Effect of Dietary Supplementation of Immunobiotic Lactiplantibacillusplantarum N14 Fermented Rakkyo (Allium chinense) Pickled Juice on the Immunocompetence and Production Performance of Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Rakkyo Pickled Juice by Lactic Acid Fermentation
2.2. Evaluation of Nutritional Composition of Fermented Rakkyo Residues
2.3. Quantitative Analysis of Polysaccharide Composition
2.4. Amino Acid Analyses
2.5. Estimation of Microbial Loads in the Fermented Rakkyo Pickled Juice
2.6. Selection and Management of Study Animals
2.7. Experimental Design for In Vivo Feeding Trial
2.8. Measuring Body Growth Rate
2.9. Blood Sampling and Cell Counting
2.10. Western Blotting for Detecting Enteropathogenic Escherichia coli (ETEC)
2.11. Evaluation of Plasma C-Reactive Protein (CRP) Level
2.12. Evaluation of Phagocytic Activity
2.13. Evaluation of Complement Activity
2.14. Evaluation of Carcass Weight and Quality
2.15. Statistical Analysis
3. Results
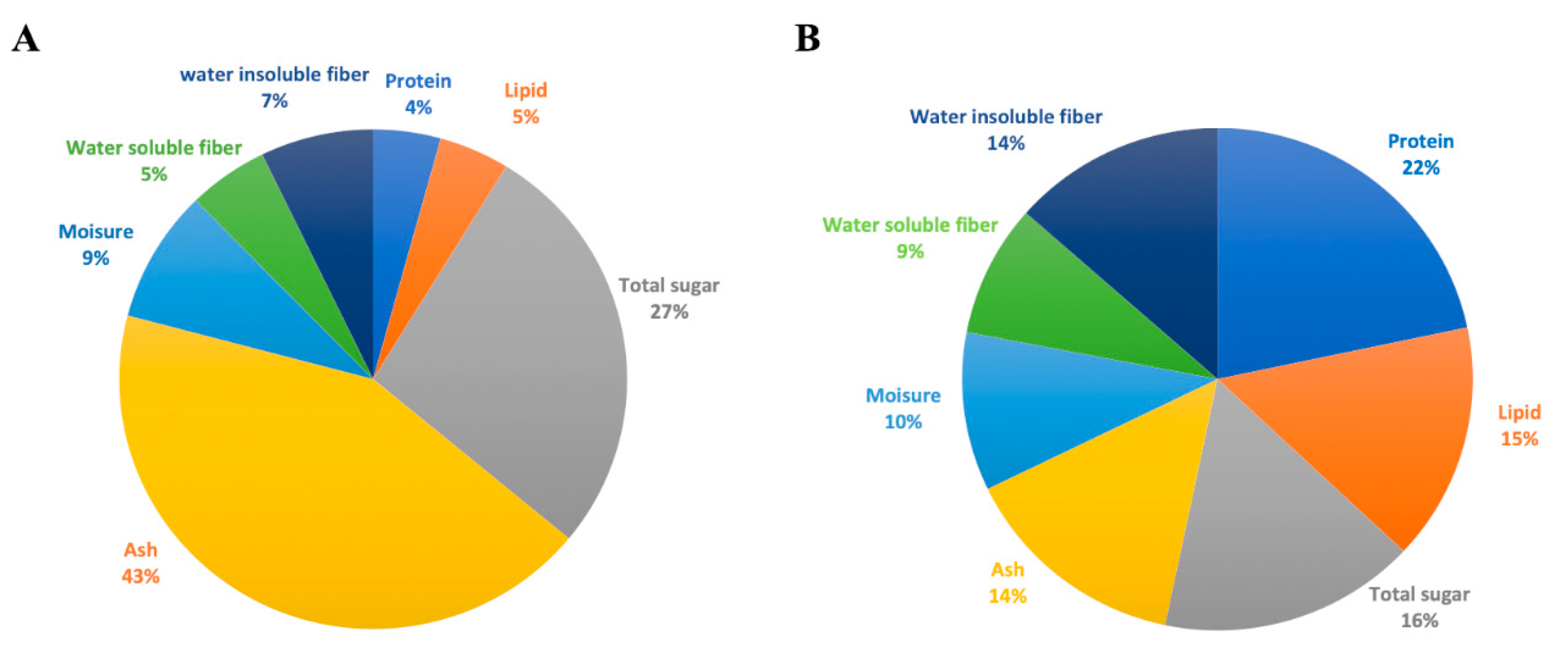
3.1. Nutritional Composition of Fermented Rakkyo Pickled Juice and Residues
3.1.1. Distribution of Major Nutrients
3.1.2. Individual Sugar Contents
3.1.3. Amino Acid Components
3.2. Microbial Loads of Fermented Rakkyo Pickled Juice
3.3. Effect of Immunobiotic Pickled Juice Feeding on Growth Rate
3.4. Effect of Immunobiotic Pickled Juice Feeding on Porcine Gut-Health
3.5. Effect of Immunobiotic Feeding on Immune Response Traits
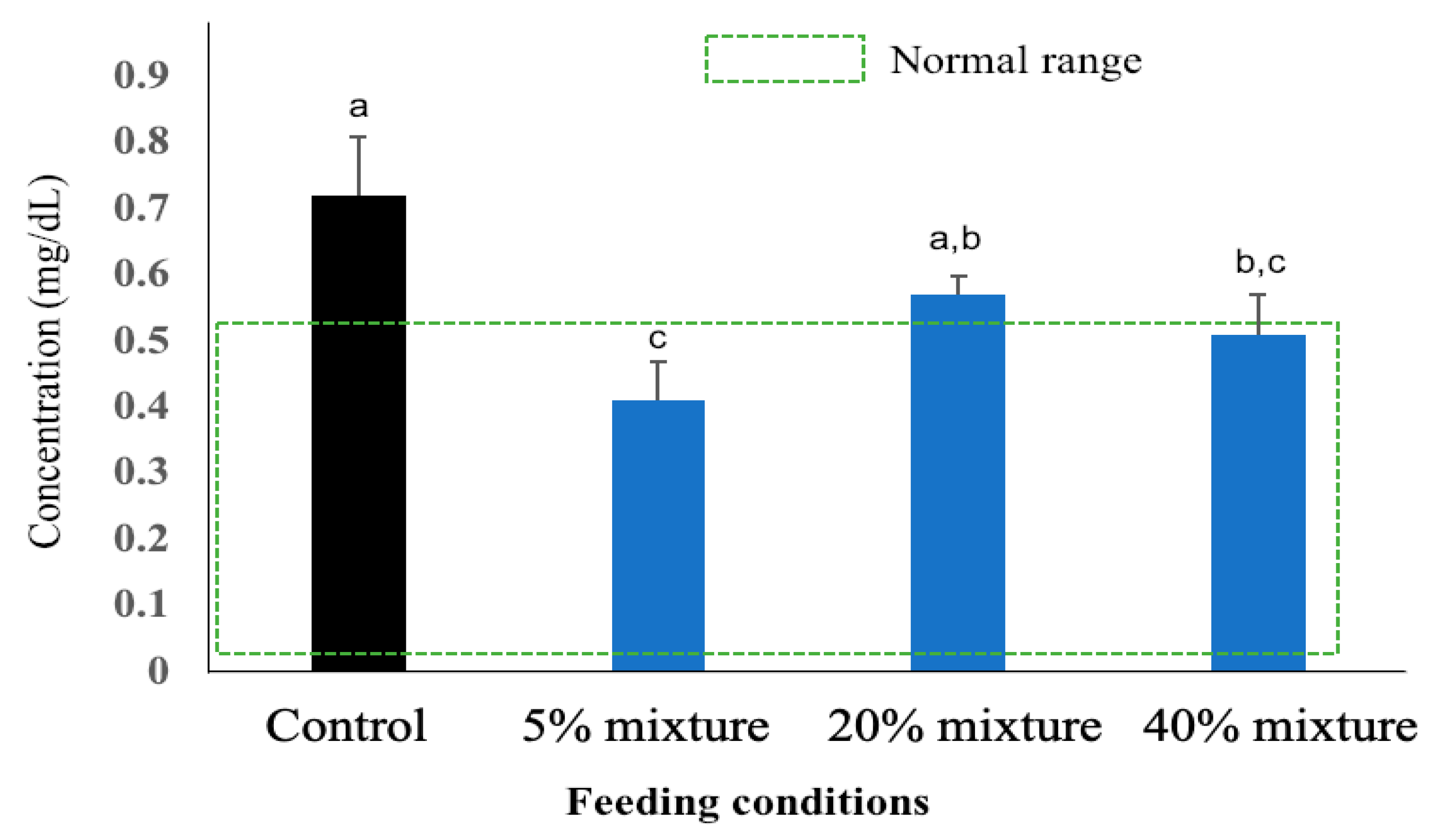
3.5.1. Plasma CRP Concentration
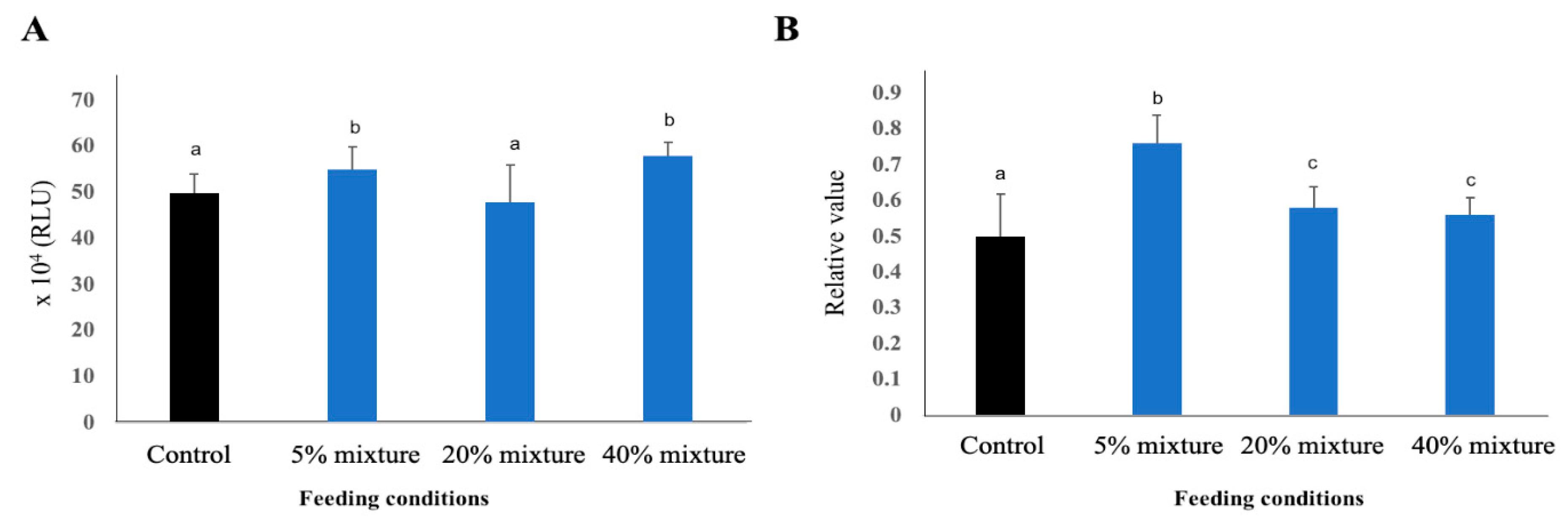
3.5.2. Phagocytosis and Complement Activity
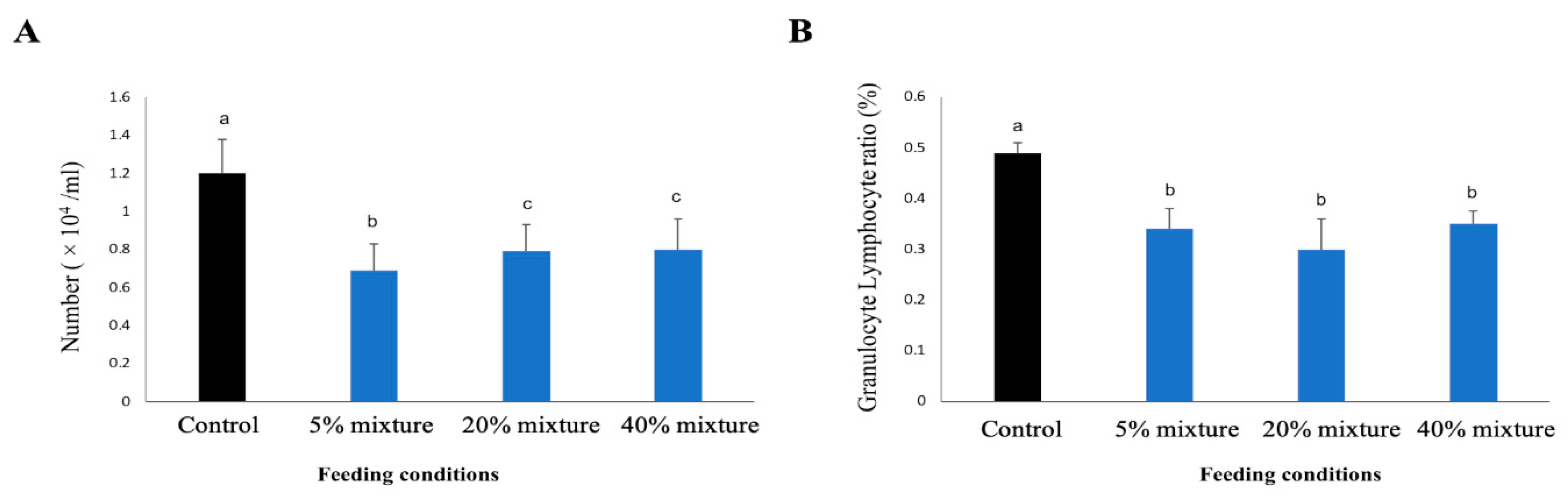
3.5.3. WBC Counts and the Ratio of Granulocyte and Lymphocytes
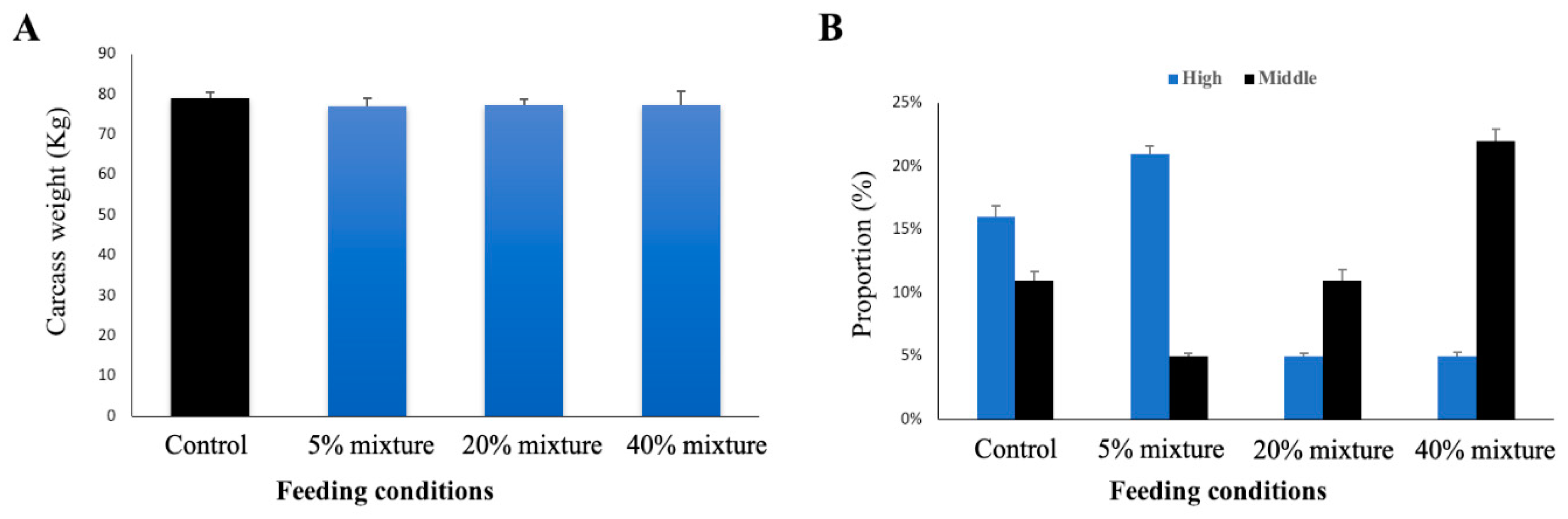
3.6. Effect of Immunobiotic Feeding on Carcass Weight and Carcass Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen, R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- FAO. Fermented Fruits and Vegetables—A Global Perspective; FAO Agricultural Services Bulletin: Rome, Italy, 1998; Volume 134. [Google Scholar]
- Devison, J. Pickles: A Global History; Reaktion Books Ltd.: London, UK, 2018. [Google Scholar]
- Montaño, A.; Casado, F.J.; de Castro, A.; Sánchez, A.H.; Rejano, L. Vitamin content and amino acid composition of pickled garlic processed with and without fermentation. J. Agric. Food Chem. 2004, 52, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wouters, D.; Bernaert, N.; Anno, N.; Droogenbroeck, V.B.; Loose, M.D.; Bockstaele, E.V.; Vuyst, L.D. Application’ and validation of autochthonous lactic acid bacteria starter cultures for controlled leek fermentations and their influence on the antioxidant properties of leek. Int. J. Food Microbiol. 2013, 165, 121–133. [Google Scholar] [CrossRef]
- McFeeters, R.F. Effects on fermentation on the nutritional properties of food. In Nutritional Evaluation of Food Processing; Karmas, E., Harris, R.S., Eds.; Avi: New York, NY, USA, 1988; pp. 423–446. [Google Scholar]
- Beato, V.M.; Sánchez, A.H.; de Castro, A.; Montaño, A. Effect of processing and storage time on the contents of organosulfur compounds in pickled blanched garlic. J. Agric. Food Chem. 2012, 60, 3485–3491. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Otani, H. Stimulatory effect of lactic acid bacteria from commercially available Nozawana-zuke pickle on cytokine expression by mouse spleen cells. Biosci. Biotechnol. Biochem. 2006, 70, 411–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demir, N.; Bahçeci, K.S.; Acar, J. The effects of different initial Lactobacillus plantarum concentrations on some properties of fermented carrot juice. J. Food Process. Preserv. 2006, 30, 352–363. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Li, S.; Li, C.; Li, D.; Yang, Z. Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World J. Microbiol. Biotechnol. 2013, 29, 489–498. [Google Scholar] [CrossRef]
- Murofushi, Y.; Villena, J.; Morie, K.; Kanmani, P.; Tohno, M.; Shimazu, T.; Aso, H.; Suda, Y.; Hashiguchi, K.; Saito, T.; et al. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol. Immunol. 2015, 64, 63–75. [Google Scholar] [CrossRef]
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.; Liong, M.T. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567–576. [Google Scholar] [CrossRef]
- Chang, S.Y.; Belal, S.A.; Kang, D.R.; Choi, Y.; Kim, Y.H.; Choe, H.S.; Heo, J.Y.; Shim, K.S. Influence of probiotics-friendly pig production on meat quality and physicochemical characteristics. Korean J. Food Sci. Anim. Resour. 2018, 38, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sudikas, G.; Kulpys, J.; Juškienė, V.; Juškienė, V.; Leikus, R.; Norvilienė, J. The influence of probiotics on carcass, meat and fat quality in pigs. Vet. Med. Zoot. 2010, 52, 79–86. [Google Scholar]
- Liu, T.Y.; Su, B.C.; Wang, J.L.; Zhang, C.; Shan, A.S. Effects of probiotics on growth, pork quality and serum metabolites in growing-finishing pigs. J. Northeast Agric. Univ. 2013, 20, 57–63. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Villena, J.; Takahashi, Y.; Hosoya, S.; Tomosoda, Y.; Tsukida, K.; Shimazu, T.; Aso, H.; Tohno, M.; Ishida, M.; et al. Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs. BMC Immunol. 2014, 15, 24. [Google Scholar] [CrossRef]
- Nagata, Y.; Yoshida, M.; Kitazawa, H.; Araki, E.; Gomyo, T. Improvements in seasonal allergic diseases with Lactobacillus plantarum No. 14. Biosci. Biotechnol. Biochem. 2010, 74, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Kamimura, Y.; Saka, T.; Yoshida, M.; Saito, M.; Kudo, H.; Kunisaki, N.; Gomyo, T. Lactobacillus plantarum strain No. 14 reduces human allergic reaction. J. Jpn. Soc. Food Sci. Technol. 2008, 55, 625–631. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Hashiguchi, K.; Nagata, Y.; Yoshida, M.; Murohushi, Y.; Kitazawa, H. Chemical and immunological characterization of exopolysaccharides produced by Lactobacillus plantarum No. 14. Jpn. J. Lact. Acid Bact. 2011, 22, 100–105. (In Japanese) [Google Scholar] [CrossRef]
- Masumizu, Y.; Zhou, B.; Kober, A.K.M.H.; Islam, M.A.; Iida, H.; Ikeda-Ohtsubo, W.; Suda, Y.; Albarracin, L.; Nochi, T.; Aso, H.; et al. Isolation and immunocharacterization of Lactobacillus salivarius from the intestine of wakame-fed pigs to develop novel “immunosynbiotics”. Microorganisms 2019, 7, 167. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Bchir, B.; Besbes, S.; Paquot, M.; Richel, A.; Blecker, C.; Attia, H. Chemical composition and functional properties of dietary fibre extracted by Englyst and Prosky methods from the alga Ulva lactuca collected in Tunisia. Algal Res. 2015, 9, 65–73. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. 1957, 226, 497–509. [Google Scholar]
- AOAC. Official Methods of Analyses of Association of Analytical Chemist, 15th ed.; Arlington VA: Washington, DC, USA, 1990. [Google Scholar]
- Official Method AOAC. 976.05. Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds; Agricultural Chemicals; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Menezes, E.W.; de Melo, A.T.; Lima, T.G.H.; Lajolo, F.M. Measurement of carbohydrate components and their impact on energy value of foods. J. Food Compos. Anal. 2004, 17, 331–338. [Google Scholar] [CrossRef]
- Bartolomeo, M.P.; Maisano, F. Validation of a reversed-phase HPLC method for quantitative amino acid analysis. J. Biomol. Tech. 2006, 17, 131–137. [Google Scholar]
- Caffaro-Filho, R.A.; Fantinatti-Garboggini, F.; Durrant, L.R. Quantitative analysis of Terminal Restriction Fragment Length Polymorphism (T-RFLP) microbial community profiles: Peak height data showed to be more reproducible than peak area. Braz. J. Microbiol. 2007, 38, 736–738. [Google Scholar] [CrossRef]
- Pomorska-Mól, M.; Markowska-Daniel, I.; Kwit, K.; Stępniewska, K.; Pejsak, Z. C-reactive protein, haptoglobin, serum amyloid A and pig major acute phase protein response in pigs simultaneously infected with H1N1 swine influenza virus and Pasteurella multocida. BMC Vet. Res. 2013, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Lin, C.S.K. Valorisation of food waste in biotechnological processes. Sustain. Chem. Process. 2013, 1, 13–19. [Google Scholar]
- Russ, W.; Meyer-Pittroff, R. Utilizing waste products from the food production and processing industries. Crit. Rev. Food Sci. Nutr. 2004, 44, 57–62. [Google Scholar] [CrossRef]
- Duff, S.J.B.; Murray, W.D. Bioconversion of forest products industry waste cellulosics to fuel ethanol: A review. Bioresour. Technol. 1996, 55, 1–33. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Desai, B.B. Effects of agricultural practices, handling, processing, and storage on vegetables. In Nutritional Evaluation of Food Processing; Karmas, E., Harris, R.S., Eds.; Avi: New York, NY, USA, 1988; pp. 23–71. [Google Scholar]
- Kyriazakis, I.; Sandberg, F.B. The problem of predicting the partitioning of scarce resources during sickness and health in pigs. In Mechanistic Modeling in Pig and Poultry Production; Gous, R., Morris, T., Fischer, C., Eds.; CAB International: Wallingford, UK, 2006; pp. 117–142. [Google Scholar]
- Van Heugten, E.; Coffey, M.T.; Spears, J.W. Effects of immune challenge, dietary energy density, and source of energy on performance and immunity in weanling pigs. J. Anim. Sci. 1996, 74, 2431–2440. [Google Scholar] [CrossRef]
- Montet, D.; Loiseau, G.; Zakhia-Rozis, N. Microbial technology of fermented vegetables. In Microbial Biotechnology in Horticulture; Ray, R.C., Ward, O.P., Eds.; Science Publishers: Enfield, NH, USA, 2006; Volume 1, pp. 309–343. [Google Scholar]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. Jpn. Clin. Nutr. 2001, 73, 374S–379S. [Google Scholar] [CrossRef]
- Gollop, N.; Zakin, V.; Weinberg, Z.G. Antibacterial activity of lactic acid bacteria included in inoculants for silage and in silages treated with these inoculants. J. Appl. Microbiol. 2005, 98, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Chen, W.; Zhou, Z.; Meng, Q.; Wu, H. Effects of adding various silage additives to whole corn crops at ensiling on performance, rumen fermentation, and serum physiological characteristics of growing-finishing cattle. Animals 2019, 9, 695. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, O.; Dong, M. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.T.; Langlois, B.E.; Cromwell, G.L.; Hays, V.W. Effect of chlortetracycline on the spread of R-100 plasmid-containing Escherichia coli BEL15R from experimentally infected pigs to uninfected pigs and chicks. J. Anim. Sci. 1984, 58, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Agga, G.E.; Scott, H.M.; Amachawadi, R.G.; Nagaraja, T.G.; Vinasco, J.; Bai, J.; Norby, B.; Renter, D.G.; Dritz, S.S.; Nelssen, J.L.; et al. Effects of chlortetracycline and copper supplementation on antimicrobial resistance of fecal Escherichia coli from weaned pigs. Prev. Vet. Med. 2014, 114, 231–246. [Google Scholar] [CrossRef]
- Melanie, L.B.; Davies, H.E.; Glynn, C.; Thompson, C.; Madden, M.; Wiseman, J.; Dood, C.E.R.; Hurdidge, L.; Payne, G.; Treut, Y.L.; et al. Influence of probiotics on gut health in the weaned pig. Livest. Sci. 2010, 133, 179–181. [Google Scholar]
- Shimazu, T.; Villena, J.; Tohno, M.; Fujie, H.; Hosoya, S.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; Saito, T.; et al. Immunobiotic Lactobacillus jensenii elicit anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the toll-like receptor signaling pathway. Infect. Immun. 2012, 80, 276–288. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Zhang, J.; Yang, Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res. Vet. Sci. 2012, 94, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Suzuki, R.; Fujie, H.; Chiba, E.; Takahashi, T.; Shimazu, T.; Aso, H.; Ohwada, S.; Suda, Y.; Ikegami, S.; et al. Immunobiotic Lactobacillus jensenii modulates toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen presenting cells. Clin. Vaccine Immunol. 2012, 19, 1038–1053. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhu, Y.H.; Zhang, H.F.; Yue, Y.; Cai, Z.X.; Lu, Q.P.; Zhang, L.; Weng, X.G.; Zhang, F.J.; Zhou, D.; et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: Intestinal microbiota and immune imbalances. PLoS ONE 2012, 7, e40666. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Li, T.; Kim, I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. R. Bras. Zootec. 2016, 45, 93–100. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [PubMed]



| Study Groups | Dietary Treatment Given through Drinking Water |
|---|---|
| Control or 0% mixture | Only drinking water |
| 5% mixture | Drinking water + 5% juice mixture (2.5% fermented rakkyo pickled juice and 2.5% residual liquid) |
| 20% mixture | Drinking water + 20% juice mixture (10% fermented rakkyo pickled juice and 10% residual liquid) |
| 40% mixture | Drinking water + 40% juice mixture (20% fermented rakkyo pickled juice and 20% residual liquid) |
| Sugar Name | Pure Bacterial Cells | Polysaccharides of Bacterial Starter Culture | Fermented Rakkyo Pickled Juice | Fermented Rakkyo Residual Liquid |
|---|---|---|---|---|
| Glucose | 2.1 | 5.6 | 0.9 | 2.8 |
| Arabinose | - | - | - | 0.3 |
| Xylose | - | - | - | 0.4 |
| Mannose | - | 4.4 | 0.4 | - |
| Galactose | 1.1 | 1.7 | - | 2 |
| Rhamnose | 0.5 | 0.4 | - | - |
| Ribose | 1.2 | 1.7 | - | - |
| Fucose | - | - | - | - |
| Total (g) | 4.9 | 13.8 | 1.3 | 5.5 |
| Range of MW | ||||
| 1,000,000 or more | 2 | 2 | 1 | 2 |
| 300,000 ~ | 1 | 1 | 1 | 1 |
| 100,000 ~ | - | 5 | 1 | - |
| 30,000 ~ | 2 | 22 | - | - |
| 10,000 ~ | 2 | 19 | - | 2 |
| 3000 ~ | 3 | 15 | 1 | 8 |
| 1000 ~ | 21 | 15 | 5 | 16 |
| <1000 | 69 | 21 | 91 | 71 |
| Total (%) | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Hashiguchi, K.; Kober, A.K.M.H.; Morie, K.; Zhou, B.; Tomokiyo, M.; Shimazu, T.; Aso, H.; Villena, J.; Suda, Y.; et al. Effect of Dietary Supplementation of Immunobiotic Lactiplantibacillusplantarum N14 Fermented Rakkyo (Allium chinense) Pickled Juice on the Immunocompetence and Production Performance of Pigs. Animals 2021, 11, 752. https://doi.org/10.3390/ani11030752
Islam MA, Hashiguchi K, Kober AKMH, Morie K, Zhou B, Tomokiyo M, Shimazu T, Aso H, Villena J, Suda Y, et al. Effect of Dietary Supplementation of Immunobiotic Lactiplantibacillusplantarum N14 Fermented Rakkyo (Allium chinense) Pickled Juice on the Immunocompetence and Production Performance of Pigs. Animals. 2021; 11(3):752. https://doi.org/10.3390/ani11030752
Chicago/Turabian StyleIslam, Md. Aminul, Kenji Hashiguchi, A.K.M. Humayun Kober, Kyoko Morie, Binghui Zhou, Mikado Tomokiyo, Tomoyuki Shimazu, Hisashi Aso, Julio Villena, Yoshihito Suda, and et al. 2021. "Effect of Dietary Supplementation of Immunobiotic Lactiplantibacillusplantarum N14 Fermented Rakkyo (Allium chinense) Pickled Juice on the Immunocompetence and Production Performance of Pigs" Animals 11, no. 3: 752. https://doi.org/10.3390/ani11030752
APA StyleIslam, M. A., Hashiguchi, K., Kober, A. K. M. H., Morie, K., Zhou, B., Tomokiyo, M., Shimazu, T., Aso, H., Villena, J., Suda, Y., & Kitazawa, H. (2021). Effect of Dietary Supplementation of Immunobiotic Lactiplantibacillusplantarum N14 Fermented Rakkyo (Allium chinense) Pickled Juice on the Immunocompetence and Production Performance of Pigs. Animals, 11(3), 752. https://doi.org/10.3390/ani11030752










