Microbiological Hazards in Dry Dog Chews and Feeds
Abstract
Simple Summary
Abstract
1. Introduction
2. Microbiological Hazards in Treats
3. Pet Foods—Contamination with Mycotoxins
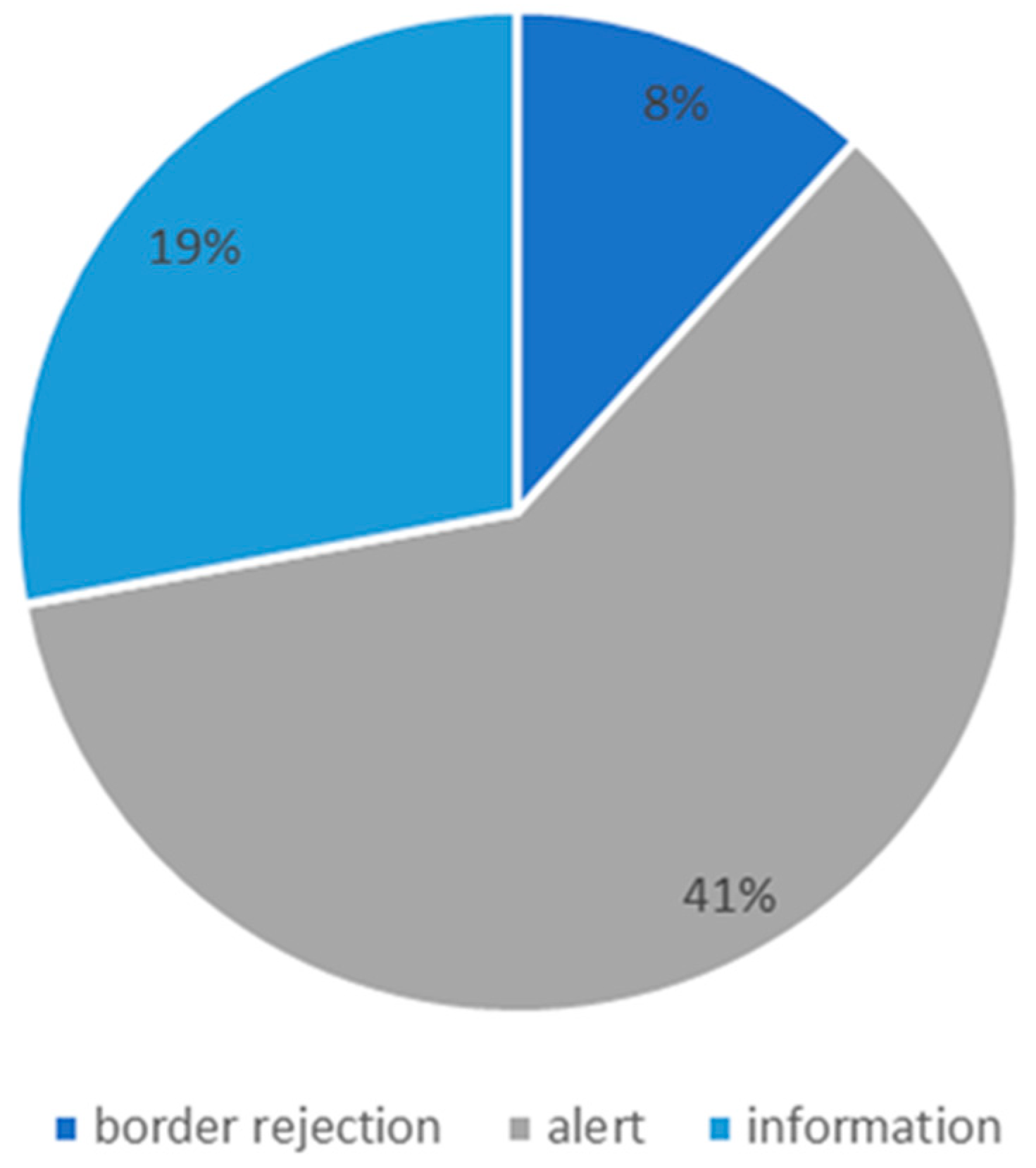
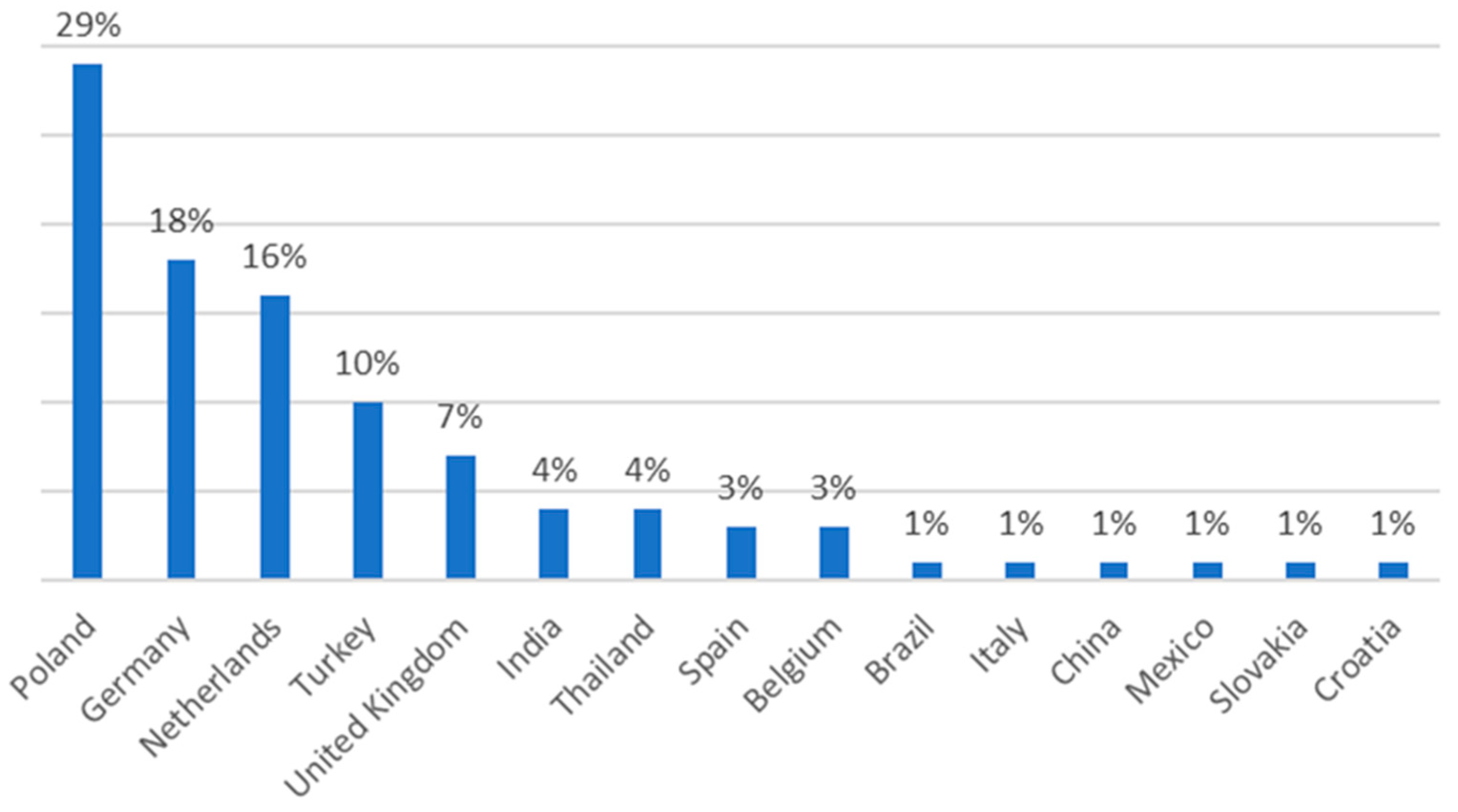
4. Analysis of the RASFF System
- –
- Alerts are sent when a food or feed presenting a serious health risk is on the market and when rapid action is required;
- –
- Information is sent when hazardous food or feeds or materials/articles met food are identified, but it is not necessary to take immediate action in this regard in another country being a member of the network, e.g., because a given product is no longer available on the market or is only on the market of the country notifying the notification; and
- –
- Border rejection means notification of rejection of a batch, container or food cargo.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FCI. The Fédération Cynologique Internationale. Available online: http://www.fci.be/en/ (accessed on 4 February 2021).
- The European Pet Food Industry Federation. Annual Report. The Europeran Pet Food Industry; FEDIAF: Bruxelles, Belgium, 2020. [Google Scholar]
- Jansen, W.; Müller, A.; Grabowski, N.T.; Kehrenberg, C.; Muylkens, B.; Al Dahouk, S. Foodborne diseases do not respect borders: Zoonotic pathogens and antimicrobial resistant bacteria in food products of animal origin illegally imported into the European Union. Vet. J. 2019, 244, 75–82. [Google Scholar] [CrossRef]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Caro-Hernández, P.A.; Tobar, J.A. Microbiological analysis of surfaces in contact with food. Entramado 2020, 16, 240–249. [Google Scholar] [CrossRef]
- RASFF. The Rapid Alert System for Food and Feed. Annual Report; Publications Office of the European Union: Rue Mercier, Luxemburg, 2019. [Google Scholar]
- Dodd, S.; Cave, N.; Abood, S.; Shoveller, A.K.; Adolphe, J.; Verbrugghe, A. An observational study of pet feeding practices and how these have changed between 2008 and 2018. Vet. Rec. 2020, 186, 643. [Google Scholar] [CrossRef]
- Domesle, K.J.; Young, S.R.; Ge, B. Rapid screening for Salmonella in raw pet food by loop-mediated isothermal amplification. J. Food Prot. 2020. [Google Scholar] [CrossRef]
- Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation). Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32009R1069 (accessed on 25 December 2020).
- Soffer, N.; Abuladze, T.; Woolston, J.; Li, M.; Hanna, L.F.; Heyse, S.; Charbonneau, D.; Sulakvelidze, A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage 2016, 6, e1220347. [Google Scholar] [CrossRef] [PubMed]
- Behravesh, C.B.; Ferraro, A.; Deasy, M.; Dato, V.; Moll, M.; Sandt, C.; Rea, N.K.; Rickert, R.; Marriott, C.; Warren, K.; et al. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 2010, 126, 477–483. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.; Reid-Smith, R.; Weese, J.S.; Angulo, F.J. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin. Infect. Dis. 2006, 42, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Maciorowski, K.G.; Herrera, P.; Kundinger, M.M.; Ricke, S.C. Animal feed production and contamination by foodborne Salmonella. J. Für Verbrauch. Leb. 2006, 1, 197–209. [Google Scholar] [CrossRef]
- Hołda, K.; Głogowski, R.; Hac-Szymańczuk, E.; Wiczuk, W. Comprehensive microbiological evaluation of dry foods for growing dogs marketed in Poland. Ann. Wars. Univ. Life Sci. SGGW Anim. Sci. 2017, 56, 81–89. [Google Scholar] [CrossRef]
- Pigłowski, M. Pathogenic and non-pathogenic microorganisms in the Rapid Alert System for food and feed. Int. J. Environ. Res. Public Health 2019, 16, 477. [Google Scholar] [CrossRef] [PubMed]
- FEDIAF. Guide to Good Practice for the Manufacture of Safe Pet Foods; The European Pet Food Industry: Bruxelles, Belgium, 2018. [Google Scholar]
- Commission Regulation (EU) No 142/2011, 2011. Commission Regulation (EU) No 142/2011 of 25 February 2011. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32011R0142 (accessed on 25 December 2020).
- Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 Laying down Requirements for Feed Hygiene. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0183 (accessed on 25 December 2020).
- Commission Recommendation of 17 August 2006 on the Prevention and Reduction of Fusarium Toxins in Cereals and Cereal Products (2006/583/EC). Off. J. Eur. Union 2006, L234, 35–40.
- Yukawa, S.; Uchida, I.; Tamura, Y.; Ohshima, S.; Hasegawa, T. Characterization of antibiotic resistance of Salmonella isolated from dog treats in Japan. Epidemiol. Infect. 2019, 147, 1–6. [Google Scholar] [CrossRef]
- Andreoletti, O.; Budka, H.; Collins, J.; Griffin, J.; Havelaar, A.; Hope, J.; Klein, G.; Kruse, H.; Magnino, S.; López, M.; et al. Microbiological risk assessment in feedingstuffs for food-producing animals 1 Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 720, 1–84. [Google Scholar]
- Bree, F.P.J.; Bokken, G.C.A.M.; Mineur, R.; Franssen, F.; Opsteegh, M.; Giessen, J.W.B.; Lipman, L.J.A.; Overgaauw, P.A.M. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec. 2018, 182, 50. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018. [Google Scholar] [CrossRef]
- Stull, J.W.; Peregrine, A.S.; Sargeant, J.M.; Weese, J.S. Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada. BMC Public Health 2013, 13, 520. [Google Scholar] [CrossRef]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Cunningham, J.; Ahmed, R. Characterization of Salmonella associated with pig ear dog treats in Canada. J. Clin. Microbiol. 2001, 39, 3962–3968. [Google Scholar] [CrossRef]
- Michael, G.B.; Schwarz, S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: An alarming trend? Clin. Microbiol. Infect. 2016, 22, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.D.; Reisbig, M.D.; Mulvey, M. Association between handling of pet treats and infection with Salmonella enterica serotype Newport expressing the AmpC b-lactamase, CMY-2. J. Clin. Microbiol. 2003, 39, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, S.J.; Daly, E.R.; Seiferth, J.; Nadeau, A.M.; Mahoney, J.; Finnigan, J.; Wikoff, P.; Kiebler, C.A.; Simmons, L. Human outbreak of Salmonella Typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Pathog. Dis. 2015, 12, 441–446. [Google Scholar] [CrossRef]
- Barrow, P.A.; Methner, U. Salmonella in Domestic Animals; CABI: Wallingford, UK, 2013; pp. 318–335. [Google Scholar]
- Stolle, I.; Prenger-Berninghoff, E.; Stamm, I.; Scheufen, S.; Hassdenteufel, E.; Guenther, S.; Bethe, A.; Pfeifer, Y.; Ewers, C. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J. Antimicrob. Chemother. 2013, 68, 2802–2808. [Google Scholar] [CrossRef]
- Andruzzi, M.N.; Krath, M.L.; Lawhon, S.D.; Boudreau, B. Salmonella enterica subspecies houtenae as an opportunistic pathogen in a case of meningoencephalomyelitis and bacteriuria in a dog. BMC Vet. Res. 2020, 16, 1–5. [Google Scholar] [CrossRef]
- Oki, M.; Ueda, A.; Tsuda, A.; Yanagi, H.; Ozawa, H.; Takagi, A. Salmonella enterica serotype enteritidis vertebral osteomyelitis and epidural abscess complicated with meningitis. Tokai J. Exp. Clin. Med. 2016, 41, 169–171. [Google Scholar] [PubMed]
- Schotte, U.; Borchers, D.; Wulff, C.; Geue, L. Salmonella Montevideo outbreak in military kennel dogs caused by contaminated commercial feed, which was only recognized through monitoring. Vet. Microbiol. 2007, 119, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kukier, E.; Kwiatek, K. Phenotypical and genotypical characterisation of Clostridium perfringens strains isolated from feedingstuffs. Bull. Vet. Inst. Pulawy 2010, 54, 501–511. [Google Scholar]
- Milanov, D.; Petrović, T.; Todorović, D.; Aleksić, N.; Čabarkapa, I. Toxin genotypes of Clostridium perfringens in animal feed and their role in the ethiology of enterotoxemia in domestic animals. Food Feed Res. 2018, 45, 67–76. [Google Scholar] [CrossRef]
- Pirs, T.; Ocepek, M.; Rupnik, M. Isolation of Clostridium difficile from food animals in Slovenia. J. Med. Microbiol. 2008, 57, 790–792. [Google Scholar] [CrossRef]
- Wojdat, E.; Kwiatek, K.; Kozak, M. Occurrence and characterization of some Clostridium species isolated from animal feedingstuffs. Bull. Vet. Inst. Pulawy 2006, 50, 63–67. [Google Scholar]
- Liubakka, A.; Vaughn, B.P. Clostridium difficile Infection and Fecal Microbiota Transplant. AACN Adv. Crit. Care 2016, 27, 324–337. [Google Scholar] [CrossRef]
- Gould, L.H.; Limbago, B. Clostridium difficile in food and domestic animals: A new foodborne pathogen? Clin. Infect. Dis. 2010, 51, 577–582. [Google Scholar] [CrossRef]
- Broda, D.M.; Delacy, K.M.; Bell, R.G.; Braggins, T.J.; Cook, R.L. Psychrotrophic Clostridium spp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 1996, 29, 335–352. [Google Scholar] [CrossRef]
- Freeman, L.M.; Janecko, N.; Weese, J.S. Nutritional and microbial analysis of bully sticks and survey of opinions about pet treats. Can. Vet. J. Rev. Vet. Can. 2013, 54, 50–54. [Google Scholar]
- Bilung, L.M.; Ulok, V.; Tesfamariam, F.M. Assessment of Listeria monocytogenes in pet food. Agric. Food Secur. 2018, 7, 23. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Panseri, S.; Cami, M.; Balzaretti, C.M. When pet snacks look like children’s toys! The potential role of pet snacks in transmission of bacterial zoonotic pathogens in the household. Foodborne Pathog. Dis. 2020. [Google Scholar] [CrossRef]
- Lambertini, E.; Buchanan, R.L.; Narrod, C.; Ford, R.M.; Baker, R.C.; Pradhan, A.K. Quantitative assessment of human and pet exposure to Salmonella associated with dry pet foods. Int. J. Food Microbiol. 2016, 216, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Adley, C.; Dillon, C.; Morris, C.P.; Delappe, N.; Cormican, M. Prevalence of Salmonella in pig ear pet treats. Int. Food Res. J. 2011, 44, 193–197. [Google Scholar] [CrossRef]
- Lambertini, E.; Buchanan, R.L.; Narrod, C.; Pradhan, A.K. Transmission of bacterial zoonotic pathogens between pets and humans: The role of pet food. Crit. Rev. Food Sci. Nutr. 2015, 56, 364–418. [Google Scholar] [CrossRef] [PubMed]
- Magossi, G.; Cernicchiaro, N.; Dritz, S.; Houser, T.; Woodworth, J.; Jones, C.; Trinetta, V. Evaluation of Salmonella presence in selected United States feed mills. Microbiologyopen 2019, 8, e00711. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the substrate and transmission to the larvae. Food Control 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Kukier, E.; Goldsztejn, M.; Grenda, T.; Kwiatek, K.; Wasyl, D.; Hoszowski, A. Microbiological quality of compound feed used in Poland. Bull. Vet. Inst. Pulawy 2012, 56, 349–354. [Google Scholar] [CrossRef]
- Pigłowski, M. Comparative analysis of notifications regarding mycotoxins in the Rapid Alert System for Food and Feed (RASFF). Qual. Assur. Saf. Crop. Foods 2019, 11, 725–735. [Google Scholar] [CrossRef]
- Vudathala, D.; Klobut, J.; Cummings, M.; Tkachenko, A.; Reimschuessel, R.; Murphy, L. Collaborators, multilaboratory evaluation of a lateral flow method for aflatoxin B1 analysis in dry dog food. J. AOAC Int. 2020, 103, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Commission Recommendation (EU) 2016/1319 of 29 July 2016 Amending Recommendation 2006/576/EC as Regards Deoxynivalenol, Zearalenone and Ochratoxin A in Pet Food (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016H1319 (accessed on 25 December 2020).
- Los, A.; Ziuzina, D.; Bourke, P. Current and future technologies for microbiological decontamination of cereal grains. J. Food. Sci. 2018, 83, 1484–1493. [Google Scholar] [CrossRef]
- Laca, A.; Mousia, Z.; Diaz, M.; Webb, C.; Pandiella, S.S. Distribution of microbial contamination within cereal grains. J. Food Eng. 2006, 72, 332–338. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiol. 2013, 37, 78–95. [Google Scholar] [CrossRef]
- Atanda, S.A.; Pessu, P.O.; Agoda, S.; Isong, I.U.; Adekalu, O.A.; Echendu, M.A.; Falade, T.C. Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 2011, 5, 4373–4382. [Google Scholar] [CrossRef]
- Santos Pereira, C.C.; Cunha, S.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Vudathala, D.; Cummings, M.; Tkachenko, A.; Guag, J.; Reimschuessel, R.; Murphy, L. A lateral flow method for aflatoxin B1 in dry dog food: An inter-laboratory trial. J. AOAC Int. 2021, qsaa175. [Google Scholar] [CrossRef]
- Barany, A.; Guilloto, M.; Cosano, J.; de Boevre, M.; Oliva, M.; de Saeger, S.; Fuentes, J.; Martínez-Rodriguez, G.; Mancera, J. Dietary aflatoxin B1 (AFB1) reduces growth performance, impacting growth axis, metabolism, and tissue integrity in juvenile gilthead sea bream (Sparus aurata). Aquaculture 2021, 533, 736189. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- da Rocha, M.E.B.; Freire, F.D.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Cheli, F. Mycotoxin contamination management tools and efficient strategies in feed industry. Toxins 2020, 12, 480. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Huertero, F.; Zaragoza-Ojeda, M.; Sánchez-Alarcón, J.; Milić, M.; Šegvić Klarić, M.; Montiel-González, J.M.; Valencia-Quintana, R. Involvement of ahr pathway in toxicity of aflatoxins and other mycotoxins. Front. Microbiol. 2019, 10, 2347. [Google Scholar] [CrossRef]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin successfully inhibited the computationally identified CYP2A6 enzyme-mediated bioactivation of aflatoxin B1 in arbor acres broiler. Front. Pharmacol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Chukwuka, O.K.; Okoli, I.C.; Opara, M.N.; Omede, A.A.; Ogbuewu, I.P.; Iheshiulor, O.O.M. The growing problems of mycotoxins in animal feed industry in West Africa. Asian J. Poult. Sci. 2010, 4, 122–134. [Google Scholar] [CrossRef]
- Singh, S.D.; Chuturgoon, A.A. A comparative analysis of mycotoxin contamination of supermarket and premium brand pelleted dog food in Durban, South Africa. J. S. Afr. Vet. Assoc. 2017, 88, 1–6. [Google Scholar] [CrossRef]
- Leiva, A.; Molina, A.; Redondo-Solano, M.; Artavia, G.; Rojas-Bogantes, L.; Granados-Chinchilla, F. Pet food quality assurance and safety and quality assurance survey within the Costa Rican pet food industry. Animals 2019, 9, 980. [Google Scholar] [CrossRef]
- Leiva, A.; Méndez, G.; Rodriguez, C.; Molina, A.; Granados-Chinchilla, F. Chemical assessment of mycotoxin contaminants and veterinary residues in Costa Rican animal feed. Int. J. Food Contam. 2019, 6. [Google Scholar] [CrossRef]
- Witaszak, N.; Waśkiewicz, A.; Bocianowski, J.; Stępień, Ł. Contamination of pet food with mycobiota and Fusarium mycotoxins—Focus on dogs and cats. Toxins 2020, 12, 130. [Google Scholar] [CrossRef]
- Shao, M.; Li, L.; Gu, Z.; Yao, M.; Xu, D.; Fan, W.; Yan, L.; Song, S. Mycotoxins in commercial dry pet food in China. Food Addit. Contam. 2018, 11, 237–245. [Google Scholar] [CrossRef]
- Tegzes, J.H.; Oakley, B.B.; Brennan, G. Comparison of mycotoxin concentrations in grain versus grain-free dry and wet commercial dog food. Toxicol. Commun. 2019, 3, 61–66. [Google Scholar] [CrossRef]
- Witaszak, N.; Stępień, Ł.; Bocianowski, J.; Waśkiewicz, A. Fusarium species and mycotoxins contaminating veterinary diets for dogs and cats. Microorganisms 2019, 7, 26. [Google Scholar] [CrossRef]
- Macías-Montes, A.; Rial-Berriel, C.; Acosta-Dacal, A.; Henríquez-Hernández, L.A.; Almeida-González, M.; Rodríguez-Hernández, A.; Zumbado, M.; Boada, L.D.; Zaccaroni, A.; Luzardo, O.P. Risk assessment of the exposure to mycotoxins in dogs and cats through the consumption of commercial dry food. Sci. Total Environ. 2019, 708, 134592. [Google Scholar] [CrossRef]
- Okuma, T.A.; Huynh, T.P.; Hellberg, R.S. Use of enzyme-linked immunosorbent assay to screen for aflatoxins, ochratoxin A, and deoxynivalenol in dry pet foods. Mycotoxin Res. 2018, 34, 69–75. [Google Scholar] [CrossRef]
- Gazzotti, T.; Biagi, G.; Pagliuca, G.; Pinna, C.; Scardilli, M.; Grandi, M.; Zaghini, G. Occurrence of mycotoxins in extruded commercial dog food. Anim. Feed Sci. Technol. 2015, 202, 81–89. [Google Scholar] [CrossRef]
- De Leo, F.; Coluccia, B.; Miglietta, P.P.; Serio, F. Food contact materials recalls and international trade relations: An analysis of the nexus between RASFF notifications and product origin. Food Control 2021, 120, 107518. [Google Scholar] [CrossRef]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 25 December 2020).
- RASFF. RASFF Portal. 2020. Available online: https://www.ec.europa.eu/food/safety/rasff_en (accessed on 25 December 2020).
- RASFF. RASFF Portal. 2020. Available online: https://webgate.ec.europa.eu/rasff-window/portal/?event=SearchForm&cleanSearch=1 (accessed on 25 December 2020).
- Nemser, S.M.; Doran, T.; Grabenstein, M.; McConnell, T.; McGrath, T.; Pamboukian, R.; Smith, A.C.; Achen, M.; Danzeisen, G.; Kim, S.; et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis. 2014, 11, 706–709. [Google Scholar] [CrossRef]
- Kowalska, A.; Manning, L. Using the rapid alert system for food and feed: Potential benefits and problems on data interpretation. Crit. Rev. Food Sci. Nutr. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Petróczi, A.; Nepusz, T.; Naughton, D.P. The Procrustean bed of EU food safety notifications via the Rapid Alert System for Food and Feed: Does one size fit all? Food Chem. Toxicol. 2013, 56, 411–418. [Google Scholar] [CrossRef]
- D’Amico, P.; Armani, A.; Castigliego, L.; Sheng, G.; Gianfaldoni, D.; Guidi, A. Seafood traceability issues in Chinese food business activities in the light of the European provisions. Food Control 2014, 35, 7–13. [Google Scholar] [CrossRef]
| Mycotoxin | Pet Foods Product | Guide Value in mg/kg for a Feed with a Moisture Content of 12% |
|---|---|---|
| deoxynivalenol | cereals and cereal products with the exception of maize by-products | 8 |
| maize-by products | 12 | |
| compound feed | 5 | |
| zearalenone | cereals and cereal products with the exception of maize by-products | 2 |
| maize-by products | 3 | |
| compound feed for adult dogs and cats other than those intended for reproduction | 0.2 | |
| compound feed for puppies, kittens, dogs and cats intended for reproduction | 0.1 | |
| ochratoxin A | cereals and cereal products | 0.25 |
| compound feed for dogs and cats | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kępińska-Pacelik, J.; Biel, W. Microbiological Hazards in Dry Dog Chews and Feeds. Animals 2021, 11, 631. https://doi.org/10.3390/ani11030631
Kępińska-Pacelik J, Biel W. Microbiological Hazards in Dry Dog Chews and Feeds. Animals. 2021; 11(3):631. https://doi.org/10.3390/ani11030631
Chicago/Turabian StyleKępińska-Pacelik, Jagoda, and Wioletta Biel. 2021. "Microbiological Hazards in Dry Dog Chews and Feeds" Animals 11, no. 3: 631. https://doi.org/10.3390/ani11030631
APA StyleKępińska-Pacelik, J., & Biel, W. (2021). Microbiological Hazards in Dry Dog Chews and Feeds. Animals, 11(3), 631. https://doi.org/10.3390/ani11030631






