Sexual Interference Behaviors in Male Adult and Subadult Tibetan Macaques (Macaca thibetana)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Subjects
2.2. Behavior Definitions
2.3. Behavioral Data Collection
2.4. Data Analysis
3. Results
3.1. Copulation Events
3.2. Interference Events
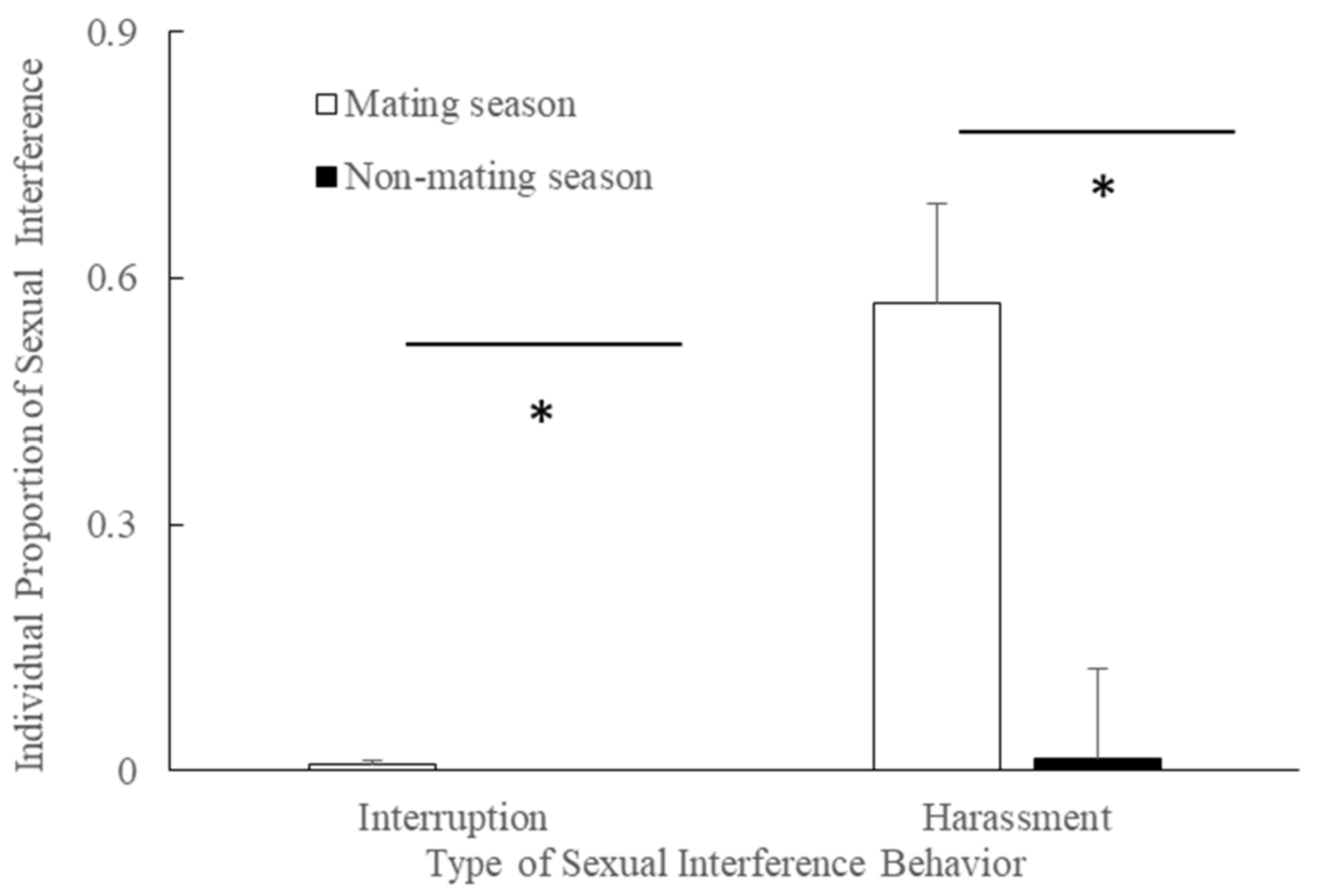
3.3. Season and Interruption/Harassment
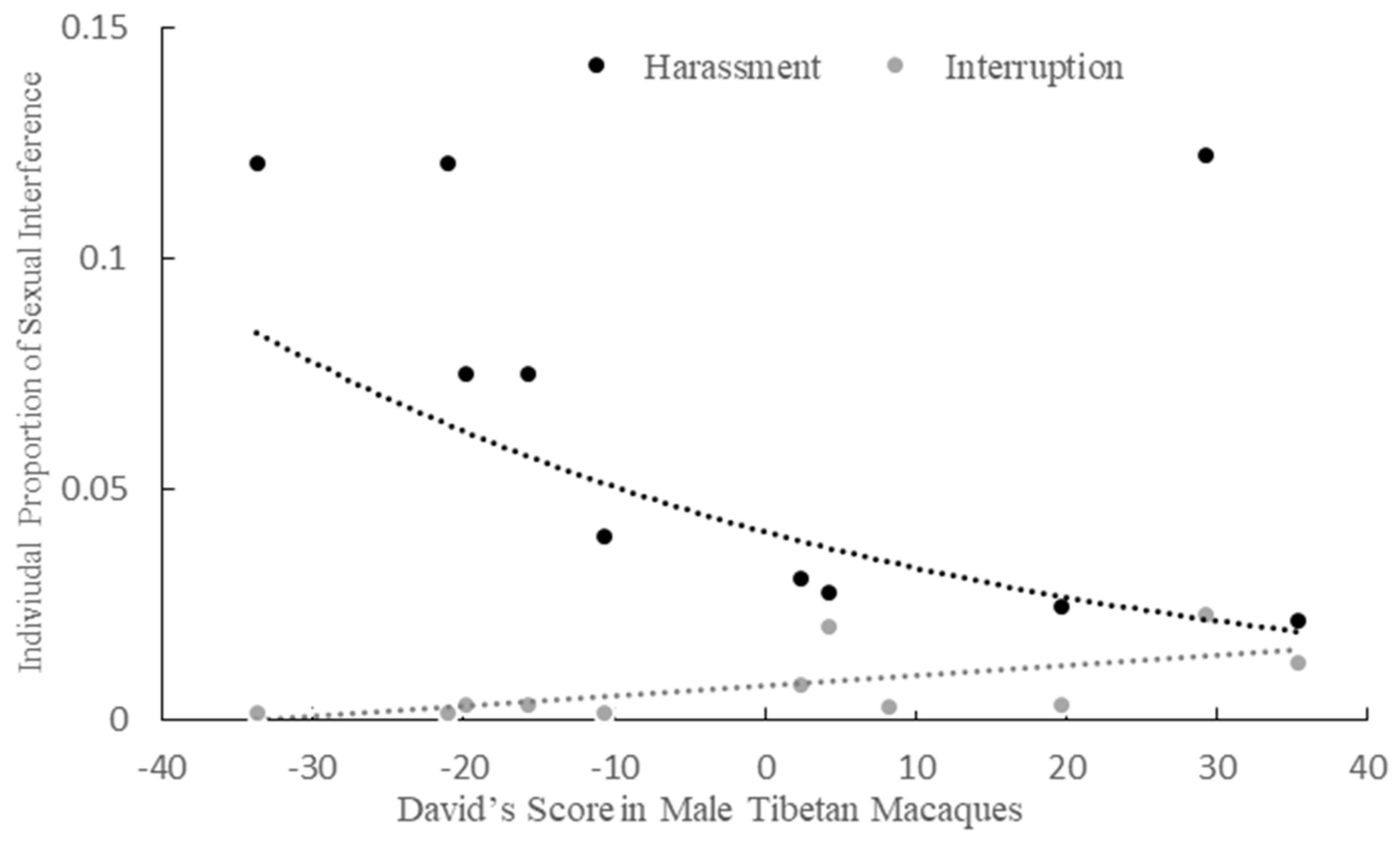
3.4. Dominance Rank and Interruption/Harassment in the Mating Season
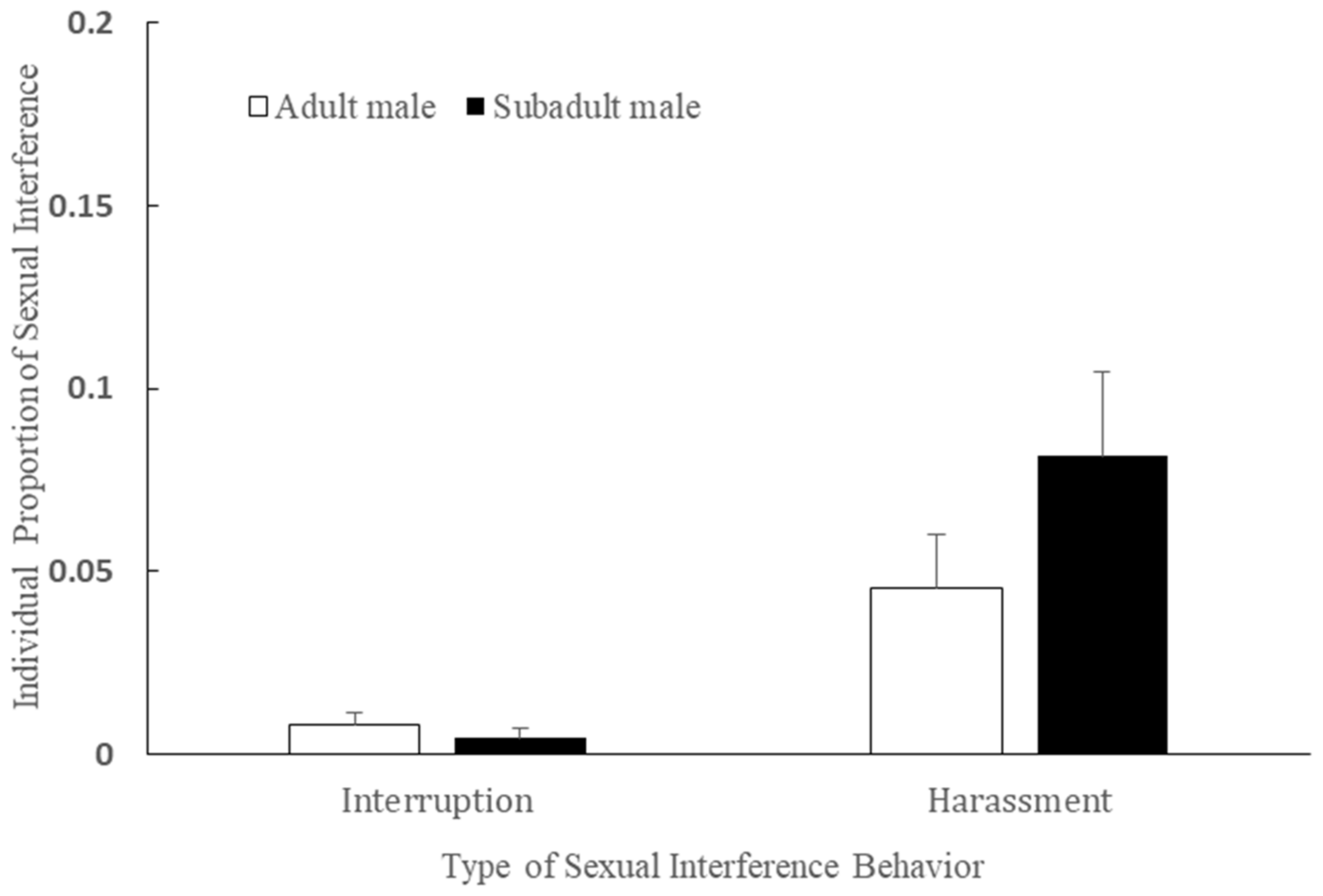
3.5. Age and Interruption/Harassment in the Mating Season
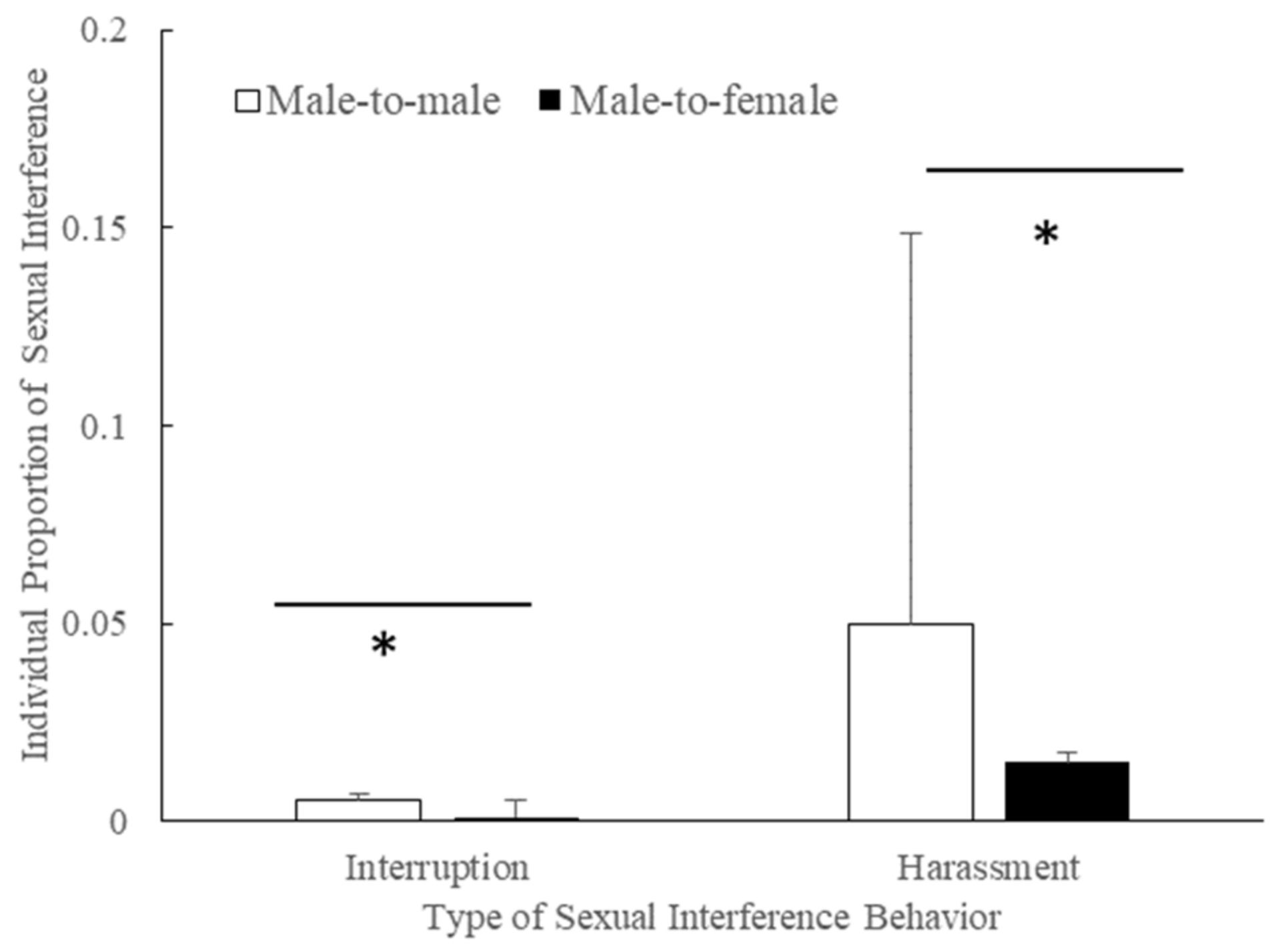
3.6. Interruption/Harassment Direction in the Mating Season
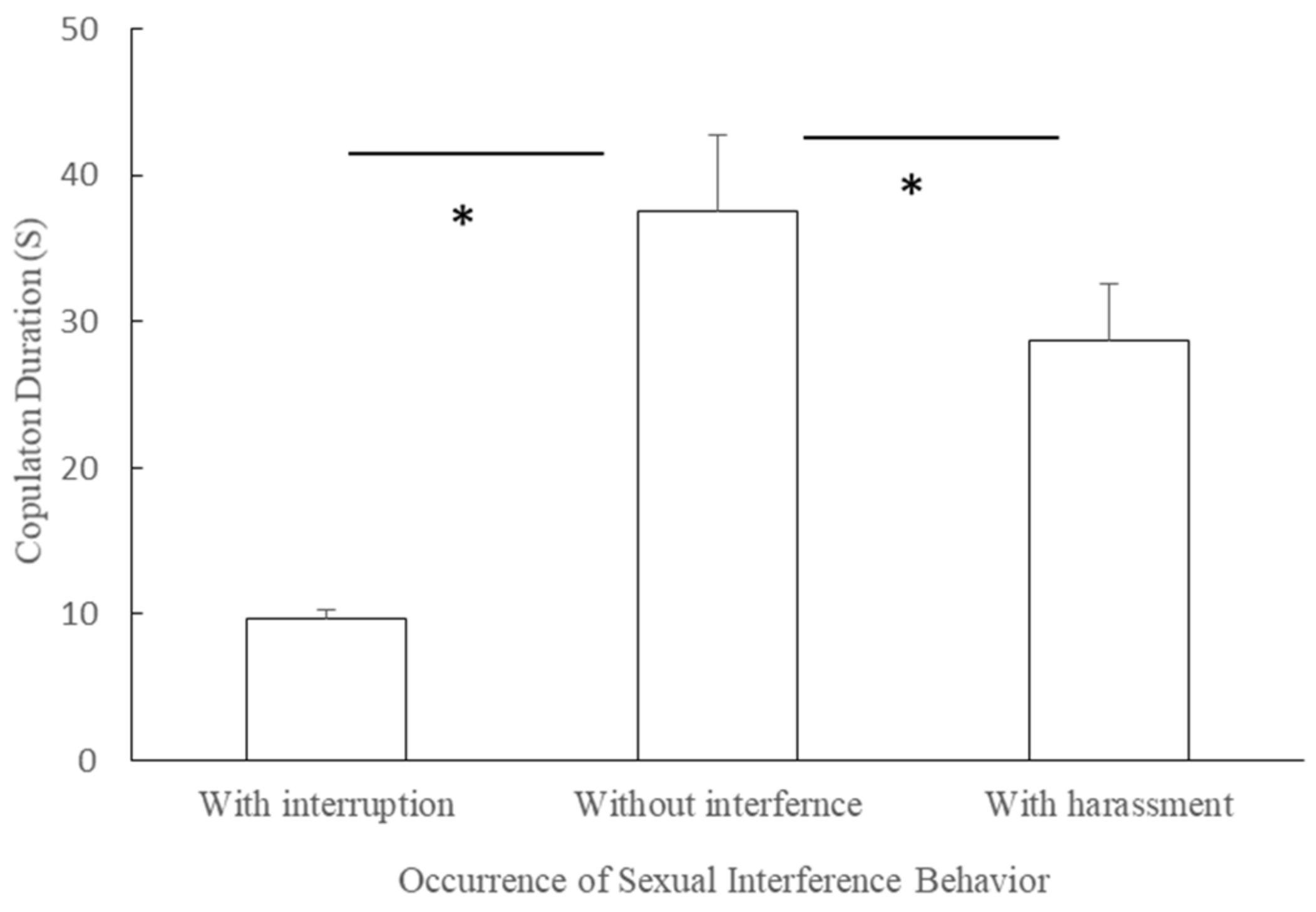
3.7. Effect of Interruption/Harassment Behaviors on Copulation Duration in the Mating Season
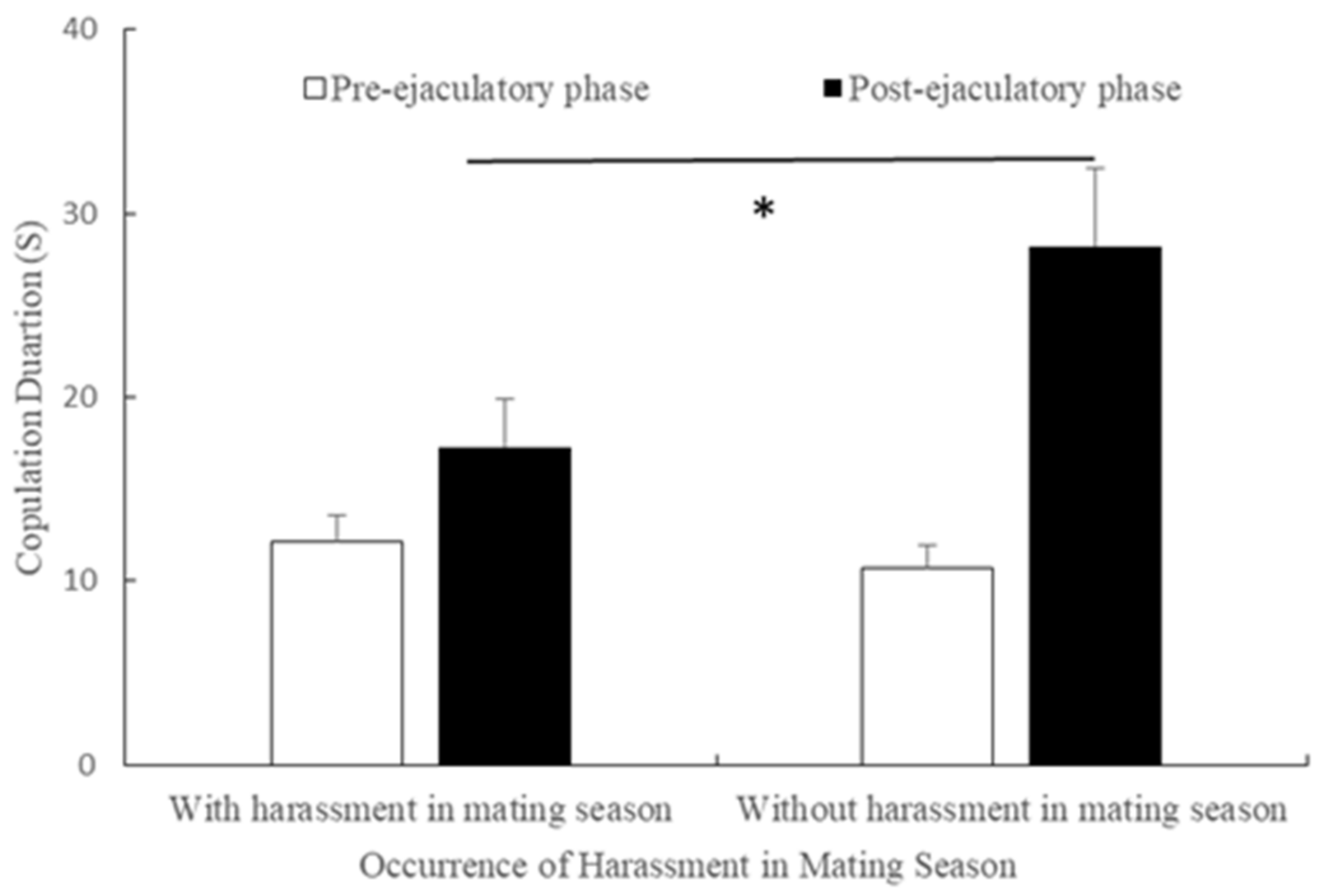
3.8. Durations of Pre- and Post-Ejaculatory Phases of Copulation in Mating Season
4. Discussion
4.1. Seasonal Variation in Male Sexual Competition
4.2. Effect of Male Rank on Sexual Interference in Mating Competition
4.3. Effect of Interference Direction in Mating Competition
4.4. Post-Ejaculatory Phased and Sperm Transport
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006, 21, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Makowicz, A.M.; Schlupp, I. The direct costs of living in a sexually harassing environment. Anim. Behav. 2013, 85, 569–577. [Google Scholar] [CrossRef]
- Arnold, S.J. Sexual behavior, sexual interference and sexual defense in the salamanders Ambystoma maculatum, Ambystoma tigrinum and Plethodon jordani. Z. Tierpsychol. 1976, 42, 247–300. [Google Scholar] [CrossRef]
- Powell, D.M. Female–female competition or male mate choice? Patterns of courtship and breeding behavior among feral horses (Equus caballus) on Assateague Island. J. Ethol. 2008, 26, 137–144. [Google Scholar] [CrossRef]
- Ziv, E.B.; Ilany, A.; Demartsev, V.; Barocas, A.; Geffen, E.; Koren, L. Individual, social, and sexual niche traits affect copulation success in a polygynandrous mating system. Behav. Ecol. Sociobiol. 2016, 70, 901–912. [Google Scholar]
- Dixson, A.F. Primate Sexuality; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Cappozzo, H.L.; Túnez, J.I.; Cassini, M.H. Sexual harassment and female gregariousness in the South American sea lion. Otaria Flavescens. Naturwissenschaften 2008, 95, 625–630. [Google Scholar] [CrossRef]
- Gouzoules, H. Harassment of sexual behavior in the stumptail macaque. Macaca arctoides. Folia. Primatol. 1994, 22, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T. Sexual behavior of adult male chimpanzees of the Mahale Mountains National Park, Tanzania. Primates 1997, 38, 379–398. [Google Scholar] [CrossRef]
- Li, B.G.; Zhao, D. Copulation behavior within one-male groups of wild Rhinopithecus roxellana in the Qinling Mountains of China. Primates 2007, 48, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, L.V.P.; Stone, A.I. Jealous of Mom? Interactions between infants and adult 26 males during the mating season in wild squirrel monkeys (Saimiri collinsi). Neotrop. Primates 2014, 21, 165–170. [Google Scholar] [CrossRef]
- Thierry, B. Affiliative interference in mounts in a group of Tonkean macaques (Macaca tonkeana). Am. J. Primatol. 1986, 11, 89–97. [Google Scholar] [CrossRef]
- Niemeyer, C.L.; Anderson, J.R. Primate harassment of matings. Ethol. Sociobiol. 1980, 4, 205–220. [Google Scholar] [CrossRef]
- Brereton, A.R. Return-benefit spite hypothesis: An explanation for sexual interference in stumptail macaques (Macaca arctoides). Primates 1994, 35, 123–136. [Google Scholar] [CrossRef]
- Bruce, K.E.; Estep, D.Q. Interruption of and harassment during copulation by stumptail macaques. Macaca arctoides. Anim. Behav. 1992, 44, 1029–1044. [Google Scholar] [CrossRef]
- Vervaecke, H.; Stevens, J.; Van Elsacker, L. Interfering with Others: Female-Female Reproductive Competition in Pan Paniscus.Sexual Selection and Reproductive Competition in Primates: New Perspectives and Directions; Jones, C.B., Ed.; American Society of Primatologists: Norman, OK, USA, 2003; pp. 231–253. [Google Scholar]
- Qi, X.G.; Yang, B.; Garber, P.A.; Ji, W.; Watanabe, K.; Li, B.G. Sexual interference in the golden snub-nosed monkey (Rhinopithecus roxellana): A test of the sexual competition hypothesis in a polygynous species. Am. J. Primatol. 2011, 73, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Okamoto, K. Frequent harassment of mounting after a takeover of a group of Moor macaques (Macaca maurus). Primates. 1998, 39, 225–230. [Google Scholar] [CrossRef]
- Overduin-de Vries, A.M.; Massen, J.J.M.; Spruijt, B.M.; Sterck, E.H.M. Sneaky monkeys: An audience effect of male Rhesus macaques (Macaca mulatta) on sexual Behavior. Am. J. Primatol. 2012, 74, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.L.; Chamove, A.S. Motivation of harassment of matings in stumptailed macaques. Behaviour 1983, 87, 298–322. [Google Scholar] [CrossRef]
- Wilson, M.E. Social dominance and female reproductive behavior in Rhesus monkeys (Macaca mulatta). Anim. Behav. 1981, 29, 472–482. [Google Scholar] [CrossRef]
- Stone, A.I. Is fatter sexier? Reproductive strategies of male Squirrel monkeys (Saimiri sciureus). Int. J. Primatol. 2014, 35, 628–642. [Google Scholar] [CrossRef]
- Estep, D.Q.; Gordon, T.P.; Wilson, M.E.; Walker, M.L. Social stimulation and the resumption of copulation in Rhesus (Macaca mulatto) and stumptail Macaques (Macaca arctoides). Int. J. Primatol. 1986, 7, 507–517. [Google Scholar] [CrossRef]
- Sommer, V. Sexual harassment in langur monkeys (Presbytis entellus): Competition for ova, sperm, and nurture? Ethology 1989, 80, 205–217. [Google Scholar] [CrossRef]
- Hrdy, S.B. The Langurs of Abu: Female and Male Strategies of Reproduction; Harvard University Press: Cambridge, UK, 1980; pp. 527–528. [Google Scholar]
- Overduin-de Vries, A.M.; Olesen, C.U.; de Vries, H.; Spruijt, B.M.; Sterck, E.H.M. Sneak copulations in long-tailed macaques (Macaca fascicularis): No evidence for tactical deception. Behav. Ecol. Sociobiol. 2013, 67, 101–111. [Google Scholar] [CrossRef]
- Thompson, C.L. Non-monogamous copulations and potential within-group mating competition in white-faced saki monkeys (Pithecia pithecia). Am. J. Primatol. 2013, 75, 817–824. [Google Scholar] [CrossRef]
- Zhao, Q.K. Sexual behavior of Tibetan macaques at Mt. Emei, China. Primates 1993, 34, 431–444. [Google Scholar] [CrossRef]
- Xiong, C.P.; Wang, Q.C. A comparative study on the male sexual behavior in Thibetan and Japanese monkeys. Acta Theiol. Sin. 1991, 11, 13–22. (In Chinese) [Google Scholar]
- Li, J.H. The Tibetan Macaque Society: A Field Study; Anhui University Press: Hefei, China, 1999. (In Chinese) [Google Scholar]
- Usui, R.; Sheeran, L.K.; Li, J.H.; Sun, L.; Wang, X.; Pritchard, A.J.; DuVall-Lash, A.S.; Wagner, R.S. Park rangers’ behaviors and their effects on tourists and Tibetan macaques (Macaca thibetana) at Mt. Huangshan, China. Animals 2014, 4, 546–561. [Google Scholar] [CrossRef]
- Li, J.H.; Yin, H.B.; Zhou, L.Z. Non-reproductive copulation behavior among Tibetan macaques (Macaca thibetana) at Huangshan, China. Primates 2007, 48, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H. Seasonality of reproduction and sexual activity in female Tibetan macaques (Macaca thibetana) at Huangshan, China. Acta Zool. Sin. 2005, 51, 365–375. (In Chinese) [Google Scholar]
- Xia, D.P.; Li, J.H.; Zhu, Y.; Sun, B.H.; Sheeran, L.K. Seasonal variation and synchronization of sexual behaviors in free-ranging male Tibetan macaques (Macaca thibetana) at Huangshan, China. Zool. Res. 2010, 31, 509–515. [Google Scholar]
- Xia, D.P.; Li, J.H.; Sun, B.H.; Weed, J.L.; Kyes, R.C. Evaluation of fecal testosterone, rank and copulatory behavior in wild male Macaca thibetana at Huangshan, China. Pakistan. J. Zool. 2015, 47, 1445–1454. [Google Scholar]
- Xiong, C.P. Sexual harassment of copulation in Thibetan monkeys (Macaca thibetana). Acta Theiol. Sin. 1993, 13, 172–180. [Google Scholar]
- Xiong, C.P. Sexual behavioral pattern in the female thibetan monkeys (Macaca thibetana). Acta Theiol. Sin. 1998, 18, 247–253. (In Chinese) [Google Scholar]
- Wang, Q.C.; Li, J.H.; Yang, Z.F. The Tibetan macaque (Macaca thibetana) in China. Bull. Biology. 1994, 29, 19–22. [Google Scholar]
- Berman, C.M.; Li, J.H. Impact of translocation, provisioning and range restriction on a group of Macaca thibetana. Int. J. Primatol. 2002, 23, 383–397. [Google Scholar] [CrossRef]
- Pritchard, A.J.; Sheeran, L.K.; Gabriel, K.I.; Li, J.H.; Wagner, R.S. Behaviors that predict personality components in adult free-ranging Tibetan macaques Macaca thibetana. Curr. Zool. 2014, 60, 362–372. [Google Scholar] [CrossRef]
- Wang, X.; Xia, D.P.; Sun, L.; Garber, P.A.; Kyes, R.C.; Sheeran, L.K.; Li, J.H. Infant attraction: Why social bridging matters for female leadership in Tibetan macaques. Curr. Zool. 2020, 66, 635–642. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhu, Y.; Wang, S. Male mate choice in Tibetan macaques Macaca thibetana at Mt. Huangshan, China. Curr. Zool. 2010, 56, 213–221. [Google Scholar]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.K.; Li, J.H.; Sun, L.; Sheeran, L.K.; Wagner, R.S.; Xia, D.P.; Chen, R. Collective decision making in Tibetan macaques: How followers affect the rules and speed of group movement. Anim. Behav. 2018, 146, 51–61. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Li, J.H.; Xia, D.P.; Sun, B.H.; Zhang, D. Collective movement in the Tibetan macaques (Macaca thibetana): Early joiners write the rule of the game. PLoS ONE 2015, 10, e0127459. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Sheeran, L.K.; Sun, B.H.; Zhang, Q.X.; Zhang, D.; Li, J.H. Social rank versus affiliation: Which is more closely related to leadership of group movements in Tibetan macaques (Macaca thibetana)? Am. J. Primatol. 2016, 78, 816–824. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.; Stevens, J.M.G.; Vervaecke, H. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 2006, 71, 585–592. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.H.; Xia, D.P.; Zhu, Y.; Wang, X.; Sun, B.H.; Wang, S. Stability of the female dominance hierarchy in free-ranging, provisioned adult Tibetan macaques (Macaca thibetana) at Mt. Huangshan, China. Acta Theiol. Sin. 2013, 33, 238–245. (In Chinese) [Google Scholar]
- Berman, C.M.; Ionica, C.S.; Li, J.H. Dominance style among Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 2004, 25, 1283–1312. [Google Scholar] [CrossRef]
- Berman, C.M.; Ionica, C.; Li, J.H. Supportive and tolerant relationships among male Tibetan macaques at Huangshan, China. Behaviour 2007, 144, 631–661. [Google Scholar]
- Berman, C.; Yin, H.; Ogawa, H.; Li, J.H.; Ionica, C. Variation in kin bias over time in a group of Tibetan macaques at Huangshan, China: Contest competition, time constraints or risk response? Behaviour 2008, 145, 863–896. [Google Scholar] [CrossRef]
- Berman, C.M.; Ionica, C.S.; Dorner, M.; Li, J.H. Postconflict affiliation between former opponents in Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 2006, 27, 827–854. [Google Scholar] [CrossRef]
- Norusis, M. SPSS 13.0 Advanced Statistical Procedures Companion; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Yang, B.; Wang, C.L.; Ji, W.H. Sexual interference in nonhuman primates. Acta Ecol. Sin. 2013, 19, 5973–5980. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Loy, J.; Loy, K. Sexual harassment among captive patas monkeys (Erythrocebus patas). Primates 1977, 18, 691–699. [Google Scholar] [CrossRef]
- Robbins, M.M. Male mating patterns in wild multimale mountain gorilla groups. Anim. Behav. 1999, 57, 1013–1020. [Google Scholar] [CrossRef][Green Version]
- Alfaro, J.W.L. Male mating strategies and reproductive constraints in a group of wild tufted capuchin monkeys (Cebus apella nigritus). Am. J. Primatol. Off. J. Am. Soc. Primatol. 2005, 67, 313–328. [Google Scholar] [CrossRef]
- Xia, D.P.; Wang, X.; Zhang, Q.X.; Sun, B.H.; Sun, L.; Sheeran, L.K.; Li, J.H. Progesterone levels in seasonally breeding, free-ranging male Macaca thibetana. Mamm. Res. 2018, 63, 99–106. [Google Scholar] [CrossRef]
- Wu, W.; Sun, B.H.; Wang, X.; Li, W.B.; Li, B.W.; Li, J.H. Correlations among dominance rank, testosterone levels and intestinal parasitic infection individual male Tibetan macaque (Macaca thibetana). Acta Theiol. Sin. 2018, 38, 451–457. (In Chinese) [Google Scholar]
- Lynch, J.W.; Ziegler, T.E.; Strier, K.B. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm. Behav. 2002, 41, 275–287. [Google Scholar] [CrossRef]
- Lappan, S.; Morino, L. Mating in the presence of a competitor: Audience effects may promote male social tolerance in polyandrous siamang (Symphalangus syndactylus) groups. Behaviour 2014, 151, 1067–1089. [Google Scholar] [CrossRef]
- Inoue, M.; Mitsunaga, F.; Nozaki, M.; Ohsawa, H.; Takenaka, A.; Sugiyama, Y.; Takenaka, O. Male dominance rank and reproductive success in an enclosed group of Japanese macaques: With special reference to post-conception mating. Primates 1993, 34, 503–511. [Google Scholar] [CrossRef]
- Cowlishaw, G.; Dunbar, R.I. Dominance rank and mating success in male primates. Anim. Behav. 1991, 41, 1045–1056. [Google Scholar] [CrossRef]
- Reichard, U.H.; Boesch, C.E. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Vervaecke, H.; Van Elsacker, L. Sexual competition in a group of captive bonobos (Pan paniscus). Primates 2000, 41, 109–115. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, K.; Slob, A.K.; Ten, J.J. Gender-related behaviors in group-living stumptail macaques. Psychobiology 1988, 16, 357–371. [Google Scholar]
- Drukker, B.; Nieuwenhuijsen, K.; ten Bosch, J.V.D.W.; Van Hooff, J.A.R.A.M.; Slob, A.K. Harassment of sexual interactions among stumptail macaques, Macaca arctoides. Anim. Behav. 1991, 42, 171–182. [Google Scholar] [CrossRef]
- Linn, G.S.; Mase, D.; Lafrancois, D.; Okeeffe, R.T.; Lifshitz, K. Social and menstrual cycle phase influences on the behavior of group-housed Cebus apella. Am. J. Primatol. 1995, 35, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Smuts, B.B.; Smuts, R.W. Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Adv. Stud. Behav. 1993, 22, 1–63. [Google Scholar]
- Gilby, I.C.; Brent, L.J.N.; Wroblewski, E.E. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 2013, 67, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Von Rohr, C.R.; Koski, S.E.; Burkart, J.M. Impartial third-party interventions in captive chimpanzees: A reflection of community concern. PLoS ONE 2012, 7, e32494. [Google Scholar] [CrossRef]
- Matthews, M.; Adler, N.T. Facilitative and inhibitory influences of reproductive behavior on sperm transport in rats. J. Comp. Physio. Psychol. 1977, 91, 727. [Google Scholar] [CrossRef]
- Stockley, P.; Preston, B.T. Sperm competition and diversity in rodent copulatory behaviour. J. Evol. Biol. 2004, 17, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Busse, C.D.; Estep, D.Q. Sexual arousal in male pigtailed monkeys (Macaca nemestrina): Effects of serial matings by two males. J. Comp. Psychol. 1984, 98, 227. [Google Scholar] [CrossRef] [PubMed]

| Age Class | Rank (DS Score) | Male | Immigrate Date/Birth Date |
|---|---|---|---|
| subadult male | 1 (35.3) | HXM | 2010-02-23 (Birth Date) |
| adult male | 2 (29.18) | TG | 2003-??-?? (Immigrate Date) |
| adult male | 3 (19.71) | ZB | 2013-08-?? (Immigrate Date) |
| adult male | 4 (8.21) | GS | 1984-??-?? (Immigrate Date) |
| adult male | 5 (4.18) | YRB | 2008-01-22 (Birth Date) |
| adult male | 6 (2.3) | BT | 2011-02-?? (Immigrate Date) |
| adult male | 7 (−10.7) | HM | 2014-11-?? (Immigrate Date) |
| adult male | 8 (−15.7) | DS | 2013-08-?? (Immigrate Date) |
| subadult male | 9 (−19.8) | TRG | 2010-04-24 (Birth Date) |
| subadult male | 10 (−21) | YCLO | 2010-06-21 (Birth Date) |
| subadult male | 11 (−33.7) | YRQ | 2010-05-23 (Birth Date) |
| Total | 11 |
| Pattern | Definition |
|---|---|
| Copulation behavior | A male mounting a female with intromission and penis thrusting, with or without ejaculation [31,32]. |
| Ejaculation | Ejaculate visible on the genitalia or perineum of the male or female after the pair dismounted or when the male ceases intravaginal thrusting, shows muscular body spasms and rhythmical pants and has a frowning, round mouth expression [31,32]. |
| Pre-ejaculatory phase | The period in which copulation began when a female was mounted by a male to the time of ejaculation [31,32]. |
| Post-ejaculatory phase | The period following ejaculation, ending when the male’s penis was removed from the female’s vagina [31,32]. |
| Interruption | A individual approaches a copulating pair (a mating male or a mating female) with aggressive contact (e.g., slapping, touching, grabbing), that separates the copulating pair [31,32]. |
| Harassment | An individual approaches a copulating pair (a mating male or a mating female) with vocalizations, facial expressions, or rarely with aggressive contact (e.g., slapping, touching, grabbing) that does not end in separation of the copulating pair prior to ejaculation [31,32]. |
| Approach | Any movement into the 1m radius of a copulating pair regardless of the patterns of interference behavior [31]. |
| Facial expressions | Lip-smacking and bared-teeth display (e.g., closed-mouth and bared-teeth face, and open-mouth and bared-teeth face directed toward the mounter or mountee) [31,32]. |
| Contact | Any physical contact between an outsider and a copulating pair (e.g., clasping, slapping, touching the head of a mating male and a female) [31,32]. |
| Performer | Adult or subadult male who performed interference behaviors within the distance of 1 m of a copulating pair, and the actor has a social interaction (affiliative or agonistic behaviors) directed at the copulating pair [31,32]. |
| Receiver | Mating male or mating female was harassed or interrupted within 1 m of the sexually-interfering individual(s) [31,32]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-H.; Rowe, A.K.; Sheeran, L.K.; Xia, D.-P.; Sun, L.; Li, J.-H. Sexual Interference Behaviors in Male Adult and Subadult Tibetan Macaques (Macaca thibetana). Animals 2021, 11, 663. https://doi.org/10.3390/ani11030663
Pang K-H, Rowe AK, Sheeran LK, Xia D-P, Sun L, Li J-H. Sexual Interference Behaviors in Male Adult and Subadult Tibetan Macaques (Macaca thibetana). Animals. 2021; 11(3):663. https://doi.org/10.3390/ani11030663
Chicago/Turabian StylePang, Kui-Hai, Amanda K. Rowe, Lori K. Sheeran, Dong-Po Xia, Lixing Sun, and Jin-Hua Li. 2021. "Sexual Interference Behaviors in Male Adult and Subadult Tibetan Macaques (Macaca thibetana)" Animals 11, no. 3: 663. https://doi.org/10.3390/ani11030663
APA StylePang, K.-H., Rowe, A. K., Sheeran, L. K., Xia, D.-P., Sun, L., & Li, J.-H. (2021). Sexual Interference Behaviors in Male Adult and Subadult Tibetan Macaques (Macaca thibetana). Animals, 11(3), 663. https://doi.org/10.3390/ani11030663








