Effect of Season and Social Environment on Semen Quality and Endocrine Profiles of Three Endangered Ungulates (Gazella cuvieri, G. dorcas and Nanger dama)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
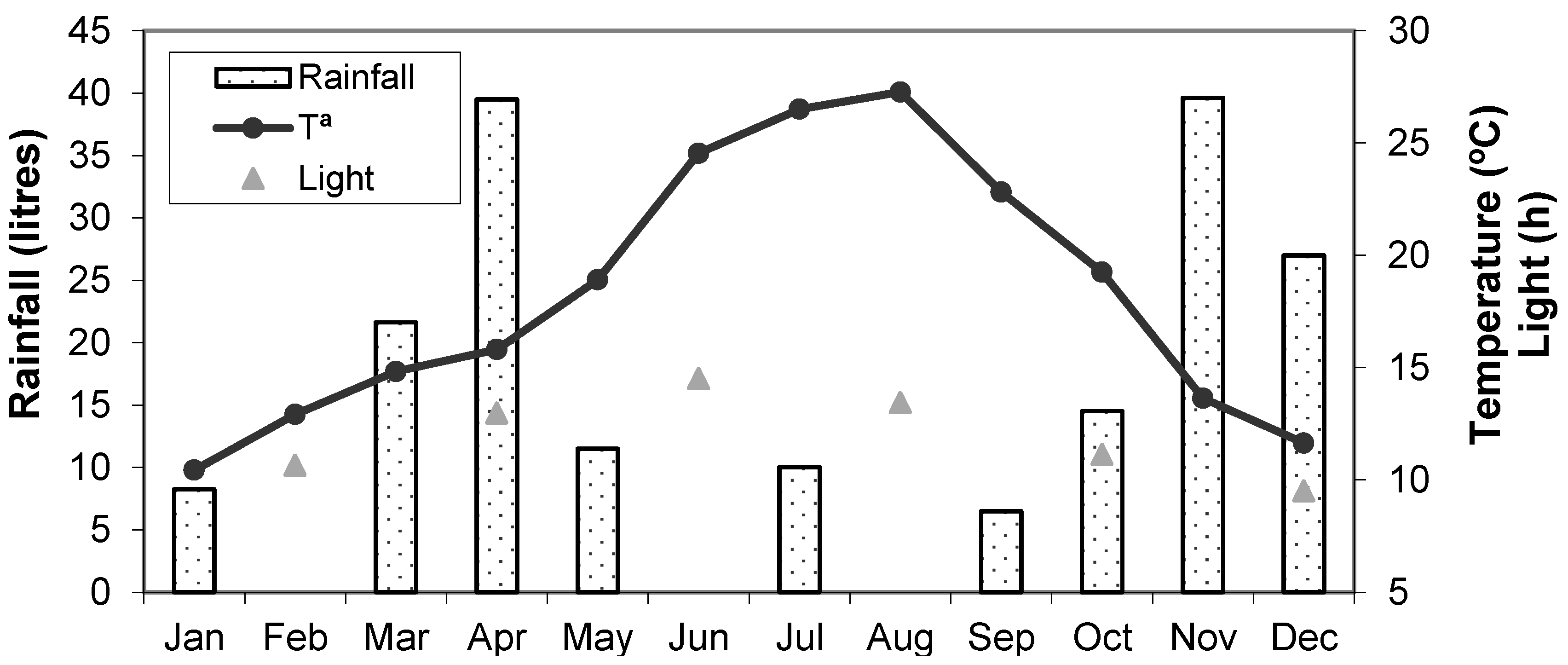
2.1. Animal Maintenance and Environment
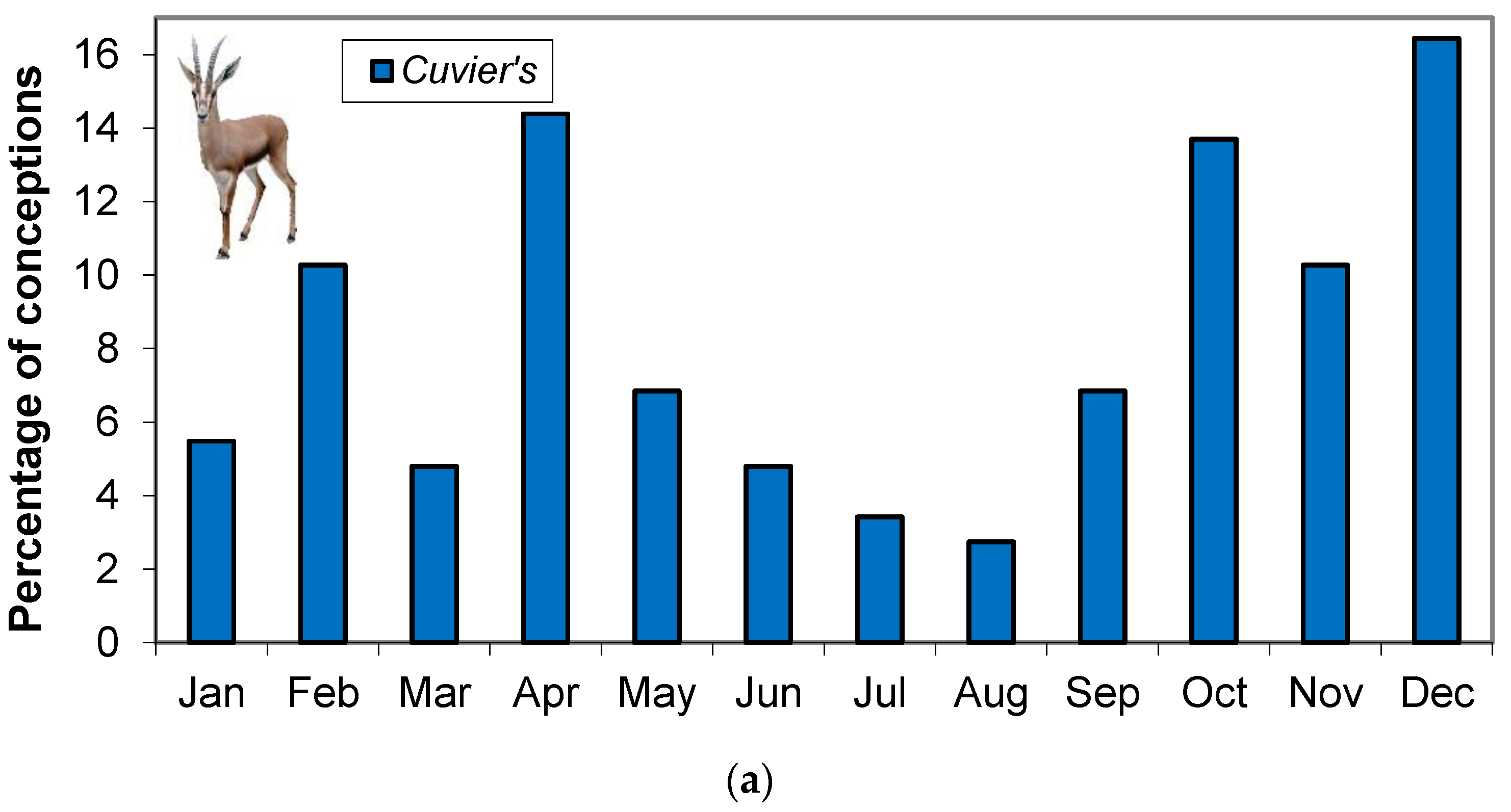
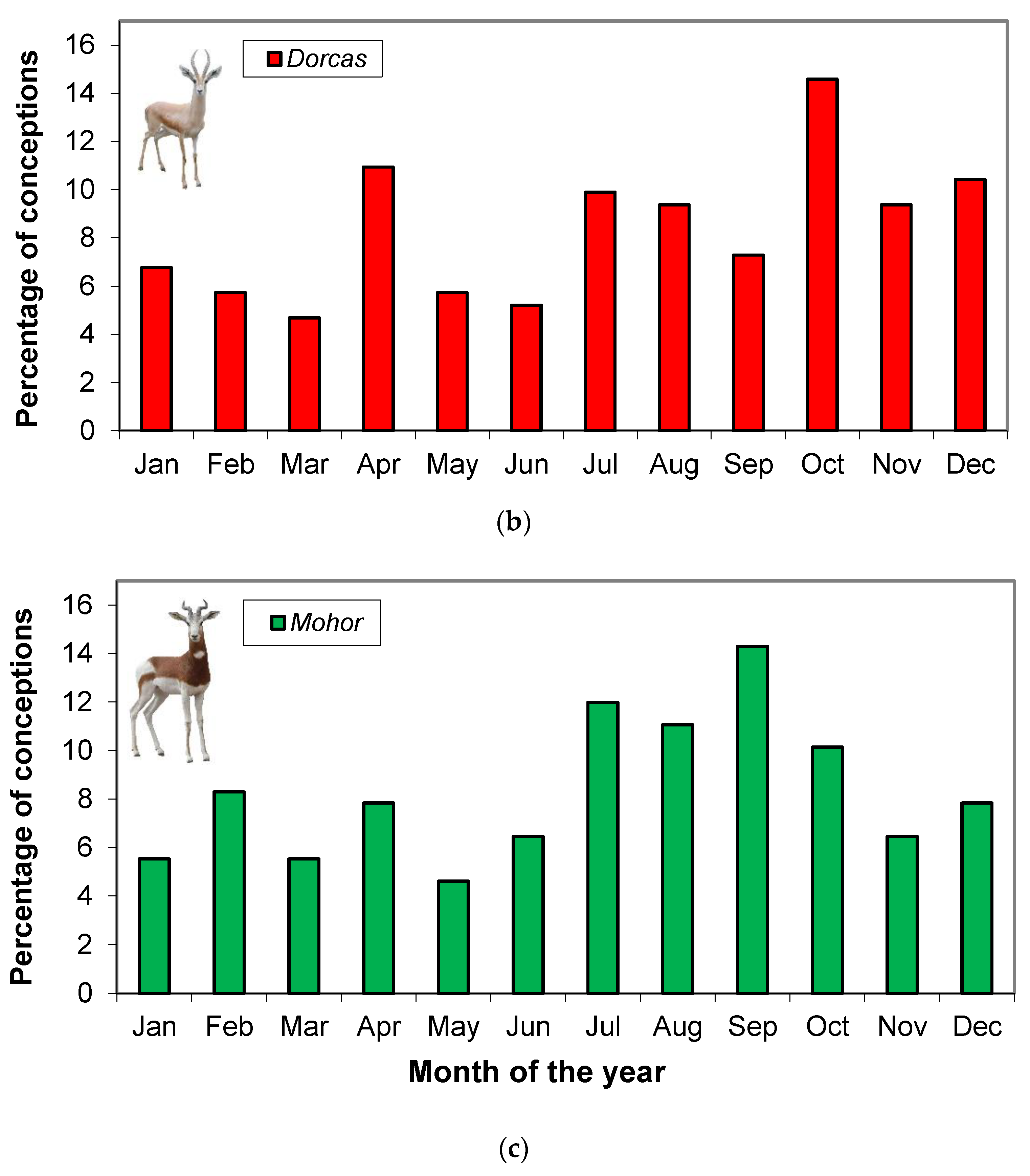
2.2. Distributions of Conceptions Dates throughout the Year
2.3. Effect of Seasonality
2.3.1. Animals and Body Measures
2.3.2. Hormone Analyses
2.3.3. Semen Collection and Evaluation
2.4. Effect of Housing Conditions
2.5. Statistical Analysis
3. Results
3.1. Distributions of Conception Dates throughout the Year
3.2. Effect of Seasonality
3.2.1. Body Measures
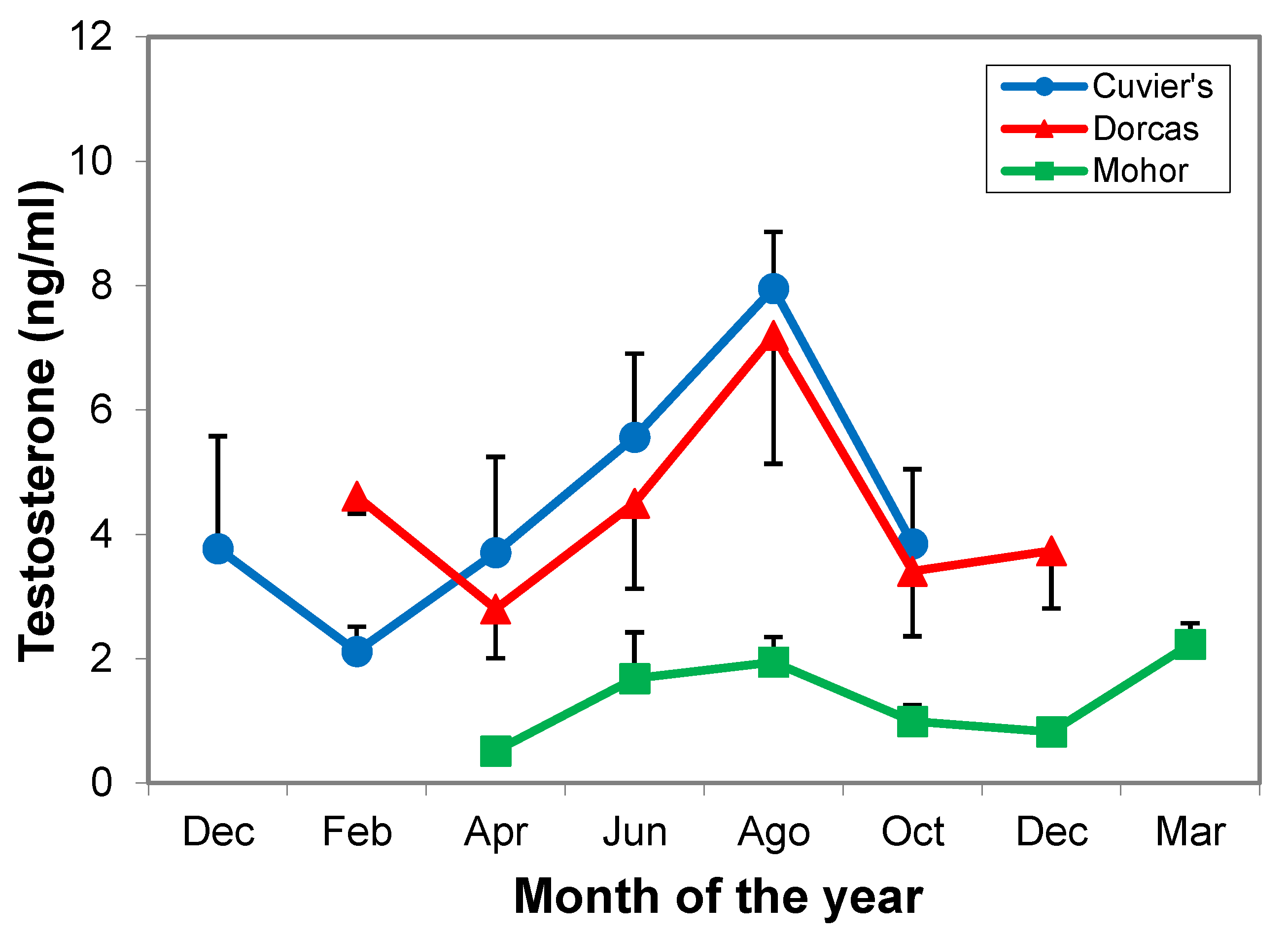
3.2.2. Testosterone Levels
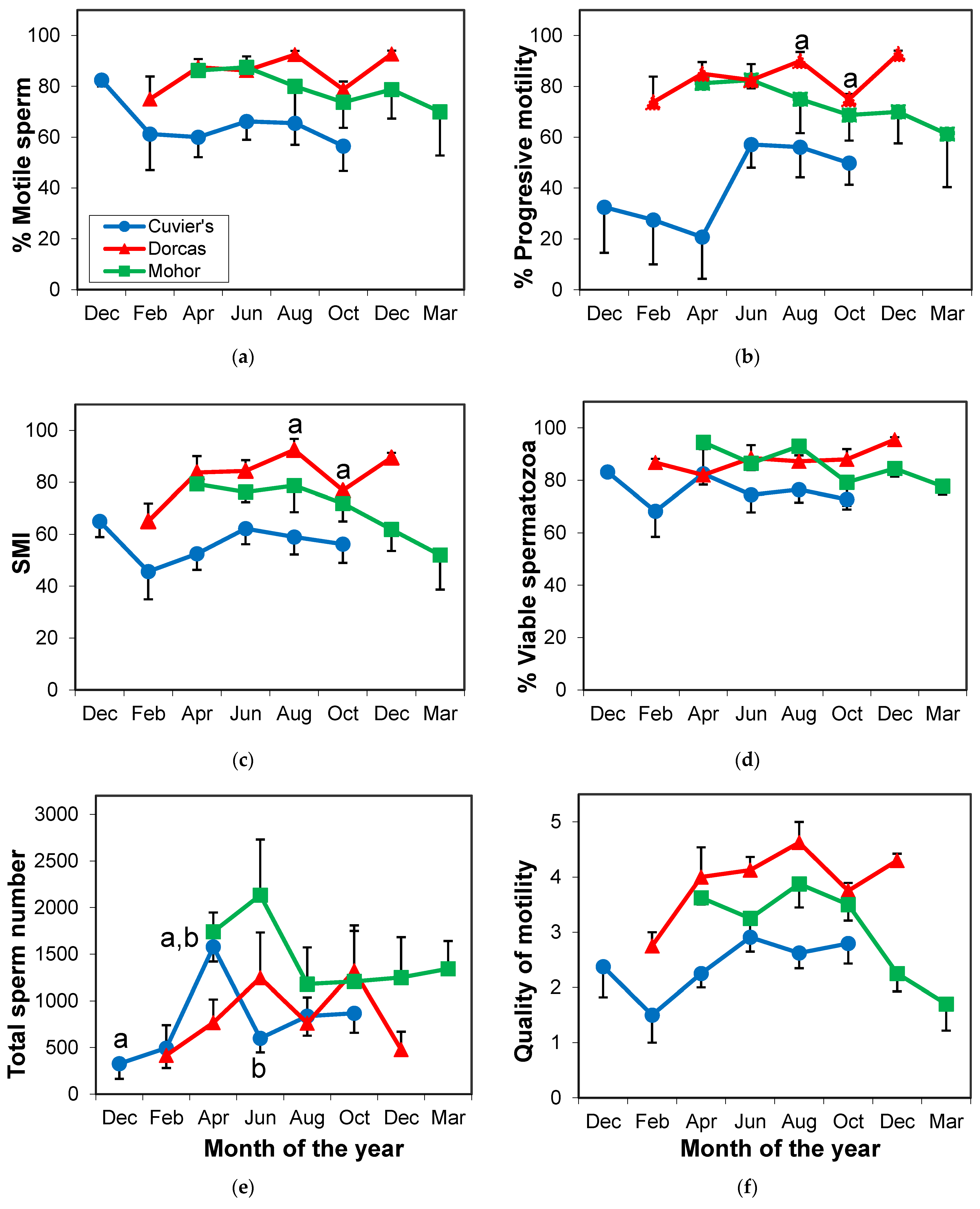
3.2.3. Seminal Parameters
3.3. Effect of Housing Conditions
3.3.1. Body Measures
3.3.2. Testosterone and Cortisol Levels
3.3.3. Seminal Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN/SSC Antelope Specialist Group. Gazella cuvieri. The Red List of Threatened Species 2016:e.T8967A50186003. Available online: https://www.iucnredlist.org/species/8967/50186003 (accessed on 19 July 2019).
- IUCN/SSC Antelope Specialist Group. Gazella dorcas. The IUCN Red List of Threatened Species 2017:e.T8969A50186334. Available online: https://www.iucnredlist.org/species/8969/50186334 (accessed on 19 July 2019).
- IUCN/SSC Antelope Specialist Group. Nager dama. The IUCN Red List of Threatened Species 2016:e.T8968A50186128. Available online: https://www.iucnredlist.org/species/8968/50186128 (accessed on 19 July 2019).
- Beudels, R.C.; Devillers, P.; Lafontaine, R.-M.; Devillers-Terschuren, J.; Beudels, M.-O. Sahelo-Saharan Antelopes. Status and Perspectives. Report on the Conservation of the Six Sahelo-Saharan Antelopes; UNEP/CMS Secretariat: Bonn, Germany, 2005. [Google Scholar]
- Abaigar, T. International Studbook. Management and Conservation of an Endangered Species in Captivity Gazella dorcas Neglecta; Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 2002. [Google Scholar]
- Abaigar, T.; Cano, M. International Studbook. Management and Conservation of Cuvier’s Gazelle (Gazella cuvieri Ogilby, 1841) in Captivity; Instituto de Estudios Almerienses: Almería, Spain, 2005. [Google Scholar]
- Barbosa, A.; Espeso, G. International Studbook for Gazella Dama Mhorr; Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 2005. [Google Scholar]
- Moreno, E.; Espeso, G. Cuvier’s Gazelle (Gazella cuvieri) International Studbook. Managing and Husbandry Guidelines; Ayuntamiento de Roquetas de Mar: Almería, Spain, 2008. [Google Scholar]
- Olmedo, G.; Escos, J.; Gomendio, M. Reproduction de Gazella cuvieri en captivité. Mammalia 1985, 49, 501–507. [Google Scholar] [CrossRef]
- Escos, J. International Studbook. The Status of Gazella Cuvieri and Recommendations for the Breeding Programme in Captivity; Instituto de Estudios Almerienses, Cuardernos Monograficos 20, Diputación Provincial de Almería: Amería, Spain, 1992.
- Essghaier, M.F.A.; Johnson, D.R. Distribution and use of dung heaps by Dorcas gazelle in western Libya. Mammalia 1981, 45, 153–155. [Google Scholar] [CrossRef]
- Mallon, D.P.; Kingswood, S.C. Antelopes. Part 4: North Africa, The Middle East and Asia. Global Survey and Regional Action Plans; SSC Antelope Specialist Group: Gland, Switzerland; IUCN: Cambridge, UK, 2001. [Google Scholar]
- Baharav, D. Reproductive strategies in female Mountain and Dorcas gazelles (Gazella gazella gazella and Gazella dorcas). J. Zool. 1983, 200, 445–453. [Google Scholar] [CrossRef]
- Cano, M.; Abaigar, T.; Vericad, J.R. Establishment of a group of Dama gazelles for reintroduction in Senegal. Int. Zoo Yearb. 1993, 32, 98–107. [Google Scholar] [CrossRef]
- Wojtusik, J.; Brown, J.L.; Pukazhenthi, B.S. Non-invasive hormonal characterization of the ovarian cycle, pregnancy, and seasonal anestrus of the female addra gazelle (Nanger dama ruficollis). Theriogenology 2017, 95, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J.C.; Pellicer-Rubio, M.T.; Malpaux, B. Seasonality of Reproduction in Mammals: Intimate Regulatory Mechanisms and Practical Implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Herbert, J.; Martensz, N.D.; Roberts, A.C. Annual reproductive rhythms in mammals: Mechanisms of light synchronization. Ann. N. Y. Acad. Sci. 1985, 453, 182–204. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B. Seasonal regulation of reproduction in mammals. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Elsevier Academic Press: London, UK, 2006. [Google Scholar]
- Skinner, J.D. The effect of season on spermatogenesis in some ungulates. J. Reprod. Fertil. 1971, 13, 29–37. [Google Scholar]
- Howard, J.G.; Wildt, D.E.; Chakraborty, P.K.; Bush, M. Reproductive traits including seasonal observations on semen quality and serum hormone concentrations in the Dorcas gazelle. Theriogenology 1983, 20, 221–234. [Google Scholar] [CrossRef]
- Brown, J.L.; Wildt, D.E.; Raath, J.R.; de Vos, V.; Howard, J.G.; Janssen, D.L.; Citino, S.B.; Bush, M. Impact of season on seminal characteristics and endocrine status of adult free-ranging African buffalo (Syncerus caffer). J. Reprod. Fertil. 1991, 91, 47–57. [Google Scholar] [CrossRef]
- Penfold, L.M.; Monfort, S.L.; Wolfe, B.A.; Citino, S.B.; Wildt, D.E. Reproductive physiology and artificial insemination studies in wild and captive gerenuk (Litocranius walleri walleri). Reprod. Fertil. Dev. 2005, 17, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Metrione, L.C.; Norton, T.M.; Beetem, D.; Penfold, L.M. Seasonal reproductive characteristics of female and male Jackson’s hartebeest (Alcelaphus buselaphus jacksoni). Theriogenology 2008, 70, 871–879. [Google Scholar] [CrossRef]
- Dufour, J.J.; Fahmy, M.H.; Minvielle, F. Seasonal changes in breeding activity, testicular size, testosterone concentration and seminal characteristics in rams with long or short breeding season. J. Anim. Sci. 1984, 58, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, R.W.; Lunstra, D.D.; Jenkins, T.G.; Berardinelli, J.G.; Guthrie, M.J.; Neuendorff, D.A.; Long, C.R.; Randel, R.D. Effect of season and location on semen quality and serum concentrations of luteinizing hormone and testosterone in Brahman and Hereford bulls. J. Anim. Sci. 1990, 68, 734–749. [Google Scholar] [CrossRef]
- Nowakowski, P.; Cwikkla, A. Seasonal variation in testes size in Polish Merino rams and its relationship to reproductive performance in spring. Theriogenology 1994, 42, 613–622. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Varsakeli, S.; Karatzas, G. Characteristics and seasonal variations in the semen of Alpine, Saanen and Damascus goat bucks born and raised in Greece. Theriogenology 2000, 53, 1285–1293. [Google Scholar] [CrossRef]
- Helbig, L.; Woodbury, M.R.; Haigh, J.C.; Collins, J.; Barth, A.D. The seasonal fertility of North American bison (Bison bison) bulls. Anim. Reprod. Sci. 2007, 97, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.A. Seasonal influence on sexual hormones and semen plasma parameters of Arabian sand gazelles (Gazalla subgutrosa marica) in Saudi Arabia. Afr. J. Biotechnol. 2014, 13, 4647–4652. [Google Scholar] [CrossRef]
- Cassinello, J.; Abaigar, T.; Gomendio, M.; Roldan, E.R.S. Characteristics of the semen of three endangered species of gazelles (Gazella dama mhorr, G. dorcas neglecta and G. cuvieri). J. Reprod. Fertil. 1998, 113, 35–45. [Google Scholar] [CrossRef][Green Version]
- Gomendio, M.; Cassinello, J.; Roldan, E.R.S. A comparative study of ejaculate traits in three endangered ungulates with different levels of inbreeding: Fluctuating asymmetry as an indicator of reproductive and genetic stress. Proc. R. Soc. Lond. 2000, 267, 875–882. [Google Scholar] [CrossRef]
- Koyama, S.; Kamimura, S. Lowered sperm motility in subordinate social status of mice. Physiol. Behav. 1999, 65, 665–669. [Google Scholar] [CrossRef]
- Koyama, S.; Kamimura, S. Influence of social dominance and female odor on the sperm activity of male mice. Physiol. Behav. 2000, 71, 415–422. [Google Scholar] [CrossRef]
- Faulkes, C.G.; Trowell, S.N.; Jarvis, J.U.M.; Bennett, N.C. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of two eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis). J. Reprod. Fertil. 1994, 100, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.A.; delBarco-Trillo, J.; Ferkin, M.H. Sperm investment in male meadow voles is affected by the condition of the nearby male conspecifics. Behav. Ecol. 2008, 19, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, R.; González-Pensado, S.P. Social rank affects reproductive development in male lambs. Anim. Reprod. Sci. 2008, 109, 161–171. [Google Scholar] [CrossRef]
- Teixeira, H.C.A.; Nascimento, N.V.; McManus, C.; Egito, A.A.; Mariante, A.d.S.; Ramos, A.F. Seasonal influence on semen traits and freezability from locally adapted Curraleiro bulls. Anim. Reprod. Sci. 2011, 125, 56–61. [Google Scholar] [CrossRef]
- Coloma, M.A.; Toledano-Díaz, A.; Castaño, C.; Velázquez, R.; Gómez-Brunet, A.; López-Sebastián, A.; Santiago-Moreno, J. Seasonal variation in reproductive physiological status in the Iberian ibex (Capra pyrenaica) and its relationship with sperm freezability. Theriogenology 2011, 76, 1695–1705. [Google Scholar] [CrossRef]
- Garde, J.J.; Soler, A.J.; Cassinello, J.; Crespo, C.; Malo, A.F.; Gomendio, M.; Roldan, E.R.S. Sperm cryopreservation in three species of endangered gazelles (Gazella cuvieri, G. dama mhorr and G. dorcas neglecta). Biol. Reprod. 2003, 69, 602–611. [Google Scholar] [CrossRef]
- Roldan, E.R.S.; Gomendio, M.; Garde, J.J.; Espeso, G.; Ledda, S.; Berlinguer, F.; del Olmo, A.; Soler, A.J.; Arregui, L.; Crespo, C.; et al. Inbreeding and reproduction in endangered ungulates: Preservation of genetic variation through the organization of genetic resource banks. Reprod. Domest. Anim. 2006, 41, 82–92. [Google Scholar] [CrossRef]
- Garde, J.J.; del Olmo, A.; Soler, A.J.; Espeso, G.; Gomendio, M.; Roldan, E.R.S. Effect of egg yolk, cryoprotectant, and various sugars on semen cryopreservation in endangered Cuvier’s gazelle (Gazella cuvieri). Anim. Reprod. Sci. 2008, 108, 384–401. [Google Scholar] [CrossRef]
- Alados, C.L. La reproducción en Gazella dorcas. Acta Vertebr. 1984, 11, 243–261. [Google Scholar]
- Harcourt, A.H.; Purvis, A.; Liles, L. Sperm competition: Mating system, not breeding season, affects testes size of primates. Funct. Ecol. 1995, 9, 468–476. [Google Scholar] [CrossRef]
- Pickard, A.R.; Holt, W.V.; Green, D.I.; Cano, M.; Abaigar, T. Endocrine correlates of sexual behavior in the Mohor gazelle (Gazella dama mhorr). Horm. Behav. 2003, 44, 303–310. [Google Scholar] [CrossRef]
- Abáigar, T.; Domené, M.A.; Palomares, F. Effects of fecal age and seasonality on steroid hormone concentration as a reproductive parameter in field studies. Eur. J. Wildl. Res. 2010, 56, 781–787. [Google Scholar] [CrossRef]
- Kusuda, S.; Nagami, H.; Kusunoki, H.; Nishikaku, T.; Nakagawa, D.; Takida, T.; Kurita, D.; Uemichi, K.; Fukai, M.; Kubota, H.; et al. Annual changes in testicular size and serum and fecal testosterone concentrations in male Bharals, Pseudois nayaur. J. Vet. Med Sci. 2006, 68, 1093–1095. [Google Scholar] [CrossRef][Green Version]
- Malfatti, A.; Barbato, O.; Todini, L.; Terzano, G.M.; Debenedetti, A.; Borghese, A. Blood testosterone levels in Italian Mediterranean buffalo bulls managed in two different breeding conditions. Theriogenology 2006, 65, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Díaz, A.; Santiago-Moreno, J.; Gómez-Brunet, A.; Pulido-Pastor, A.; López-Sebastián, A. Horn growth related to testosterone secretion in two wild Mediterranean ruminant species: The Spanish ibex (Capra pyrenaica hispanica) and European mouflon (Ovis orientalis musimon). Anim. Reprod. Sci. 2007, 102, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Synnott, A.L.; Fulkerson, W.J.; Lindsay, D.R. Sperm output by rams and distribution amongst ewes under conditions of continual mating. J. Reprod. Fertil. 1981, 61, 355–361. [Google Scholar] [CrossRef]
- Thwaites, C.J. The comparative effects of undernutrition, exercise and frequency of ejaculation on the size and tone of the testes and on semen quality in the ram. Anim. Reprod. Sci. 1995, 37, 299–309. [Google Scholar] [CrossRef]
- Preston, B.T.; Stevenson, I.R.; Pemberton, J.M.; Wilson, K. Dominant rams lose out by sperm depletion. Nature 2001, 409, 681. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Temple, D.; Abáigar, T.; Cuadrado, M.; Delclaux, M.; Enseñat, C.; Almagro, V.; Martínez-Nevado, E.; Quevedo, M.Á.; Carbajal, A.; et al. Aggressive behavior and hair cortisol levels in captive Dorcas gazelles (Gazella dorcas) as animal-based welfare indicators. Zoo Biol. 2016, 35, 467–473. [Google Scholar] [CrossRef] [PubMed]

| Cuvier’s Gazelle | Dorcas Gazelle | Mohor Gazelle | |
|---|---|---|---|
| Body weight (kg) | 34.2 ± 0.8 a | 17.2 ± 0.6 b | 63.3 ± 1.3 c |
| Testes weight (g) | 47.0 ± 3.6 a,b | 40.8 ± 4.1 a | 54.4 ± 1.4 b |
| Relative testes weight (g/kg) | 1.38 ± 0.11 a | 2.39 ± 0.28 b | 0.86 ± 0.01 c |
| Cuvier´s Gazelle | Dorcas Gazelle | Mohor Gazelle | |
|---|---|---|---|
| Volume (µL) | |||
| Alone | 626.8 ± 148.7 | 717.0 ± 209.9 | 937.0 ± 61.1 |
| with males | 1019.0 ± 288.2 | 392.1 ± 118.1 | 1566.4 ± 486.1 |
| with females | 294.7 ± 91.0 | 61.5 ± 26.4 | 2162.5 ± 589.0 |
| Sperm concentration (×106/mL) | |||
| Alone | 649.3 ± 176.2 | 1475.2 ± 436.3 a | 789.5 ± 90.4 |
| with males | 559.3 ± 11.2 | 697.9 ± 233.2 b | 564.4 ±186.6 |
| with females | 246.7 ± 168.2 | 99.6 ± 71.1 a,b | 329.1 ± 84.3 |
| Total sperm number (×106) | |||
| Alone | 547.4 ± 283.2 | 679.5 ± 137.1 a | 754.4 ± 109.1 |
| with males | 576.2 ± 169.3 | 319.0 ± 117.0 b | 961.6 ± 340.2 |
| with females | 103.2 ± 84.5 | 11.7 ± 10.5 a,b | 712.1 ± 261.0 |
| Wave motion (scale 0–5) | |||
| Alone | 1.9 ± 0.5 | 4.8 ± 0.2 a | 3.7 ± 0.3 |
| with males | 2.3 ± 1.2 | 2.8 ± 0.6 | 2.4 ± 0.6 |
| with females | 1.0 ± 1.0 | 0.5 ± 0.3 a | 2.5 ± 0.7 |
| Individual motility (%) | |||
| Alone | 49.5 ± 10.6 | 92.2 ± 2.9 a | 87.4 ± 4.0 |
| with males | 64.0 ± 29.5 | 55.8 ± 12.4 | 72.3 ± 10.3 |
| with females | 28.6 ± 28.6 | 26.0 ± 16.8 a | 78.1 ± 16.9 |
| Progressive motility (%) | |||
| Alone | 32.9 ± 10.9 | 88.2 ± 2.8 a | 75.1 ± 5.7 |
| with males | 25.3 ± 19.3 | 45.8 ± 12.8 | 47.3 ± 10.4 |
| with females | 23.8 ± 23.8 | 26.0 ± 16.8 a | 68.0 ± 13.2 |
| Quality of motility (scale 0–5) | |||
| Alone | 2.1 ± 0.5 | 4.8 ± 0.2 a | 3.5 ± 0.2 |
| with males | 2.0 ± 1.0 | 2.9 ± 0.7b | 2.3 ± 0.4 |
| with females | 1.0 ± 1.0 | 2.0 ± 1.2 a,b | 3.5 ± 0.3 |
| Viability (%) | |||
| Alone | 56.4 ± 8.3 | 91.0 ± 2.2 | 85.4 ± 2.1 |
| with males | 77.0 ± 4.4 | 73.4 ± 11.2 | 72.1 ± 6.9 |
| with females | 30.0 ± 19.6 | 55.7 ± 17.4 | 71.8 ± 7.4 |
| Intact acrosomes (%) | |||
| Alone | 66.2 ± 7.0 a | 93.4 ± 1.5 | 88.1 ± 2.4 |
| with males | 95.2 ± 0.6 b | 90.3 ± 3.4 | 78.4 ± 10.2 |
| with females | 66.1 ± 17.0 | 84.5 ± 2.5 | 86.9 ± 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arregui, L.; Garde, J.J.; Soler, A.J.; Espeso, G.; Roldan, E.R.S. Effect of Season and Social Environment on Semen Quality and Endocrine Profiles of Three Endangered Ungulates (Gazella cuvieri, G. dorcas and Nanger dama). Animals 2021, 11, 901. https://doi.org/10.3390/ani11030901
Arregui L, Garde JJ, Soler AJ, Espeso G, Roldan ERS. Effect of Season and Social Environment on Semen Quality and Endocrine Profiles of Three Endangered Ungulates (Gazella cuvieri, G. dorcas and Nanger dama). Animals. 2021; 11(3):901. https://doi.org/10.3390/ani11030901
Chicago/Turabian StyleArregui, Lucía, José Julián Garde, Ana Josefa Soler, Gerardo Espeso, and Eduardo R. S. Roldan. 2021. "Effect of Season and Social Environment on Semen Quality and Endocrine Profiles of Three Endangered Ungulates (Gazella cuvieri, G. dorcas and Nanger dama)" Animals 11, no. 3: 901. https://doi.org/10.3390/ani11030901
APA StyleArregui, L., Garde, J. J., Soler, A. J., Espeso, G., & Roldan, E. R. S. (2021). Effect of Season and Social Environment on Semen Quality and Endocrine Profiles of Three Endangered Ungulates (Gazella cuvieri, G. dorcas and Nanger dama). Animals, 11(3), 901. https://doi.org/10.3390/ani11030901







