Honey Bee Proteolytic System and Behavior Parameters under the Influence of an Electric Field at 50 Hz and Variable Intensities for a Long Exposure Time
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bees
2.2. Experimental Design
2.3. E-Field Generation
2.4. Protease Analysis
2.5. Behavior Analysis
2.6. Data Analysis
3. Results
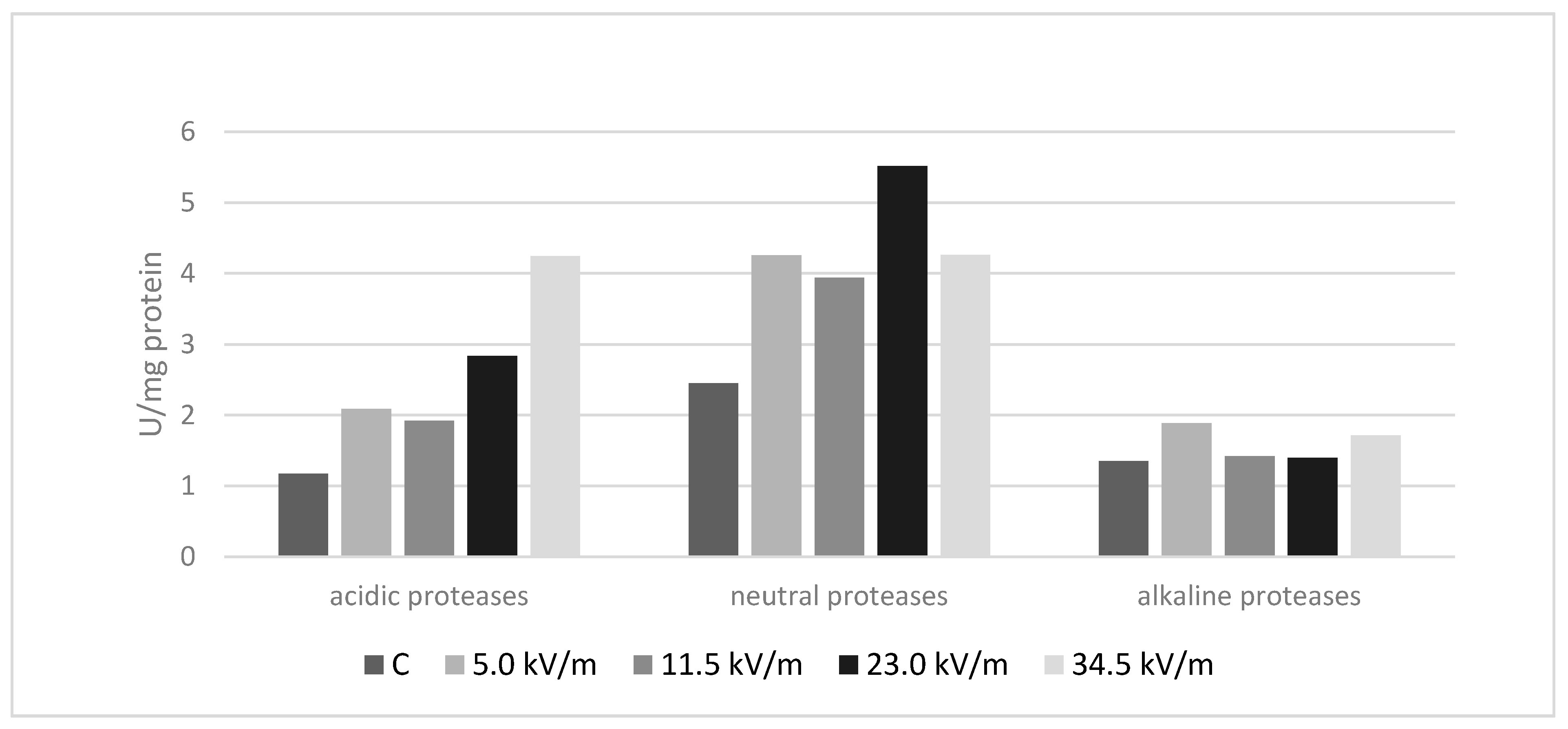
3.1. Protease Analysis
3.2. Acidic Proteases
3.3. Neutral Proteases
3.4. Alkaline Proteases
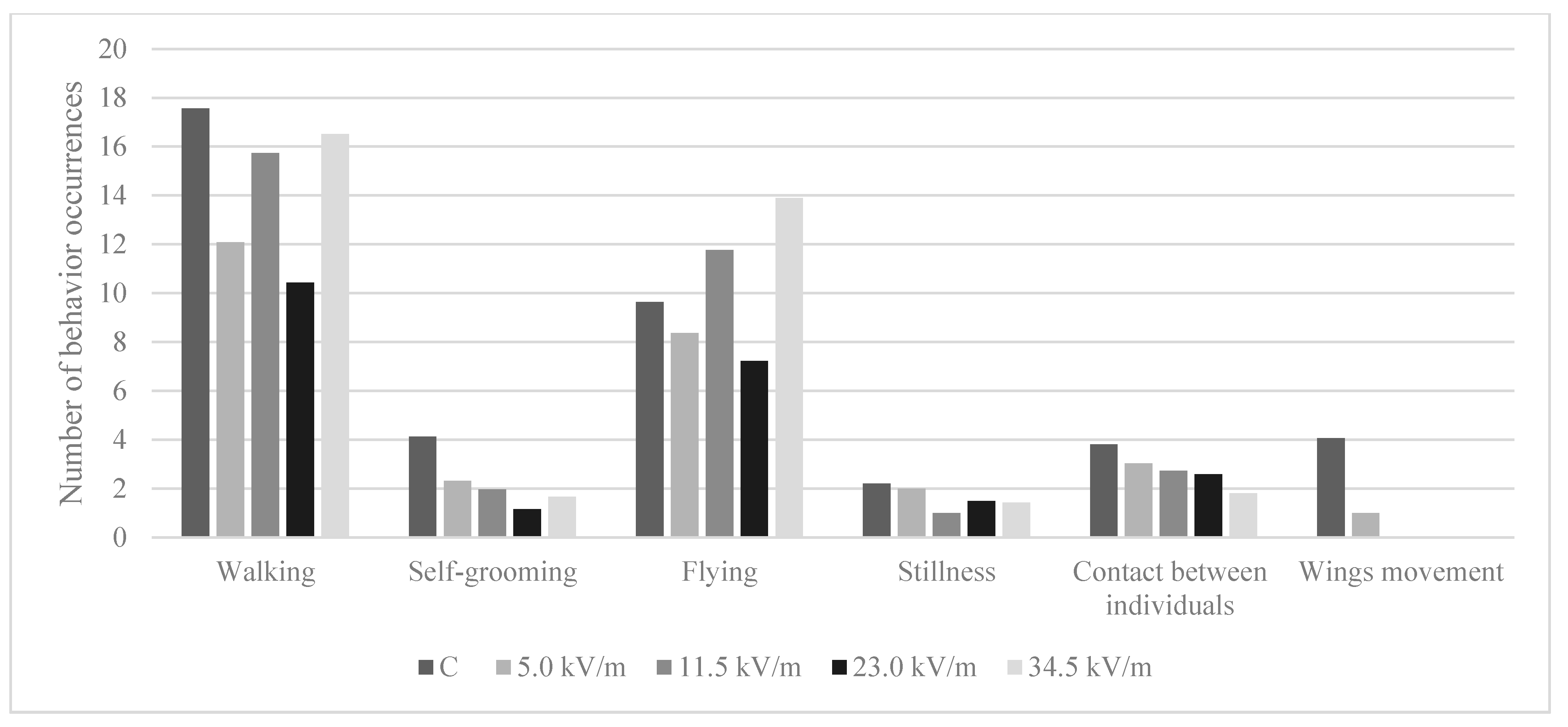
3.5. Behavior Analysis
3.5.1. Number of Behavioral Occurrences
3.5.2. Time Spent Performing Behaviors
4. Discussion
4.1. Protease Analysis
4.2. Behavior Analysis
4.3. Behavior and Protease Analysis Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loriatti, L.; Martinelli, M.; Viani, F.; Benedetti, M. Real-time distributed monitoring of electromagnetic pollution in urban environments. In Proceedings of the International Geoscience and Remote Sensing Symposium 09 (IGRASS), Cape Town, South Africa, 13–17 July 2009; Volume 5, pp. 100–103. [Google Scholar] [CrossRef]
- Gotz, M.; Rapp, M.; Dostert, K. Power line channel characteristics and their effect on communication system design. IEEE Commun. Mag. 2004, 42, 78–86. [Google Scholar] [CrossRef]
- Maslanyj, M.; Simpson, J.; Roman, E.; Schüz, J. Power frequency magnetic fields and risk of childhood leukaemia: Misclassification of exposure from the use of the ‘distance from power line’ exposure surrogate. Bioelectromagnetics 2009, 30, 183–188. [Google Scholar] [CrossRef]
- Milutinov, M.; Juhas, A.; Prša, M. Electromagnetic field underneath overhead high voltage power line. In Proceedings of the 4th International conference on Engineering Technologies–ICET, Islamabad, Pakistan, 19–20 October 2009. [Google Scholar]
- Paś, J. Linie napowietrzne wysokich napięć—środowisko elektromagnetyczne a ograniczenia w użytkowaniu terenów. Biul. Wojsk. Akad. Tech. 2014, 63, 51–63. [Google Scholar] [CrossRef]
- Schuà, L. Die Wirkung von luftelektrischen Feldern auf Tiere. Verh. Dtsch. Zool. Ges. 1954, 18, 435–440. [Google Scholar]
- Shepherd, S.; Lima, M.A.P.; Oliveira, E.E.; Sharkh, S.M.; Jackson, C.W.; Newland, P.L. Extremely Low Frequency Electromagnetic Fields impair the Cognitive and Motor Abilities of Honey Bees. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bindokas, V.; Gauger, J.; Greenberg, B. Laboratory investigations of the electrical characteristics of honey bees and their exposure to intense electric fields. Bioelectromagnetics 1989, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Berbeć, E.; Roman, A. Changes in Honeybee Behavior Parameters under the Influence of the E-Field at 50 Hz and Variable Intensity. Animals 2021, 11, 247. [Google Scholar] [CrossRef]
- Bindokas, V.P.; Gauger, J.R.; Greenberg, B. Mechanism of biological effects observed in honey bees (Apis mellifera, L.) hived under extra-high-voltage transmission lines: Implications derived from bee exposure to simulated intense electric fields and shocks. Bioelectromagnetics 1988, 9, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Odemer, R.; Odemer, F. Effects of radiofrequency electromagnetic radiation (RF-EMF) on honey bee queen development and mating success. Sci. Total Environ. 2019, 15, 553–562. [Google Scholar] [CrossRef]
- Altmann, G. Hormon-physiologische Untersuchungen des Stoffwechsels der Honigbiene. Dtsch Bienenwirtsch 1959, 2, 74–76. [Google Scholar]
- Migdał, P.; Roman, A.; Strachecka, A.; Murawska, A.; Bieńkowski, P. Changes of selected biochemical parameters of the honeybee under the influence of an electric field at 50 Hz and variable intensities. Apidologie 2020, 51, 956–967. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Changes in the Honeybee Antioxidant System after 12 h of Exposure to Electromagnetic Field Frequency of 50 Hz and Variable Intensity. Insects 2020, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Gryzińska, M.; Krauze, M. Influence of Environmental Pollution on the Protective Proteolytic Barrier of the Honey Bee Apis mellifera mellifera. Pol. J. Environ. Stud. 2010, 19, 855–859. [Google Scholar]
- Strachecka, A.; Demetraki-Paleolog, J. System proteolityczny powierzchni ciała Apis mellifera w zachowaniu zdrowotności rodzin pszczelich. Kosmos 2011, 60, 43–51. [Google Scholar]
- Buczek, K. Honey bee colony collapse disorder (CCD). Ann. UMCS 2009, 64, 1–7. [Google Scholar] [CrossRef][Green Version]
- Simone-Finstorm, M.D.; Spivak, M. Increased Resin Collection after Parasite Challenge: A Case of Self-Medication in Honey Bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef]
- Walter, R.; Clčlia, F. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. Part B Mol. Integr. Physiol. 1994, 109, 1–62. [Google Scholar]
- Rawlings, R.; Barret, A. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Otlewski, J.; Jaskólski, M.; Buczek, O.; Cierpicki, T.; Czapińska, H.; Krowarsch, D.; Smalas, A.; Stachowiak, D.; Szpineta, A.; Dadlez, M. Structure-function relationship of serine protease-protein inhibitor interaction. Acta Biochim. Pol. 2001, 48, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Krem, M.; Di Cera, E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem. Sci. 2002, 27, 67–74. [Google Scholar] [CrossRef]
- Grzywnowicz, K.; Ciołek, A.; Tabor, A.; Jaszek, M. Profiles of the body-surface proteolytic system of honey bee queens, workers and drones: Ontogenetic and seasonal changes in proteases and their natural inhibitors. Apidologie 2008, 40, 4–19. [Google Scholar] [CrossRef]
- Peng, Y.S.; Fang, Y.; Xu, S.; Ge, L. The resistance mechanism of the Asian honey bee, Apis cerana Fabr, to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Bukowicki, R.; Sanchez, A. Protection against E-field and radiation at low frequency 50 Hz. In Proceedings of the International Conference on Electromagnetic Fields, Health & Environment, Madeira Island, Portugal, 27–29 April 2006. [Google Scholar]
- Migdał, P.; Murawska, A.; Roman, A. Modified Standardized Method to Extract and Store Insect Hemolymph with Use of a Glass Capillary. J. Apic. Sci. 2020, 64, 165–168. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and Cost-Effective Biochemical Spectrophotometric Analysis of Solution of Insect “Blood” and Body Surface Elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Anson, M.L. Estimation of pepsin, trypsin papain and cathepsin with haemoglobin. J. Gen. Physiol. 1938, 22, 79–84. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 14 May 2020).
- Fruton, J. The Role of Proteolytic Enzymes in the Biosynthesis of Peptide Bonds. J. Biol. Med. 1950, 22, 263–271. [Google Scholar]
- López-Otín, C.; Bond, J. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect 2015, 11, 47–55. [Google Scholar] [CrossRef]
- Asgari, S.; Zhan, G.; Zareie, R.; Schmidt, O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog-Demetraki, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J.; Borsuk, G.; Bajda, M. Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee, Apis mellifera. Arch. Insect Biochem. Physiol. 2014, 86, 165–179. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian life-span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid markedly affects hemolymph proteolysis, biomarkers, DNA global methylation, and the cuticle proteolytic layer in western honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Lambin, M.; Armengaud, C.; Raymond, S.; Gauthier, M. Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch. Insect Biochem. Physiol. 2001, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Teteers, B.; Johnson, M.R.; Ellis, M.D.; Siegfried, B.D. Using video-tracking to asses sublethal effects of pesticides on honey bees (Apis mellifera). Environ. Toxicol. Chem. 2012, 31, 1349–1354. [Google Scholar] [CrossRef]
- Charreton, M.; Decourtye, A.; Henry, M.; Rodet, G.; Sandoz, J.C.; Charnet, P.; Collet, C. A locomotor deficit induced by sublethal doses of pyrethroid and neonicotinoid insecticides in the honeybee Apis mellifera. PLoS ONE 2015, 10, e0144879. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef] [PubMed]
- Ingram, E.M.; Augustin, J.; Ellis, M.D.; Siegfried, B.D. Evaluating sub-lethal effects of orchard-applied pyrethroids using video-tracking software to quantify honey bee behaviors. Chemosphere 2015, 135, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef] [PubMed]

| Groups | Protease Activity (U/mg Protein) | ||
|---|---|---|---|
| Acidic | Neutral | Alkaline | |
| C | 1.18 (±0.11) a | 2.45 (±0.07) a | 1.35 (±0.10) a |
| 5.0 kV/m | 2.09 (±0.24) b | 4.26 (±0.26) b | 1.89 (±0.25) b |
| 11.5 kV/m | 1.92 (±0.08) c | 3.94 (±0.08) c | 1.42 (±0.11) c |
| 23.0 kV/m | 2.84 (±0.07) d | 5.52 (±0.25) d | 1.40 (±0.09) ac |
| 34.5 kV/m | 4.24 (±0.32) e | 4.26(±0.11) b | 1.72 (0.20) d |
| Groups | Behavior Parameters | |||||
|---|---|---|---|---|---|---|
| Walking | Self-Grooming | Flying | Stillness | Contact between Individuals | Wing Movement | |
| C | 17.57 (±5.83) a | 4.13 (±2.35) a | 9.64 (±6.89) ab | 2.22 (±1.92) | 3.82 (±2.70) a | 4.07 (±3.07) |
| 5.0 kV/m | 12.08 (±9.05) b | 2.33 (±1.88) ab | 8.37 (±6.29) a | 2.00 (±1.41) | 3.03 (±2.38) ab | 1.00 |
| 11.5 kV/m | 15.74 (±10.32) bc | 1.97 (±1.97) b | 11.77 (±9.57) a | 1.00 (±0.01) | 2.73 (±1.41) ab | NO |
| 23.0 kV/m | 10.44 (±6.45) b | 1.17 (±0.39) b | 7.23 (±6.05) ab | 1.50 (±0.71) | 2.59 (±1.04) ab | NO |
| 34.5 kV/m | 16.51 (±9.28) c | 1.68 (±1.07) b | 13.90 (±9.53) b | 1.44 (±0.73) | 1.81 (±0.88) b | NO |
| Groups | Behavior Parameters | |||||
|---|---|---|---|---|---|---|
| Walking | Self-Grooming | Flying | Stillness | Contact between Individuals | Wing Movement | |
| C | 14.07 (±6.47) a | 7.43 (±8.87) a | 1.38 (±0.45) a | 5.72 (±2.86) | 5.99 (±4.00) a | 7.36 (±6.07) |
| 5.0 kV/m | 29.78 (±22.95) bc | 12.18 (±11.82) b | 1.86 (±0.52) b | 16.87 (±15.78) | 21.66 (±20.89) b | 2.40 |
| 11.5 kV/m | 22.09 (±14.20) b | 11.78 (±10.70) b | 1.60 (±0.46) ab | 17.15 (±20.43) | 8.80 (±7.93) c | NO |
| 23.0 kV/m | 32.94 (±16.75) c | 7.98 (±6.80) a | 1.73 (±0.72) ab | 47.71 (±12.52) | 6.48 (±3.73) ac | NO |
| 34.5 kV/m | 21.46 (±13.41) b | 12.75 (±10.43) b | 1.45 (±0.25) a | 14.07 (±13.54) | 8.78 (±7.19) c | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Honey Bee Proteolytic System and Behavior Parameters under the Influence of an Electric Field at 50 Hz and Variable Intensities for a Long Exposure Time. Animals 2021, 11, 863. https://doi.org/10.3390/ani11030863
Migdał P, Murawska A, Strachecka A, Bieńkowski P, Roman A. Honey Bee Proteolytic System and Behavior Parameters under the Influence of an Electric Field at 50 Hz and Variable Intensities for a Long Exposure Time. Animals. 2021; 11(3):863. https://doi.org/10.3390/ani11030863
Chicago/Turabian StyleMigdał, Paweł, Agnieszka Murawska, Aneta Strachecka, Paweł Bieńkowski, and Adam Roman. 2021. "Honey Bee Proteolytic System and Behavior Parameters under the Influence of an Electric Field at 50 Hz and Variable Intensities for a Long Exposure Time" Animals 11, no. 3: 863. https://doi.org/10.3390/ani11030863
APA StyleMigdał, P., Murawska, A., Strachecka, A., Bieńkowski, P., & Roman, A. (2021). Honey Bee Proteolytic System and Behavior Parameters under the Influence of an Electric Field at 50 Hz and Variable Intensities for a Long Exposure Time. Animals, 11(3), 863. https://doi.org/10.3390/ani11030863







