Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018) †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Anamnesis and Clinical Findings
3.2. Laboratory Analyses
3.3. Ultrasonographic Findings and Biopsy
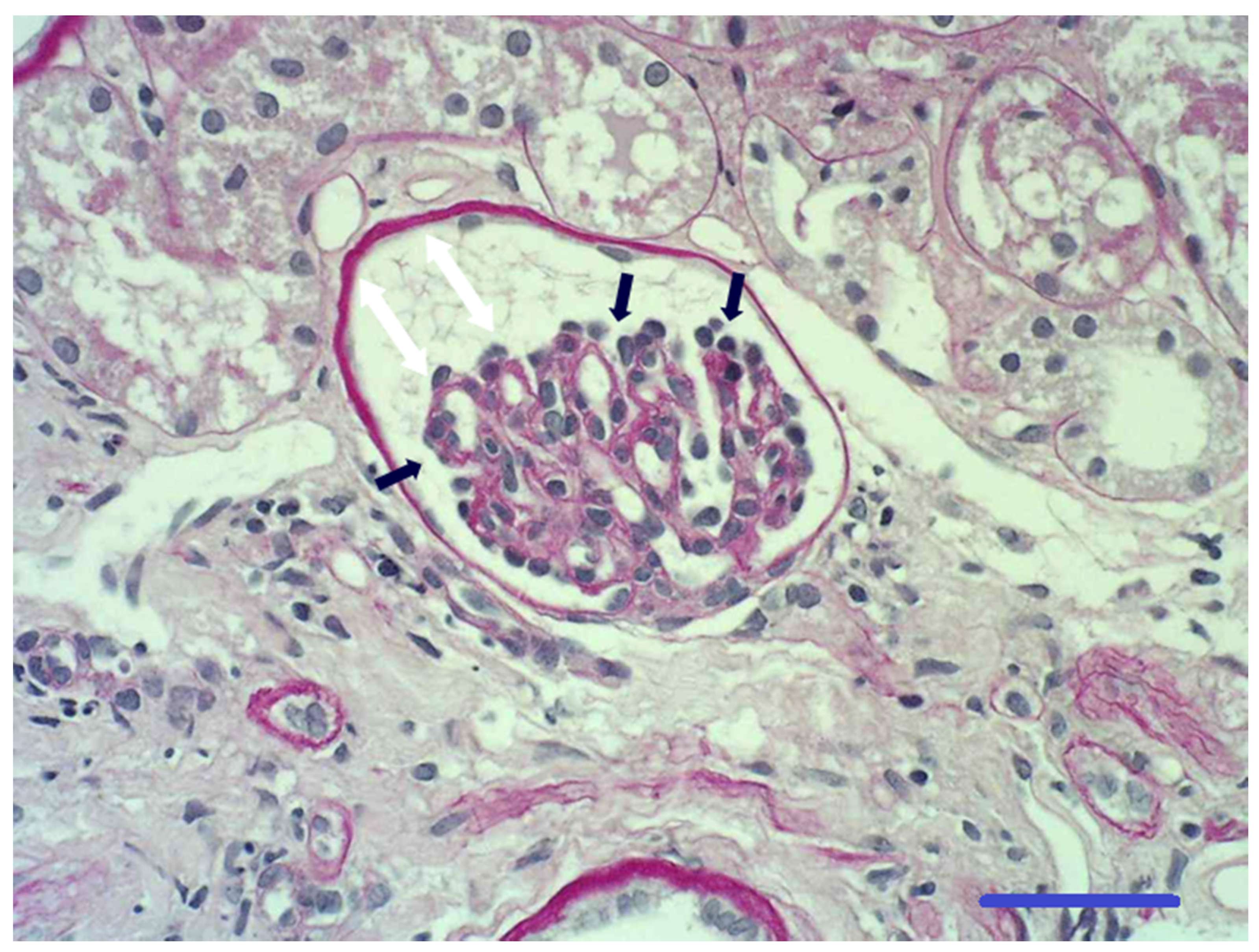
3.4. Histopathology
3.5. Therapy and Survival Time
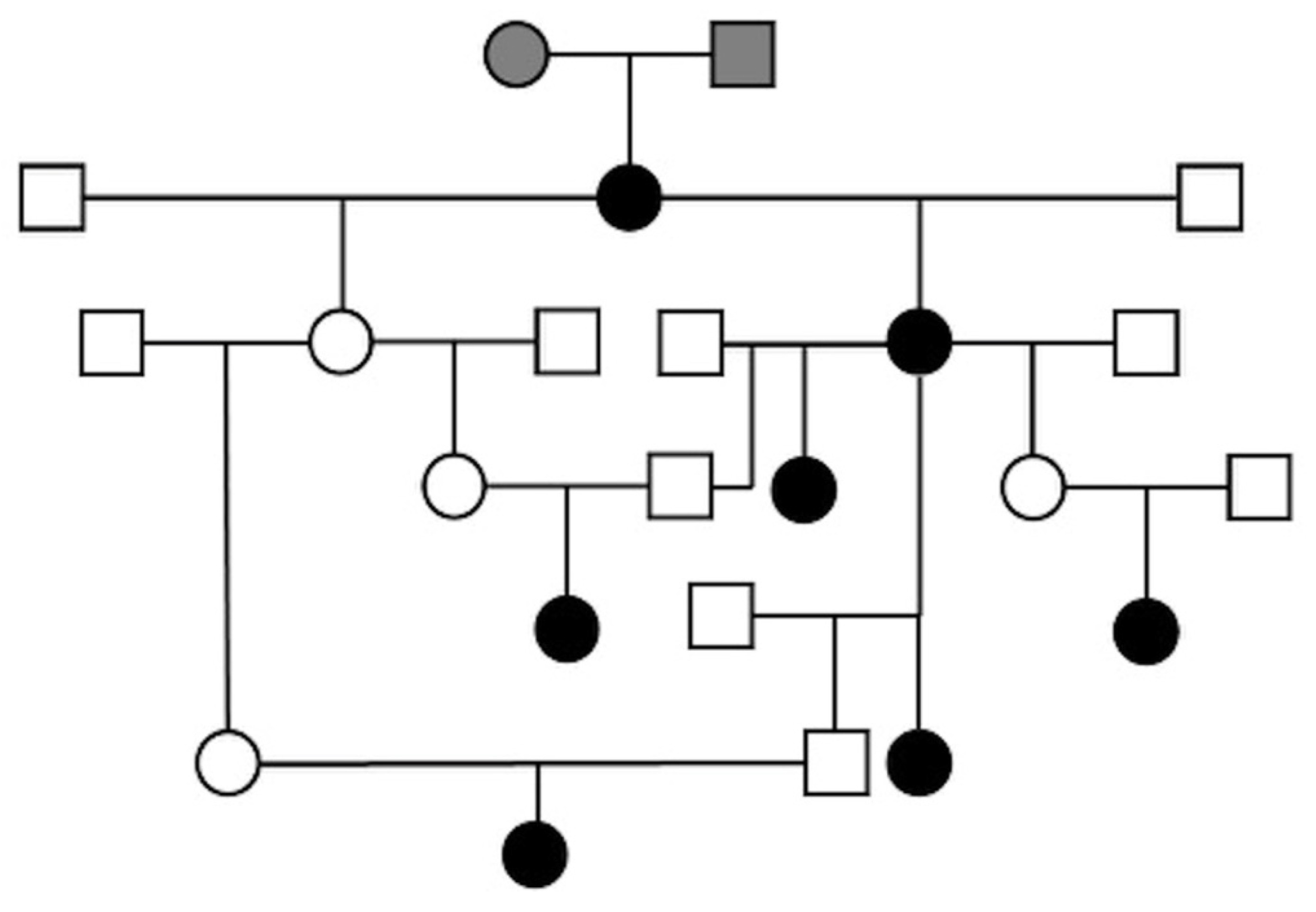
3.6. Pedigree Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greco, D.S. Congenital and inherited renal disease of small animals. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 393–399. [Google Scholar] [CrossRef]
- Picut, C.A.; Lewis, R.M. Microscopic features of canine renal dysplasia. Vet. Pathol. 1987, 24, 156–163. [Google Scholar] [CrossRef]
- Bovee, K.C. Renal Dysplasia in Shih Tzu Dogs. In Proceedings of the World Small Animal Veterinary Association World Congress, Bangkok, Thailand, 24–27 October 2003; Available online: www.vin.com/proceedings/Proceedings.plx?CID=WSAVA2003&PID=6602&O=Generic (accessed on 28 December 2020).
- DiBartola, S.P. Familial renal disease in dogs and cats. In Textbook of Veterinary Internal Medicine, 6th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Elsevier Saunders, St: Louis, MO, USA, 2005; pp. 1819–1824. [Google Scholar]
- Cianciolo, R.; Brown, C.; Mohr, C.; Nabity, M.; Van der Lugt, J.; McLeland, S.; Aresu, L.; Benali, S.; Spangler, B.; Amerman, H.; et al. Altas of Renal Lesions in Proteinuric Dogs; The Ohio State University: Columbus, Ohio, USA, 2018; Available online: https://ohiostate.pressbooks.pub/vetrenalpathatlas/ (accessed on 28 December 2020).
- Jansen, B.; Valli, V.E.O.; Thorner, P.; Baumal, R.; Lumsden, J.H. Samoyed hereditary glomerulopathy: Serial, clinical and laboratory (urine, serum biochemistry and hematology) studies. Am. J. Vet. Res. 1987, 51, 387–393. [Google Scholar]
- Hood, J.C.; Robinson, W.F.; Huxtable, C.R.; Bradley, J.S.; Sutherland, R.J.; Thomas, M.A. Hereditary nephritis in the bull terrier: Evidence for inheritance by an autosomal dominant gene. Vet. Rec. 1990, 126, 456–459. [Google Scholar]
- Freudiger, U. Die nebennierenrinden-insuffizienzen beim hund. Deut. Tierarztl. Wochensch. 1965, 72, 60–64. [Google Scholar]
- Johnson, M.E.; Denhart, J.D.; Graber, E.R. Renal cortical hypoplasia in a litter of Cocker Spaniels. J. Am. Anim. Hosp. Assoc. 1972, 8, 268–274. [Google Scholar]
- Steward, A.P.; McDougall, D.F. Familial nephropathy in the Cocker Spaniel. J. Small Anim. Pract. 1984, 25, 15–24. [Google Scholar] [CrossRef]
- Thorner, P.; Jansen, B.; Baumal, R.; Valli, V.E.; Goldberger, A. Samoyed hereditary glomerulopathy: Immunohistochemical staining of the basement membranes of kidney for laminin, collagen type IV, fibronectin, and Goodpasture antigen, and correlation with electron microscopy of glomerular capillary basement membranes. Lab. Invest. 1987, 56, 435–443. [Google Scholar] [PubMed]
- Davidson, A.G.; Bell, R.J.; Lees, G.E.; Kashtan, C.E.; Davidson, G.S.; Murphy, K.E. Genetic cause of autosomal recessive hereditary nephropathy in the English Cocker Spaniel. J. Vet. Int. Med. 2007, 21, 394–401. [Google Scholar] [CrossRef]
- Bernard, M.A.; Valli, V.E. Familial renal disease in the Samoyed dogs. Can. Vet. J. 1977, 18, 181–189. [Google Scholar] [PubMed]
- Bloedow, A.G. Familial renal disease in Samoyed dogs. Vet. Rec. 1981, 108, 167–168. [Google Scholar] [CrossRef]
- Hoppe, A.; Swenson, L.; Jonsson, L.; Hedhammar, A. Progressive nephropathy due to renal dysplasia in Shih Tzu dogs in Sweden: A clinical pathological and genetic study. J. Small Anim. Pract. 1990, 31, 83–91. [Google Scholar] [CrossRef]
- Nash, A.S.; Kelly, D.F.; Gaskell, C.J. Progressive renal disease in Soft-Coated Wheaten Terrier: Possible familial nephropathy. J. Small Anim. Pract. 1984, 25, 479–487. [Google Scholar] [CrossRef]
- Littman, M.P.; Dambach, D.M.; Vaden, S.L.; Giger, U. Familial protein-losing enteropathy and protein-losing nephropathy in Soft Coated Wheaten Terriers: 222 cases (1983–1997). J. Vet. Int. Med. 2000, 14, 68–80. [Google Scholar]
- Benali, S.L.; Lees, G.E.; Nabity, M.B.; Aricò, A.; Drigo, M.; Gallo, E.; Giantin, M.; Aresu, L. X-Linked hereditary nephropathy in navasota dogs: Clinical pathology, morphology, and gene expression during disease progression. Vet. Pathol. 2016, 53, 803–812. [Google Scholar] [CrossRef]
- Nowend, K.L.; Starr-Moss, A.N.; Lees, G.E.; Berridge, B.R.; Clubb, F.J.; Kashtan, C.E.; Nabity, M.B.; Murphy, K.E. Characterization of the genetic basis for autosomal recessive hereditary nephropathy in the English Springer Spaniel. J. Vet. Int. Med. 2012, 26, 294–301. [Google Scholar] [CrossRef]
- Minkus, G.; Breuer, W.; Wanke, R.; Reusch, C.; Leuterer, G.; Brem, G.; Hermanns, W. Familial nephropathy in Bernese mountain dogs. Vet. Pathol. 1994, 31, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Reusch, C.; Hoerauf, A.; Lechner, J.; Kirsch, M.; Leuterer, G.; Minkus, G.; Brem, G. A new familial glomerulonephropathy in Bernese mountain dogs. Vet. Rec. 1994, 134, 411–415. [Google Scholar] [CrossRef]
- Chew, D.J.; DiBartola, S.P.; Boyce, J.T.; Hayes, H.M.; Brace, J.J., Jr. Juvenile renal disease in doberman pinscher dogs. J. Am. Vet. Med. Assoc. 1983, 182, 481–485. [Google Scholar] [PubMed]
- Picut, C.A.; Lewis, R.M. Juvenile renal disease in the Doberman Pinscher: Ultrastructural changes in the glomerular basement membrane. J. Comp. Pathol. 1987, 97, 587–596. [Google Scholar] [CrossRef]
- Koeman, J.P.; Biewenga, W.J.; Gruys, E. Proteinuria associated with glomerulosclerosis and glomerular collagen formation in three Newfoundland dog littermates. Vet. Pathol. 1994, 31, 188–193. [Google Scholar] [CrossRef]
- Casal, M.L.; Dambach, D.M.; Meister, T.; Jezyk, P.F.; Patterson, D.F.; Henthorn, P.S. Familial Glomerulonephropathy in the Bullmastiff. Vet. Pathol. 2004, 41, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Karlstam, E. Renal dysplasia in boxers and Finnish harriers. J. Small Anim. Pract. 2000, 41, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Clercx, C.; Michiels, L.; Desmecht, D.; Snaps, F.; Henroteaux, M.; Day, M.J. Juvenile nephropathy in a boxer, a rottweiler, a collie and an Irish wolfhound. Aust. Vet. J. 2000, 78, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.L.; Elwood, C.; Murphy, K.F.; Gajanayake, I.; Syme, H.M. Juvenile nephropathy in 37 boxer dogs. J. Small Anim. Pract. 2007, 48, 690–694. [Google Scholar] [CrossRef]
- Kolbjørnsen, O.; Heggelund, M.; Jansen, J.H. End-stage kidney disease probably due to reflux nephropathy with segmental hypoplasia (ask-upmark kidney) in young boxer dogs in Norway. A retrospective study. Vet. Pathol. 2008, 45, 467–474. [Google Scholar] [CrossRef]
- Zatelli, A.; Bonfanti, U.; Santilli, R.; Borgarelli, M.; Bussadori, C. Echo-assisted percutaneous renal biopsy in dogs. A retrospective study of 229 cases. Vet. J. 2003, 116, 303–307. [Google Scholar] [CrossRef]
- IRIS (International Renal Interest Society) Staging of CKD (Modified 2019). Available online: http://www.iris-kidney.com/guidelines/staging.html (accessed on 28 December 2020).
- Beatrice, L.; Nizi, F.; Callegari, D.; Paltrinieri, S.; Zini, E.; D’Ippolito, P.; Zatelli, A. Comparison of urine protein-to-creatinine ratio in urine samples collected by cystocentesis versus free catch in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1221–1224. [Google Scholar] [CrossRef]
- Giraldi, M.; Paltrinieri, S.; Zatelli, A. Evaluation of the analytical variability of dipstick protein pads in canine urine. Vet. Clin. Pathol. 2018, 47, 246–251. [Google Scholar] [CrossRef]
| Dog # | Age | BUN mg/dL (RR: 8.0–20) | Creatinine mg/dL (RR: 0.5–1.4) | IRIS Stage | Albumine mg/dL (RR: 2.7–3.8) | Phosphorus mg/dL (RR: 2.5–6.0) | UPC | SAP mmHg |
|---|---|---|---|---|---|---|---|---|
| 1 | 06 | 68 | 5.6 | 4 | 1.6 | 8.9 | 5.6 | 185 |
| 2 | 09 | 56 | 4.1 | 3 | 1.7 | 9.4 | 2.8 | ND |
| 3 | 11 | 46 | 3.4 | 3 | 2.8 | 7.2 | 2.9 | ND |
| 4 | 13 | 89 | 4.8 | 3 | 1.8 | 9.3 | 3.7 | ND |
| 5 | 16 | 138 | 6.9 | 4 | 1.9 | 8.4 | 4.8 | 190 |
| 6 | 21 | 56 | 4.1 | 3 | 3.0 | 6.8 | 1.9 | 190 |
| 7 | 29 | 156 | 6.9 | 4 | 1.4 | 12.3 | 3.4 | ND |
| 8 | 71 | 120 | 6.3 | 4 | 1.6 | 6.7 | 2.5 | 205 |
| 9 | 79 | 78 | 4.2 | 3 | 1.8 | 6.5 | 2.4 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalera, M.A.; Gernone, F.; Uva, A.; D’Ippolito, P.; Roura, X.; Zatelli, A. Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018). Animals 2021, 11, 810. https://doi.org/10.3390/ani11030810
Cavalera MA, Gernone F, Uva A, D’Ippolito P, Roura X, Zatelli A. Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018). Animals. 2021; 11(3):810. https://doi.org/10.3390/ani11030810
Chicago/Turabian StyleCavalera, Maria Alfonsa, Floriana Gernone, Annamaria Uva, Paola D’Ippolito, Xavier Roura, and Andrea Zatelli. 2021. "Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018)" Animals 11, no. 3: 810. https://doi.org/10.3390/ani11030810
APA StyleCavalera, M. A., Gernone, F., Uva, A., D’Ippolito, P., Roura, X., & Zatelli, A. (2021). Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018). Animals, 11(3), 810. https://doi.org/10.3390/ani11030810







