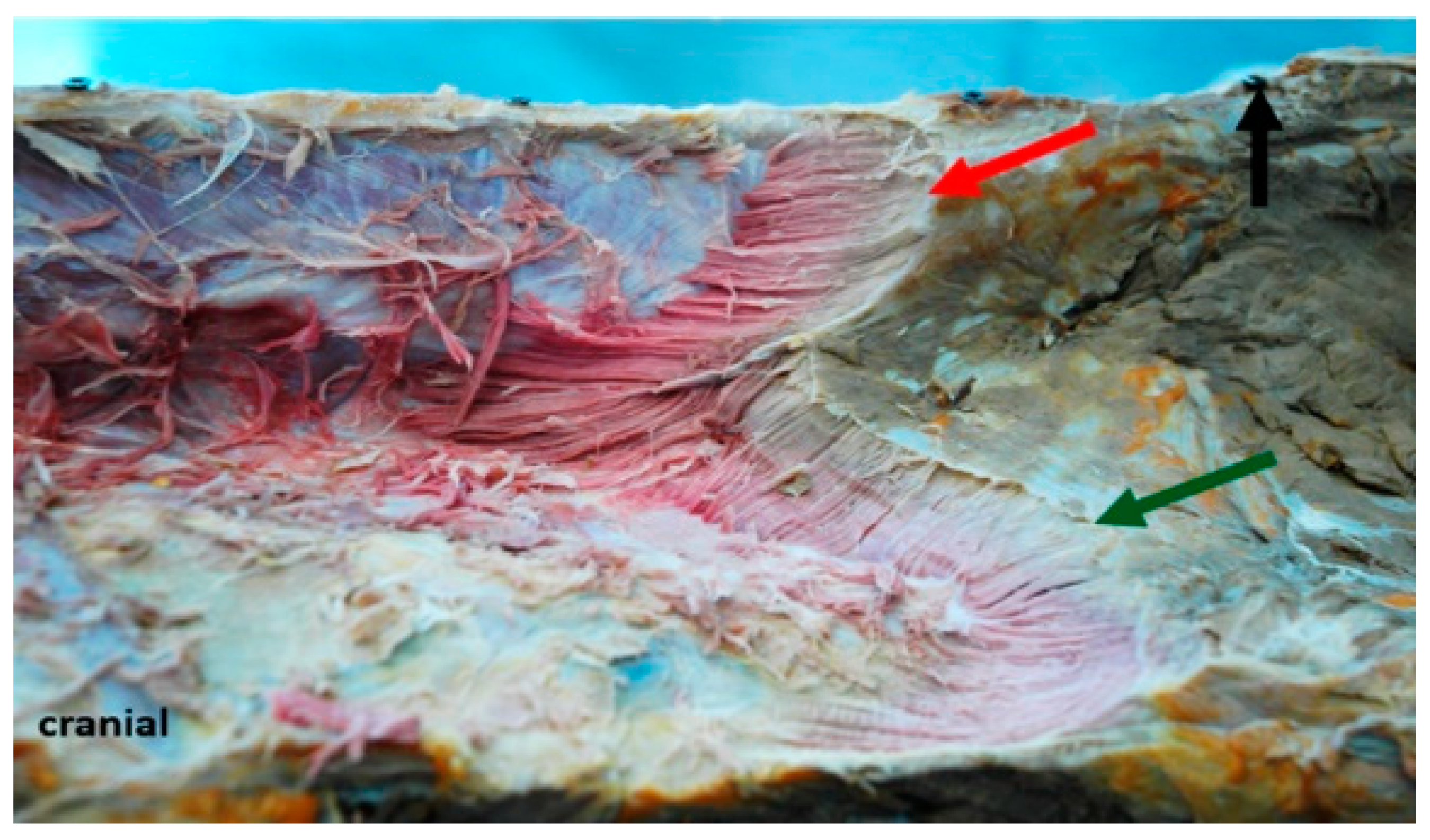
3.2. Inner Muscle Architecture
4. Discussion
Muscle Architecture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stubbs, N.C.; Hodges, P.W.; Jeffcott, L.B.; Cowin, G.; Hodgson, D.R.; McGowan, C.M. Functional anatomy of the caudal thoracolumbar and lumbosacral spine in the horse. Equine Vet. J. 2006, 36, 393–399. [Google Scholar] [CrossRef]
- Jeffcott, L.B. Disorders of the thoracolumbar spine of the horse—A survey of 443 cases. Equine Vet. J. 1980, 12, 197–210. [Google Scholar] [CrossRef]
- Haussler, K.K.; Erb, H.N. Pressure algometry for the detection of induced back pain in horses: A preliminary study. Equine Vet. J. 2006, 37, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Jeffcott, L.B. Historical perspective and clinical indications. Vet. Clin. N. Am. Equine Pract. 1999, 15, 1–11. [Google Scholar] [CrossRef]
- Pongratz, U.; Licka, T. Algometry to measure pain threshold in the horse’s back—An in vivo and in vitro study. BMC Vet. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Barnett, K.; Price, S.; Nankervis, K. Effects of manipulative therapy on the longissimus dorsi in the equine back. Equine Comp. Exerc. Physiol. 2006, 3, 153–160. [Google Scholar] [CrossRef]
- Haussler, K.K. Anatomy of the thoracolumbar vertebral region. Vet. Clin. N. Am. Equine Pract. 1999, 15, 13–26. [Google Scholar] [CrossRef]
- Ritruechai, P.; Weller, R.; Wakeling, J.M. Regionalisation of the muscle fascicle architecture in the equine longissimus dorsi muscle. Equine Vet. J. 2008, 40, 246–251. [Google Scholar] [CrossRef]
- Chiou, W.K.; Wong, M.K.; Lee, Y.H. Epidemiology of low back pain in Chinese nurses. Int. J. Nurs. Stud. 1994, 31, 361–368. [Google Scholar] [CrossRef]
- Lieber, R.L.; Friden, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 220, 1–14. [Google Scholar] [CrossRef]
- Gans, C. Fiber architecture and muscle function. Exerc. Sport Sci. Rev. 1982, 10, 160–207. [Google Scholar] [CrossRef]
- Neufuss, J.; Hesse, B.; Thorpe, S.K.S.; Vereecke, E.E.; D’Aout, K.; Fischer, M.S.; Schilling, N. Fibre type composition in the lumbar perivertebral muscles of primates: Implications for the evolution of orthogrady in hominoids. J. Anat. 2014, 224, 113–131. [Google Scholar] [CrossRef]
- Sacks, R.D.; Roy, R.R. Architecture of the Hind Limb Muscles of Cats: Functional Significance. J. Morphol. 1982, 173, 185–195. [Google Scholar] [CrossRef]
- Stark, H.; Fröber, R.; Schilling, N. Intramuscular architecture of the autochthonous back muscles in humans. J. Anat. 2013, 222, 214–222. [Google Scholar] [CrossRef]
- Licka, T.; Frey, A.; Peham, C. Electromyographic activity of the longissimus dorsi muscle in horses when walking on a treadmill. Vet. J. 2009, 180, 71–76. [Google Scholar] [CrossRef]
- Peham, C.; Schobesberger, H. A novel method to estimate the stiffness of the equine back. J. Biomech. 2006, 39, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Audigie, F.; Valette, J.P.; Pourcelot, P.; Denoix, J.M. Effects of treadmill speed on the mechanics of the back in trotting saddlehorse. Equine Vet. J. 2001, 33, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Kuraoka, A. Differences in the muscle dimensional parameters between non-formalin-fixed (freeze-thawed) and formalin-fixed specimen in gorilla (Gorilla gorilla). Mammal Study 2014, 39, 65–72. [Google Scholar] [CrossRef]
- Gerling, M.E.; Brown, S.H.M. Architectural analysis and predicted functional capability of the human latissimus dorsi muscle. J. Anat. 2013, 223, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Boynton, E.L.; Ravichandiran, K.; Fung, L.Y.; Bleakney, R.; Agur, A.M. Three- Dimensional Study of the Musculotendinous Architecture of Supraspinatus and Its Functional Correlations. Clin. Anat. 2007, 20, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ravichandiran, K.; Jackson, K.; Fiume, A.A. Robust estimation of PCSA and geometric reconstruction for human skeletal muscle. J. Biomech. 2012, 45, 1507–1513. [Google Scholar] [CrossRef]
- Von Sheven, C.C.A. The Anatomy and Function of the Equine Thoracolumbar Longissimus Dorsi Muscle. Ph.D. Thesis, Tierärztliche Fakultät der Ludwig- Maximilians-Universität München, Munich, Germany, 2010. [Google Scholar]
- Chow, R.S.; Medri, M.K.; Martin, D.C.; Leekam, R.N.; Agur, A.M.; McKee, N.H. Sonographic studies of human soleus and gastrocnemius muscle architecture: Gender variability. Eur. J. Appl. Physiol. 2000, 82, 236–244. [Google Scholar] [CrossRef]
- Crook, T.C.; Cruickshank, S.E.; McGowan, C.M.; Stubbs, N.; Wakeling, J.M.; Wilson, A.M.; Payne, R.C. Comparative anatomy and muscle architecture of selected hind limb muscles in the Quarter Horse and Arab. J. Anat. 2008, 212, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wichiewicz, T.L.; Roy, R.R.; Powell, P.L.; Perrine, J.J.; Edgerton, V.R. Muscle architecture and force-velocity relationships in humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 435–443. [Google Scholar] [CrossRef]
- Townsend, H.G.G.; Leach, D.H. Relationship between intervertebral joint morphology and mobility in the equine thoracolumbar spine. Equine Vet. J. 1984, 16, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Spinal biomechanics and functional anatomy. Vet. Clin. N. Am. Equine Pract. 1999, 15, 27–60. [Google Scholar] [CrossRef]
- Bojadsen, T.W.A.; Silva, E.S.; Rodrigues, A.J.; Amadio, A.C. Comparative study of Mm. Multifidi in lumbar and thoracic spine. J. Electromyogr. Kinesiol. 2000, 10, 143–149. [Google Scholar] [CrossRef]
- Gans, C.; Bock, W.J. The functional significance of muscle architecture: A theoretical analysis. Ergeb. Anat. Entwicklungsgesch. 1965, 38, 115–142. [Google Scholar]
- Faber, M.; Johnston, C.; Schamhardt, H.; van Weeren, R.; Roepstorff, L.; Barneveld, A. Basic three-dimensional kinematics of the vertebral column of horses trotting on a treadmill. Am. J. Vet. Res. 2001, 62, 757–764. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Dalin, G. Natural rigidity of the horse’s backbone. Equine Vet. J. 1980, 12, 101–108. [Google Scholar] [CrossRef]
- Maganaris, N.C.; Baltzopoulos, V.; Sargeant, A.J. In vivo measurements of the triceps surae complex architecture in man: Implications for muscle function. J. Physiol. 1998, 512, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Felder, A.; Ward, S.R.; Lieber, R.L. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J. Exp. Biol. 2005, 208, 3275–3279. [Google Scholar] [CrossRef]
- Powell, P.L.; Roy, R.R.; Kanim, P.; Bello, A.M.; Edgerton, V.R. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 1984, 57, 1715–1721. [Google Scholar] [CrossRef]
- Boyd-Clark, L.C.; Briggs, C.A.; Galea, M.P. Comparative histochemical composition of muscle fibers in a pre- and a postvertebral muscle of the cervical spine. J. Anat. 2001, 199, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hyytiäinen, H.K.; Mykkänen, A.K.; Hielm-Björkman, A.K.; Stubbs, N.C.; McGowan, C. Muscle fibre type distribution of the thoracolumbar and hindlimb regions of horses: Relating fibre type and functional role. Acta Vet. Scand. 2014, 56, 8. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.A.; Moseley, G.L.; Hodges, P.W. The lumbar multifidus: Does the evidence support clinical beliefs? Man. Ther. 2006, 11, 254–263. [Google Scholar] [CrossRef]
- Mannion, A.F.; Dumas, G.A.; Cooper, R.G.; Espinosa, F.J.; Faris, M.W.; Stevenson, J.M. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: Normal values and sex differences. J. Anat. 1997, 190, 505–513. [Google Scholar] [CrossRef]
- Kawai, M.; Minami, Y.; Sayama, Y.; Kuwano, A.; Hiraga, A.; Miyata, H. Muscle fiber population and biochemical properties of whole body muscles in Thoroughbred horses. Anat. Rec. 2009, 292, 1663–1669. [Google Scholar] [CrossRef]
- Rivero, J.L.L.; Ruz, M.C.; Serrano, A.L.; Diz, A.M. Effects of a 3 month endurance training programme on skeletal muscle histochemistry in Andalusian, Arabian and Anglo-Arabian horses. Equine Vet. J. 1995, 27, 51–59. [Google Scholar] [CrossRef]
- Ravara, B.; Gobbo, V.; Carraro, U.; Gelbmann, L.; Pribyl, J.; Schils, S. Functional electrical stimulation as a safe and effective treatment for equine epaxial muscle spasms: Clinical evaluations and histochemical morphometry of mitochondria in muscle biopsies. Eur. J. Transl. Myol. 2015, 25, 109–120. [Google Scholar] [CrossRef]
- Rivero, J.L.L.; Galisteo, A.M.; Agüera, E.; Miro, F. Skeletal muscle histochemistry in male and female Andalusian and Arabian horses of different ages. Res. Vet. Sci. 1993, 54, 160–169. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, J.; Handschuh, S.; Steidl, R.; Böhler, A.; Forstenpointner, G.; Egerbacher, M.; Peham, C.; Schöpper, H. Muscle Fibre Architecture of Thoracic and Lumbar Longissimus Dorsi Muscle in the Horse. Animals 2021, 11, 915. https://doi.org/10.3390/ani11030915
Dietrich J, Handschuh S, Steidl R, Böhler A, Forstenpointner G, Egerbacher M, Peham C, Schöpper H. Muscle Fibre Architecture of Thoracic and Lumbar Longissimus Dorsi Muscle in the Horse. Animals. 2021; 11(3):915. https://doi.org/10.3390/ani11030915
Chicago/Turabian StyleDietrich, Johanna, Stephan Handschuh, Robert Steidl, Alexandra Böhler, Gerhard Forstenpointner, Monika Egerbacher, Christian Peham, and Hanna Schöpper. 2021. "Muscle Fibre Architecture of Thoracic and Lumbar Longissimus Dorsi Muscle in the Horse" Animals 11, no. 3: 915. https://doi.org/10.3390/ani11030915
APA StyleDietrich, J., Handschuh, S., Steidl, R., Böhler, A., Forstenpointner, G., Egerbacher, M., Peham, C., & Schöpper, H. (2021). Muscle Fibre Architecture of Thoracic and Lumbar Longissimus Dorsi Muscle in the Horse. Animals, 11(3), 915. https://doi.org/10.3390/ani11030915





