A New Limnonectes (Anura: Dicroglossidae) from Southern Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
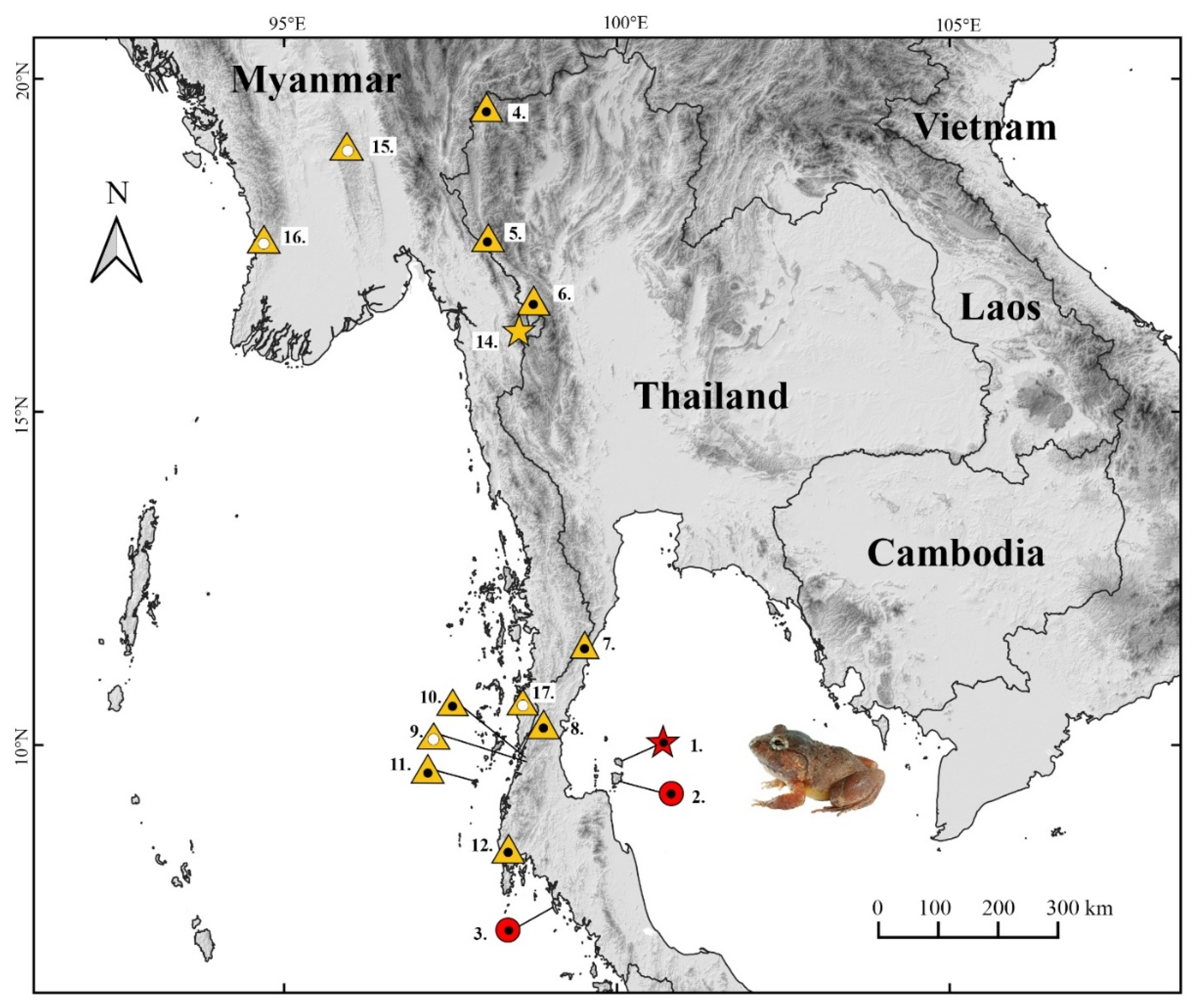
2.1. Sampling
2.2. DNA Extraction, PCR Amplification
2.3. Phylogenetic Analyses
2.4. Morphological Measurements
| SVL | snout–vent length; |
| HDL | head length from tip of snout to rear of jaws; |
| HDW | maximum head width; |
| SNT | snout length from tip of snout to anterior corner of eye; |
| EYE | eye diameter; |
| IOD | interorbital distance; |
| IND | internasal distance; |
| TMP | horizontal diameter of tympanum; |
| SHK | shank length; |
| TGH | thigh length; |
| LAL | forearm length, from elbow to base of palmar tubercle; |
| HND | manus length from tip of third digit to base of palmar tubercle; |
| FTL | pes length from tip of fourth toe to base of inner metatarsal tubercle; |
| IML | inner metatarsal tubercle length; |
| IMW | inner metatarsal tubercle width. |
2.5. Bioacoustic Analyses
3. Results
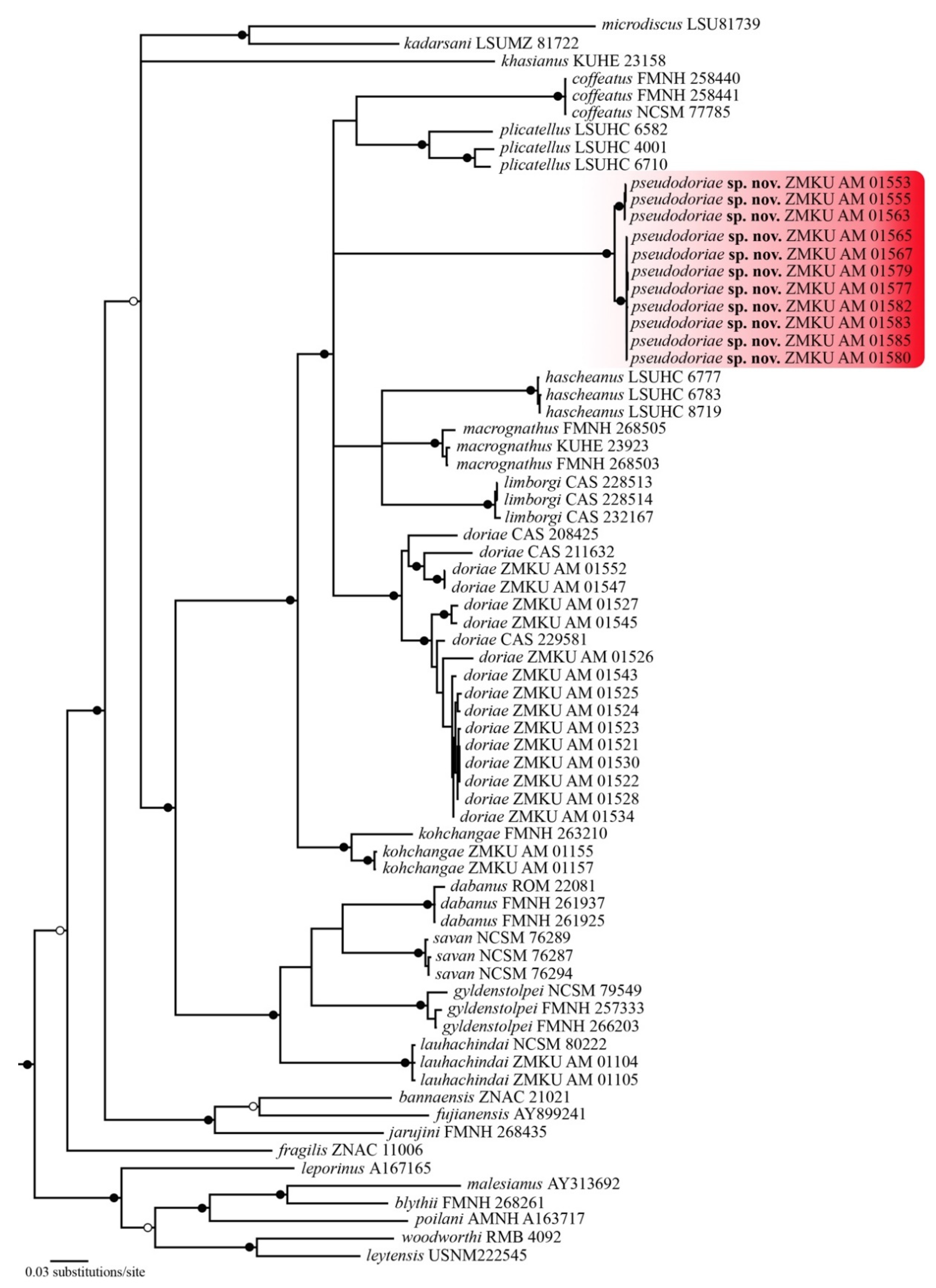
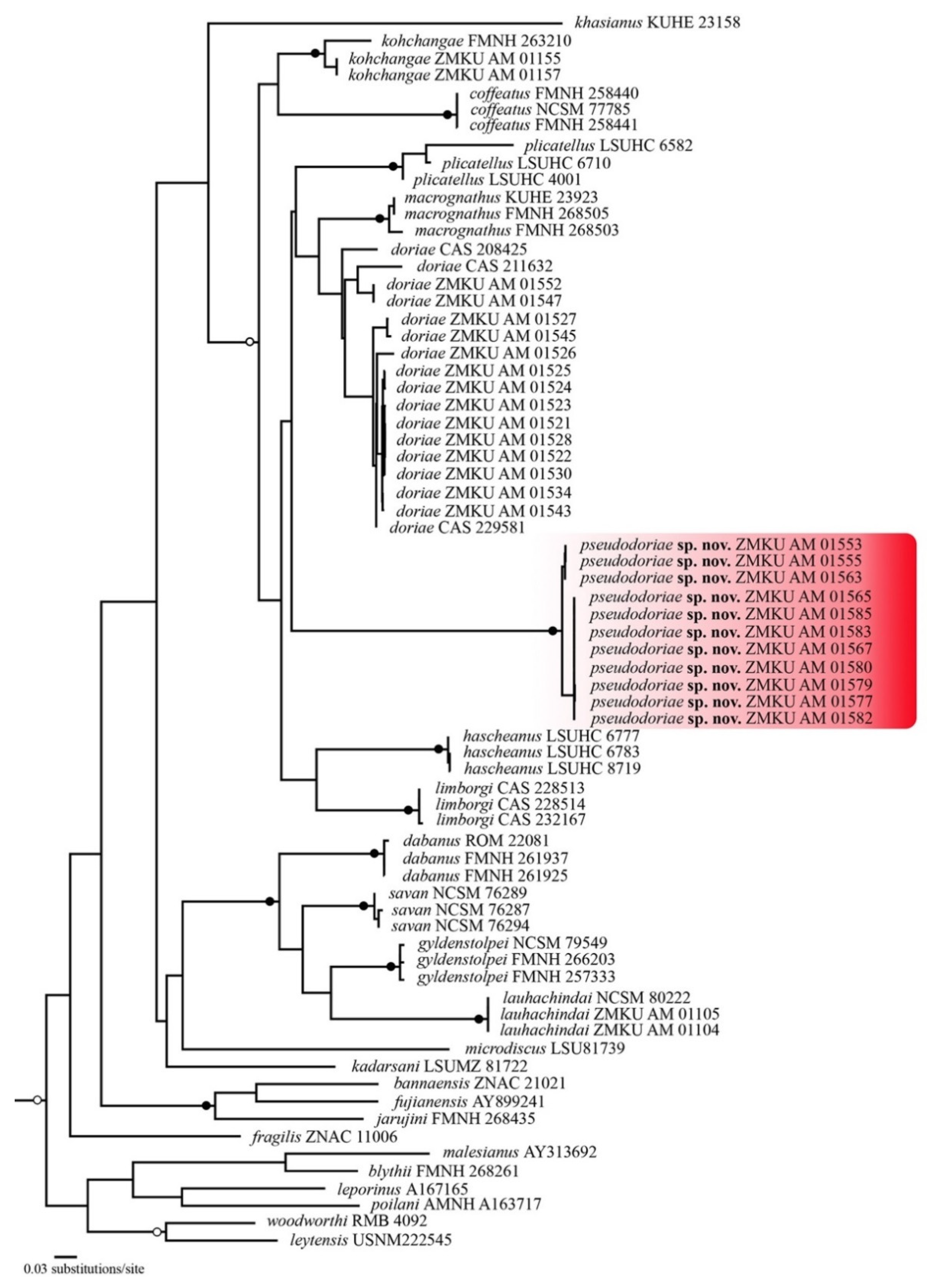
3.1. Phylogenetic Relationships
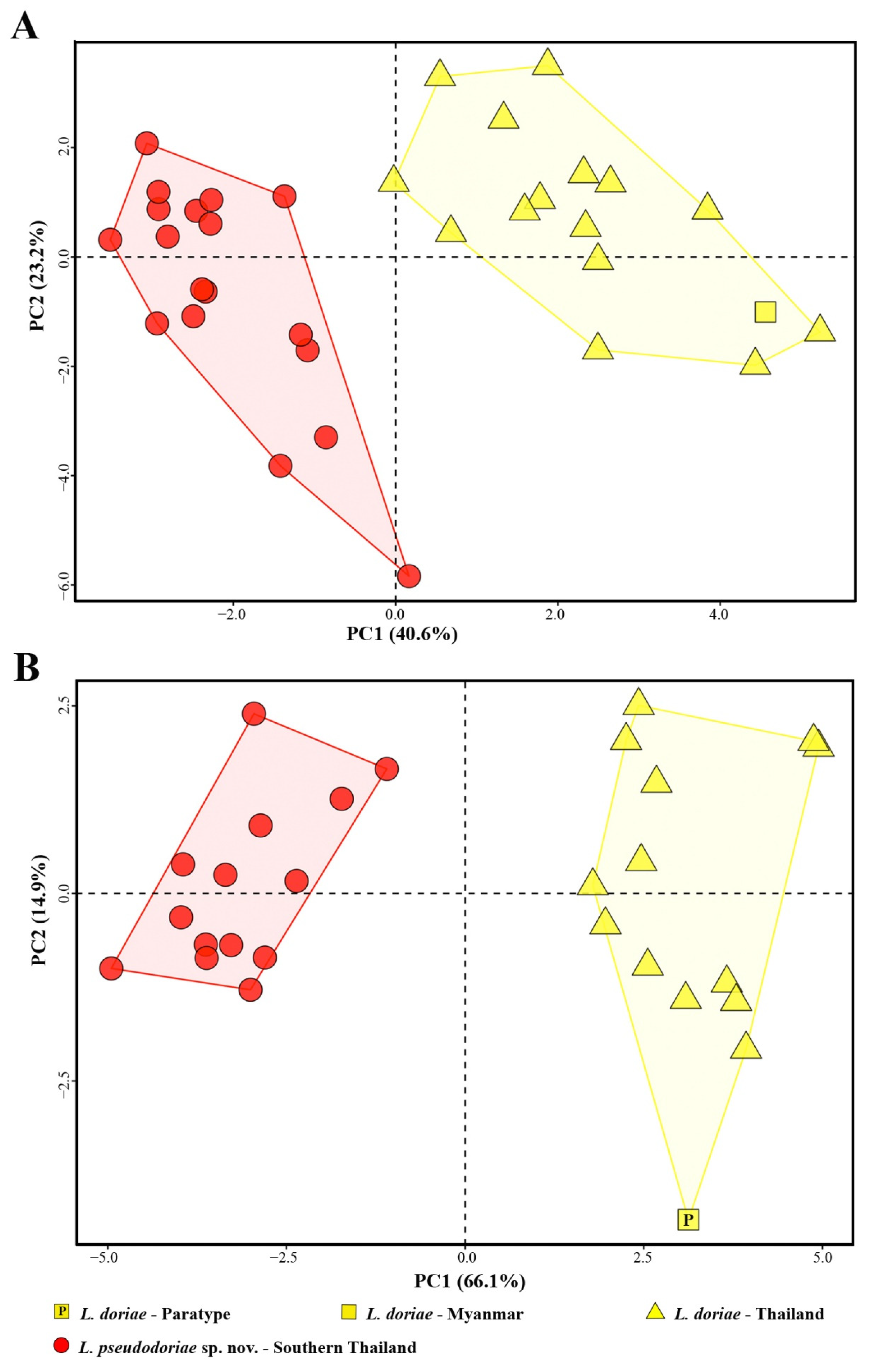
3.2. Morphology
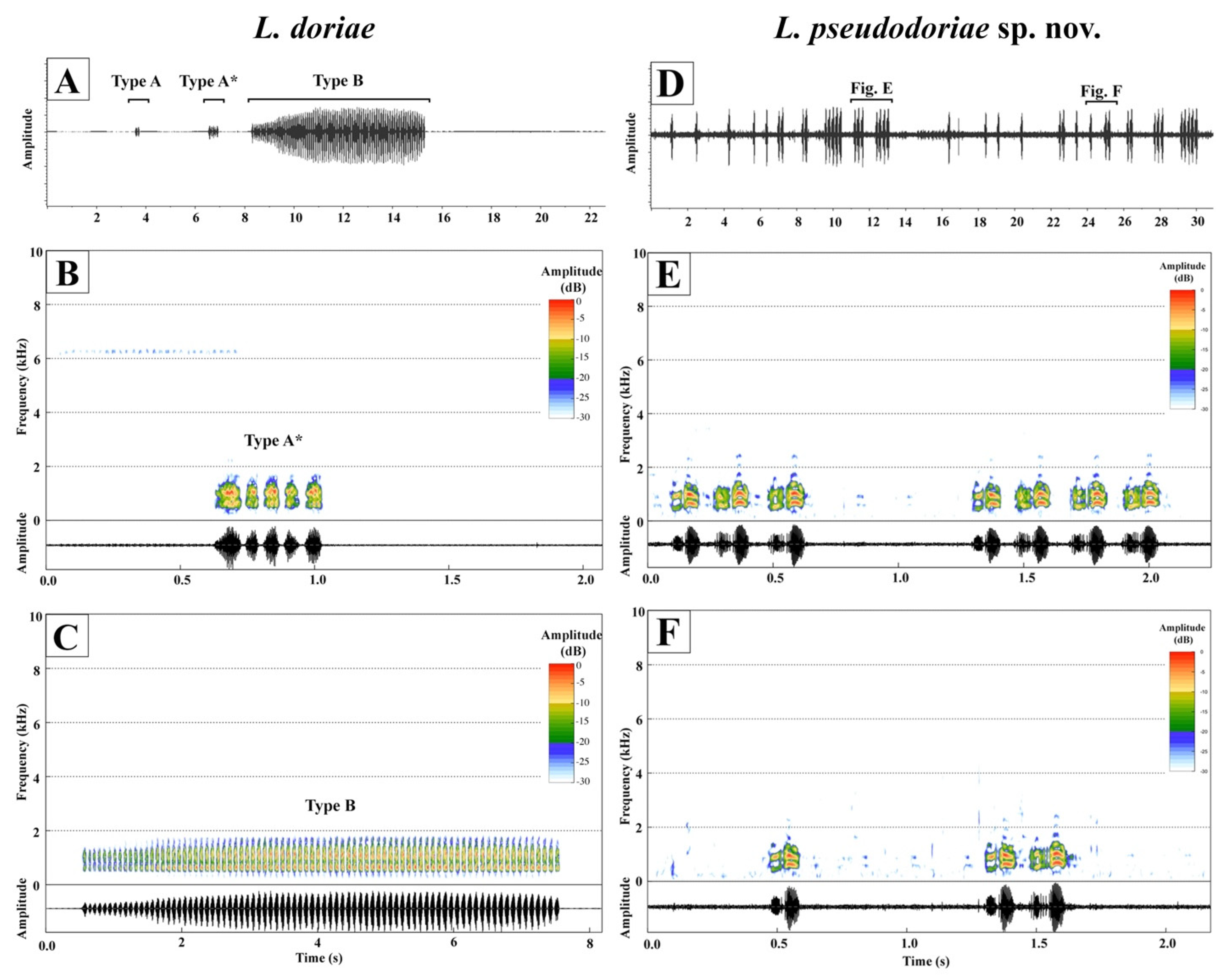
3.3. Bioacoustics
3.4. Systematic Account
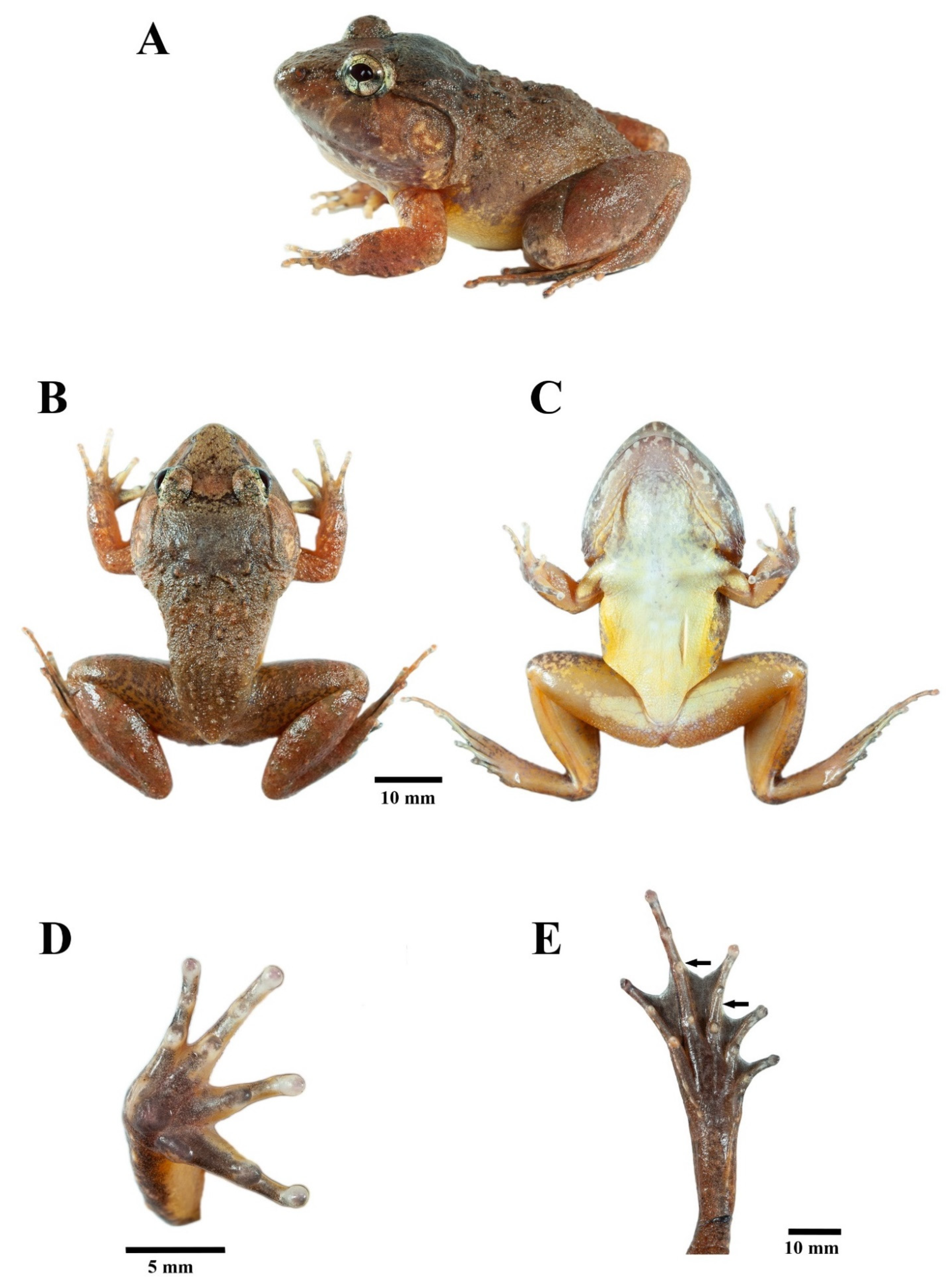
3.4.1. Diagnosis
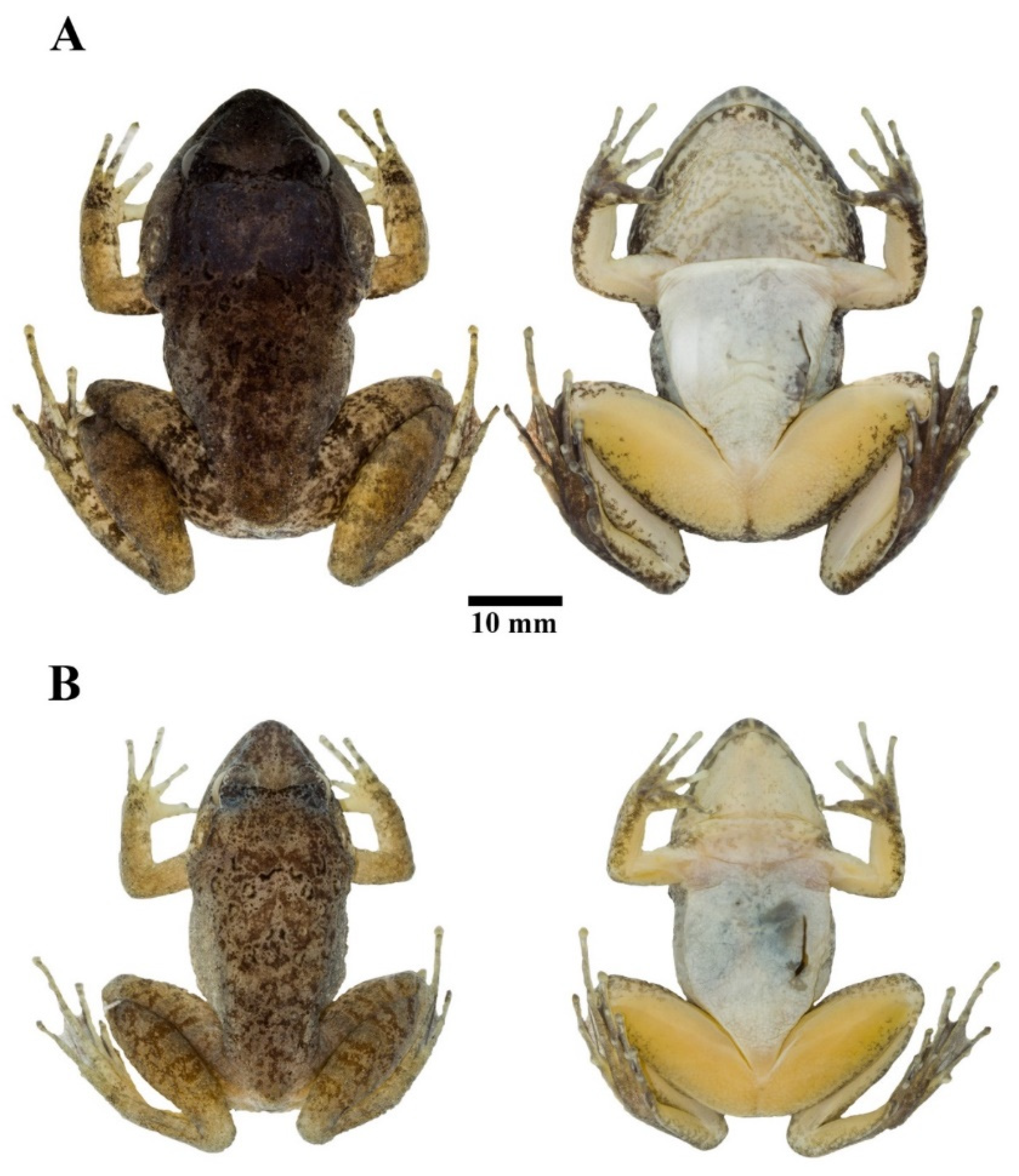
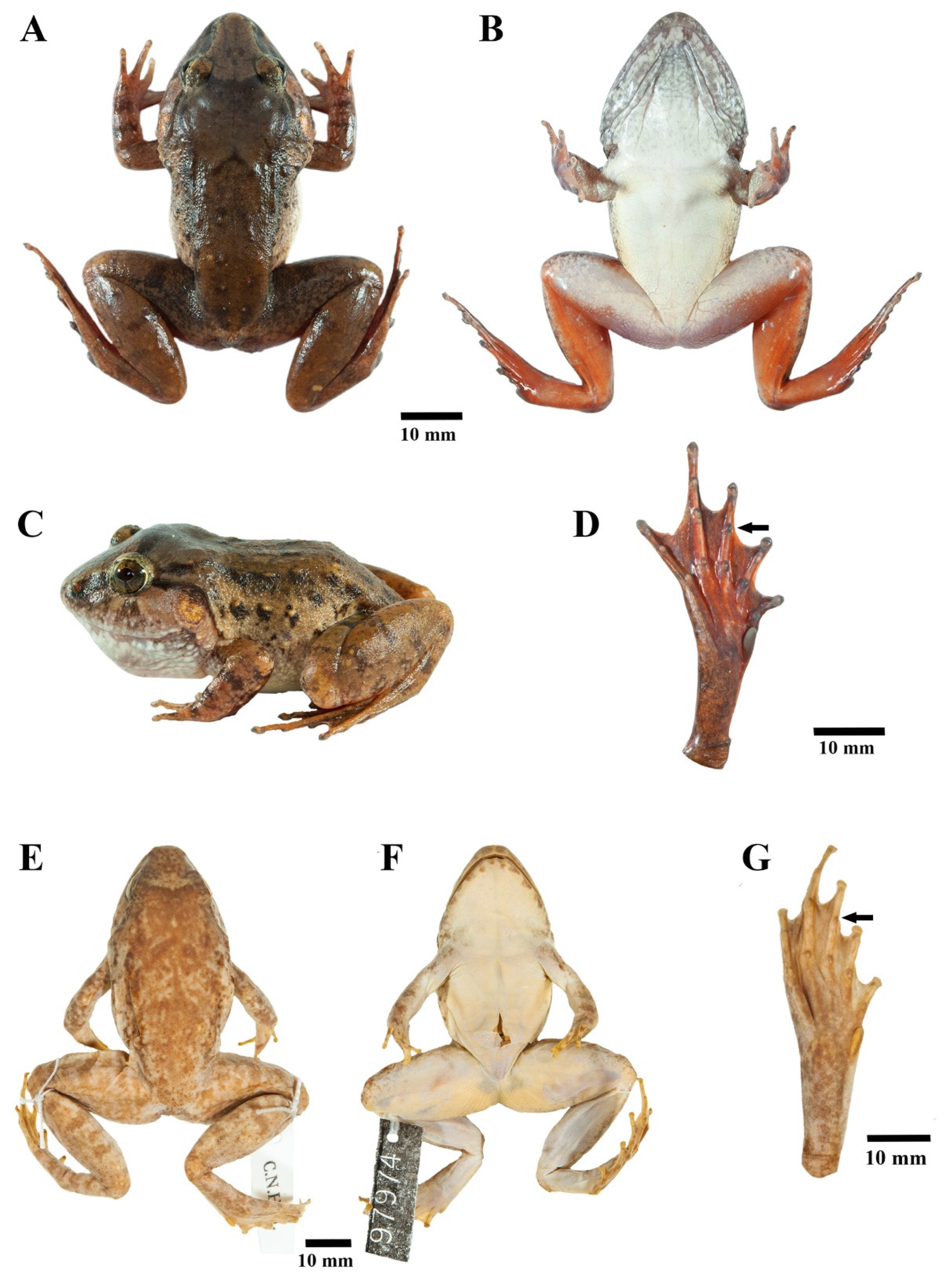
3.4.2. Description of Holotype
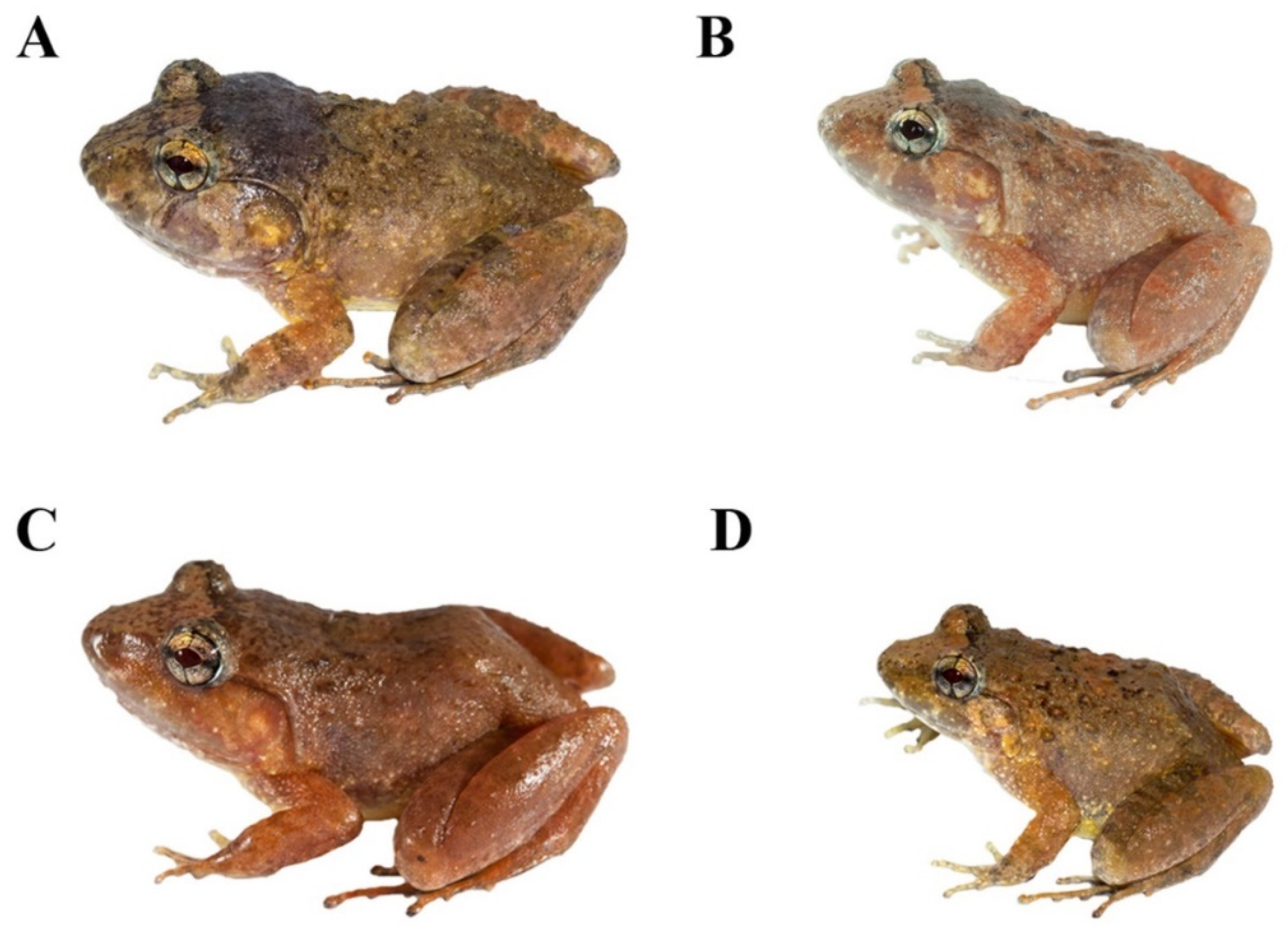
3.4.3. Color of Holotype in Life
3.4.4. Coloration of Holotype in Preservative
3.4.5. Morphological Variation
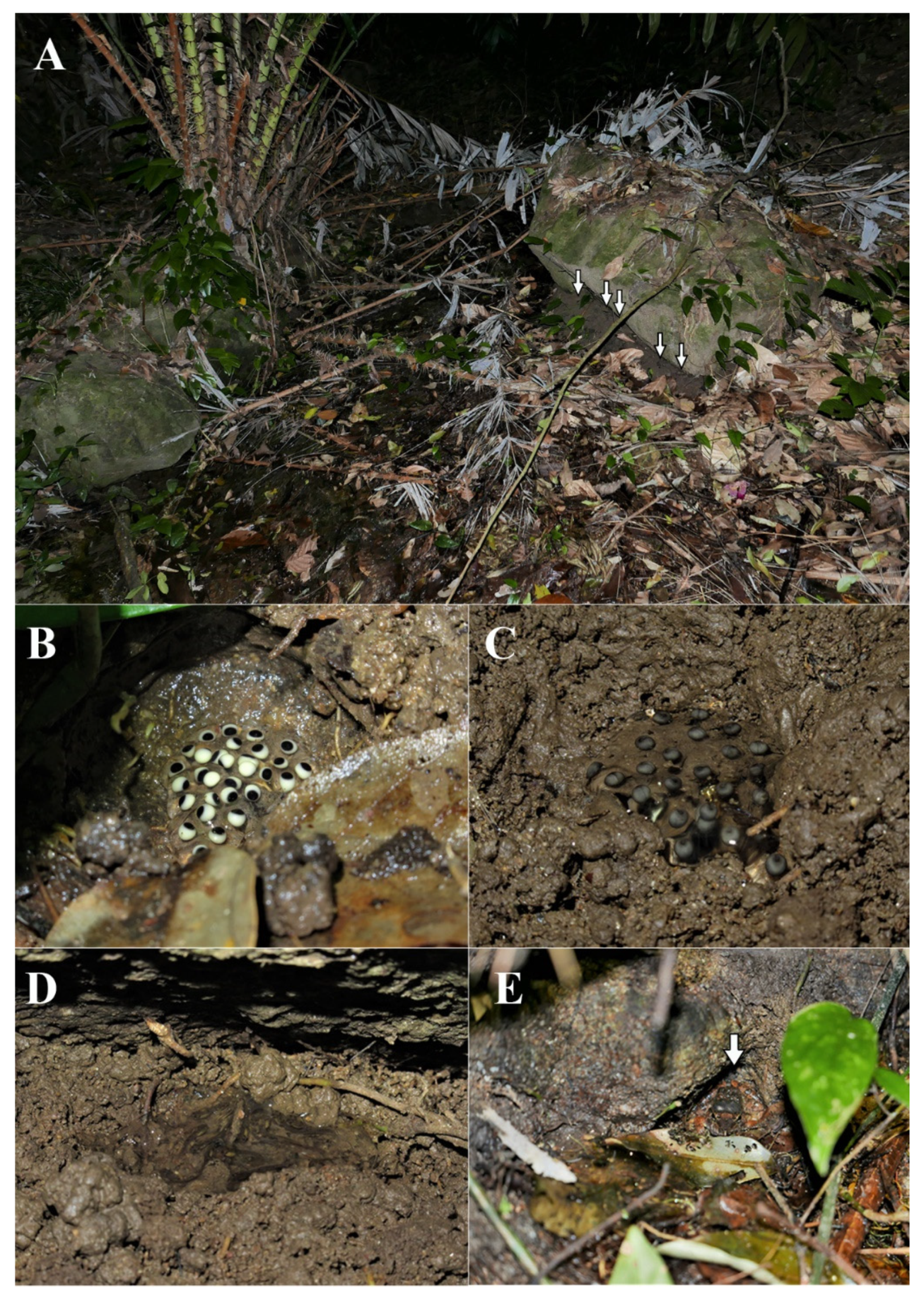
3.4.6. Habitat, Distribution, and Natural History
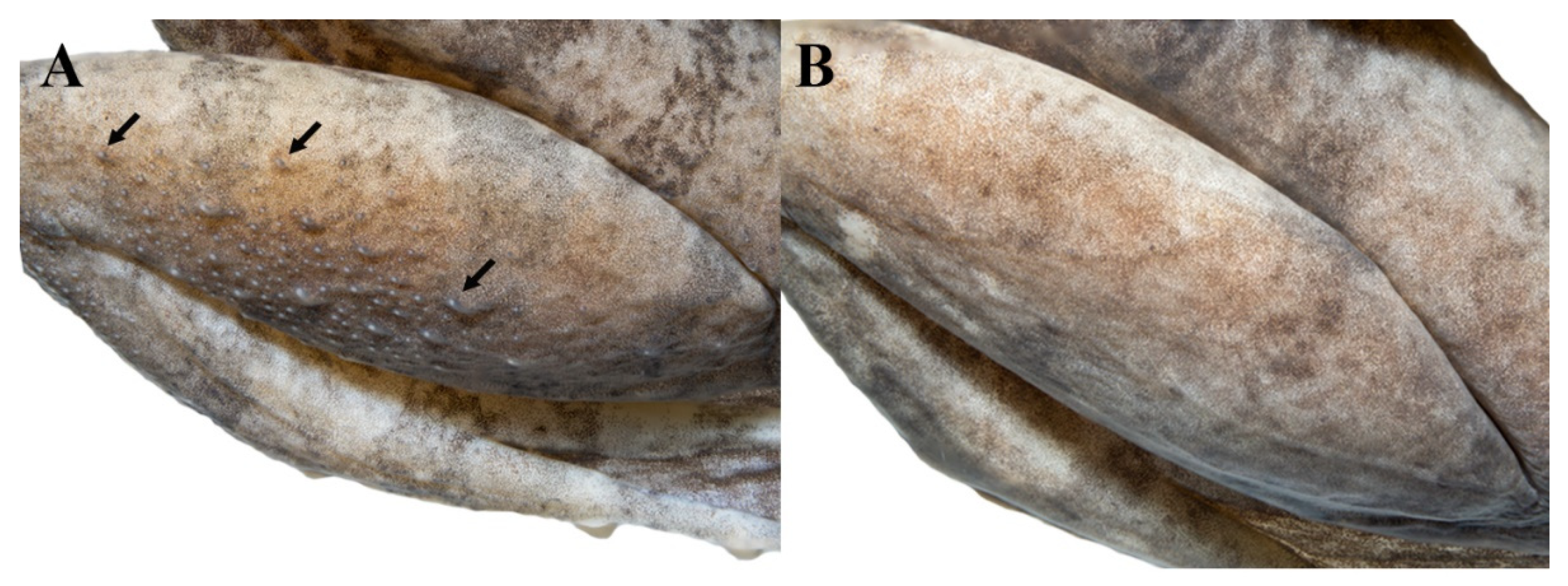
3.4.7. Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World: An Online Reference Version 6.0. 2020. Available online: http://research.amnh.org/vz/herpetology/amphibia/ (accessed on 14 December 2020).
- Boulenger, G.A. A monograph of the South Asian, Papuan, Melanesian and Australian frogs of the genus Rana. Rec. Indian Museum. 1920, 20, 1–226. [Google Scholar]
- Pope, C.H. Notes on amphibians from Fukien, Hainan, and other parts of China. Bull. Am. Mus. Nat. 1931, 61, 397–611. [Google Scholar]
- Inger, R.F. The systematic and zoogeography of the amphibia in Borneo. Fieldiana Zool. 1966, 52, 1–402. [Google Scholar]
- Emerson, S.B. A macroevolutionary study of historical contingency in the fanged frogs of Southeast Asia. Biol. J. Linn. Soc. 2001, 73, 139–151. [Google Scholar] [CrossRef]
- Lambertz, M.; Hartmann, T.; Walsh, S.; Geissler, G.; McLeod, D.S. Anatomy, histology, and systematic implications of the head ornamentation in the males of four species of Limnonectes (Anura: Dicroglossidae). Zool. J. Linn. Soc. 2014, 172, 117–132. [Google Scholar] [CrossRef]
- Rowley, J.J.L.; Le, D.T.T.; Hoang, H.D.; Altig, R. The breeding behaviour advertisement call and tadpole of Limnonectes dabanus (Anura: Dicroglossidae). Zootaxa 2014, 3881, 195–200. [Google Scholar] [CrossRef][Green Version]
- Aowphol, A.; Rujirawan, A.; Taksintum, W.; Chuaynkern, Y.; Stuart, B.L. A new caruncle-bearing Limnonectes (Anura: Dicroglossidae) from northeastern Thailand. Zootaxa 2015, 3956, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.B. Testing pattern predictions of sexual selection: A frog example. Am. Nat. 1994, 143, 848–869. [Google Scholar] [CrossRef]
- Emerson, S.B.; Inger, R.F.; Iskandar, D. Molecular systematics and biogeography of the fanged frogs of Southeast Asia. Mol. Phylogenet. Evol. 2000, 16, 131–142. [Google Scholar] [CrossRef]
- Berry, P.Y. The Amphibian Fauna of Peninsular Malaysia; Tropical Press: Kuala Lumper, Malaysia, 1975; p. 130. [Google Scholar]
- Taylor, E.H. The amphibian fauna of Thailand. Univ. Kans. sci. bull. 1962, 43, 265–599. [Google Scholar] [CrossRef]
- Chan-ard, T.A. Photographic Guide to Amphibians in Thailand; Darnsutha Press Co. Ltd.: Bangkok, Thailand, 2003; p. 175. (In Thai) [Google Scholar]
- Chuaynkern, Y.; Chuaynkern, C. A checklist of amphibians in Thailand. J. Wildl. Thail. 2012, 19, 163–211. (In Thai) [Google Scholar]
- Niyomwan, P.; Srisom, P.; Pawangkhanant, P. Amphibians of Thailand; Parbpim Limited Partnership: Bangkok, Thailand, 2019; p. 485. (In Thai) [Google Scholar]
- Inger, R.F.; Stuart, B.L. Systematics of Limnonectes (Taylorana) Dubois. Curr. Herpetol. 2010, 29, 51–86. [Google Scholar]
- Phimmachak, S.; Richards, S.J.; Sivongxay, N.; Saeteun, S.; Chuaynkern, Y.; Makchai, S.; Som, H.E.; Stuart, B.L. A caruncle-bearing fanged frog (Limnonectes, Dicroglossidae) from Laos and Thailand. Zookeys 2019, 846, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Phimmachak, S.; Sivongxay, N.; Saeteun, S.; Yodthong, S.; Rujirawan, A.; Neang, T.; Aowphol, A.; Stuart, B.L. A new Limnonectes (Anura: Dicroglossidae) from southern Laos. Zootaxa 2018, 4375, 325–340. [Google Scholar] [CrossRef]
- Simmons, J.E. Herpetological Collecting and Collections Management, 3rd ed.; Society for the Study of Amphibians and Reptiles Herpetological Circular No. 42: Salt Lake, UT, USA, 2015; p. 191. [Google Scholar]
- Chen, L.; Murphy, R.W.; Lathrop, A.; Ngo, A.; Orlov, N.L.; Ho, C.T.; Somorjai, I.L.M. Taxonomic chaos in Asian ranid frogs: An initial phylogenetic resolution. Herpetol. J. 2005, 15, 231–243. [Google Scholar]
- Palumbi, S.R. Nucleic acids II: The polymerase chain reaction. In Molecular Systematics, 2nd ed.; Hills, D.M., Moritz, C., Moble, B.K., Eds.; Sinauer Associates Ins.: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Stuart, B.L.; Chuaynkern, Y.; Chan-ard, T.; Inger, R.F. Three new species of frogs and a new tadpole from eastern Thailand. Fieldiana Zool. 2006, 111, 1–19. [Google Scholar] [CrossRef]
- Inger, R.F.; Stuart, B.L.; Iskandar, D.T. Systematics of a widespread Southeast Asian frog, Rana chalconota (Amphibia: Anura: Ranidae). Zool. J. Linn. Soc. 2009, 155, 123–147. [Google Scholar] [CrossRef]
- Evans, B.J.; Brown, R.M.; McGuire, J.A.; Supriatna, J.; Andayani, N.; Diesmos, A.; Iskandar, D.; Melnick, D.J.; Cannatella, D.C. Phylogenetics of fanged frogs: Testing biogeographical hypotheses at the interface of the Asian and Australian faunal zones. Syst. Biol. 2003, 52, 794–819. [Google Scholar] [CrossRef]
- Pyron, R.A.; Wiens, J.J. A large-scale phylogeny of amphibia including over 2800 specie, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, USA, 14 November 2010; Institute of Electrical and Electronics Engineers: New York, NY, USA, 2010; pp. 1–8. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Minh, Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.M.; Heyer, W.R. Variation and distribution in the tree-frog genus Phyllomedusa in Costa Rica, Central America. Beiträge Neotropischen Fauna 1967, 5, 111–131. [Google Scholar] [CrossRef]
- Sclater, W.L. On some specimens of frogs in the Indian Museum, Calcutta, with descriptions of several new species. Proc. Zool. Soc. Lond. 1892, 1892, 341–348. [Google Scholar]
- Stoliczka, F. Observations on some Indian and Malayan Amphibia and Reptilia. J. Asiat. Soc. Benga. 1870, 39, 134–228. [Google Scholar] [CrossRef]
- Boulenger, G.A. An account of the reptiles and batrachians obtained in Tenasserim by M. L. Fea of the Genoa Civic Museum. Ann. Mus. Civ. Stor. Nat. Genova 1887, 25, 474–486. [Google Scholar]
- Smith, M.A. The frogs allied to Rana doriae. Nat. Hist. Bull. Siam. Soc. 1922, 4, 215–225. [Google Scholar]
- Smith, M.A. The frogs allied to Rana doriae. Addendum. Nat. Hist. Bull. Siam. Soc. 1922, 4, 227–229. [Google Scholar]
- Thorpe, R.S. Quantitative handling of characters useful in snake systematics with particular reference to intraspecific variation in the Ringed Snakes Natrix natrix (L.). Biol. J. Linn. Soc. 1975, 7, 27–43. [Google Scholar] [CrossRef]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef]
- Sheridan, J.A.; Stuart, B.L. Hidden species diversity in Sylvirana nigrovittata (Amphibia: Ranidae) highlights the importance of taxonomic revisions in biodiversity conservation. PLoS ONE 2018, 13, e0192766. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Husson, F.; Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Chapman and Hall/CRC Press: New York, NY, USA, 2017; p. 262. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org (accessed on 7 February 2019).
- Sueur, J.; Aubin, T.; Simonis, C. Seewave: A free modular tool for sound analysis and synthesis. Bioacoustics 2008, 18, 213–226. [Google Scholar] [CrossRef]
- Cocroft, R.B.; Ryan, M.J. Patterns of advertisement call evolution in toads and chorus frogs. Anim. Behav. 1995, 49, 283–303. [Google Scholar] [CrossRef]
- Thomas, A.; Suyesh, R.; Biju, S.D.; Bee, M.A. Vocal behavior of the elusive purple frog of India (Nasikabatrachus sahyadrensis), a fossorial species endemic to the Western Ghats. PLoS ONE 2014, 9, e84809. [Google Scholar] [CrossRef]
- Köhler, J.; Jansen, M.; Rodríguez, A.; Kok, P.J.R.; Toledo, L.F.; Emmrich, M.; Glaw, F.; Haddad, C.F.B.; Rödel, M.O.; Vences, M. The use of bioacoustics in anuran taxonomy: Theory, terminology, methods and recommendations for best practice. Zootaxa 2017, 4251, 1–124. [Google Scholar] [CrossRef]
- Emmrich, M.; Vences, M.; Ernst, R.; Köhler, J.; Barej, M.F.; Glaw, F.; Jansen, M.; Rödel, M. A guild classification system proposed for anuran advertisement calls. Zoosyst. Evol. 2020, 96, 515–525. [Google Scholar] [CrossRef]
- Kuramoto, M.; Joshy, S.H.; Kurabayashi, A.; Sumida, M. The genus Fejervarya (Anura: Ranidae) in Central Western Ghats, India, with descriptions of four new cryptic species. Curr. Herpetol. 2007, 26, 81–105. [Google Scholar]
- Vences, M.; Köhler, J.; Crottini, A.; Glaw, F. High mitochondrial sequence divergence meets morphological and bioacoustic conservatism: Boophis quasiboehmei sp. n., a new cryptic treefrog species from south-eastern Madagascar. Bonn. Zool. Bull. 2010, 57, 241–255. [Google Scholar]
- Rowley, J.J.L.; Tran, D.T.A.; Frankham, G.J.; Dekker, A.H.; Le, D.T.T.; Nguyen, T.Q.; Dau, V.Q.; Hoang, H.D. Undiagnosed cryptic diversity in small, microendemic frogs (Leptolalax) from the Central Highlands of Vietnam. PLoS ONE 2015, 10, e0128382. [Google Scholar] [CrossRef]
- Dufresnes, C.; Mazepa, G.; Rodrigues, N.; Brelsford, A.; Litvinchuk, S.N.; Sermier, R.; Lavanchy, G.; Betto-Colliard, C.; Blaser, O.; Borzée, A.; et al. Genomic evidence for cryptic speciation in tree frogs from the Apennine Peninsula, with description of Hyla perrini sp. nov. Front. Ecol. Evol. 2018, 6, 144. [Google Scholar] [CrossRef]
- Well, K.D. The social behavior of anuran amphibians. Anim. Behav. 1977, 25, 666–693. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; The Johns Hopkins University Press: London, UK, 1994; p. 670. [Google Scholar]
- Schneider, H.; Sinsch, U. Contributions of bioacoustics to the taxonomy of the Anura. In Amphibian Biology; Heatwole, H., Tyler, M.J., Eds.; Surrey Beatty & Sons: Chipping Norton, UK, 2007; pp. 2893–2932. [Google Scholar]
- Rowley, J.J.L.; Stuart, B.L.; Thy, N.; Emmett, D.A. A new species of Leptolalax (Anura: Megophryidae) from northeastern Cambodia. Zootaxa 2010, 2567, 57–68. [Google Scholar] [CrossRef]
- Rujirawan, A.; Stuart, B.L.; Aowphol, A. A new tree frog in the genus Polypedates (Anura: Rhacophoridae) from southern Thailand. Zootaxa 2013, 3702, 545–565. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.C.; Wang, T.L.; Zhai, X.F.; Wang, J.C. Call characteristics of two sympatric and morphologically similar tree frog species, Polypedates megacephalus and Polypedates mutus (Anura: Rhacophoridae), from Hainan, China. Asian Herpetol. Res. 2018, 9, 240–249. [Google Scholar]
- Stuart, B.L.; Som, H.E.; Neang, T.; Hoang, H.D.; Le, D.T.T.; Dau, V.Q.; Potter, K.; Rowley, J.J.L. Integrative taxonomic analysis reveals a new species of Leptobrachium (Anura: Megophryidae) from north-eastern Cambodia and central Vietnam. J. Nat. Hist. 2020, 54, 225–255. [Google Scholar] [CrossRef]
- Grismer, L.L.; Grismer, J.L.; Wood Jr, P.L.; Ngo, V.T.; Neang, T.; Chan, K.O. Herpetology on the fringes of the sunda shelf: A discussion of discovery, taxonomy, and biogeography. Bonn. Zool. Monogr. 2011, 57, 57–97. [Google Scholar]
- Parnell, J. The biogeography of the Isthmus of Kra region: A review. Nord. J. Bot. 2013, 31, 001–015. [Google Scholar] [CrossRef]
- de Bruyn, M.; Nugroho, E.; Hossain, M.M.; Wilson, J.C.; Mather, P.B. Phylogeographic evidence for the existence of an ancient biogeographic barrier: The Isthmus of Kra Seaway. Heredity 2005, 94, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bohlena, J.; Dvořáka, T.; Šlechtaa, V.; Šlechtováa, V. Sea water shaping the freshwater biota: Hidden diversity and biogeographic history in the Paracanthocobitis zonalternans species complex (Teleostei: Nemacheilidae) in western Southeast Asia. Mol. Phylogenet. Evol. 2020, 148, 106806. [Google Scholar] [CrossRef] [PubMed]
- Bohlena, J.; Dvořáka, T.; Šlechtaa, V.; Šlechtováa, V. Resolving an unnoticed diversity within the Schistura robertsi species complex (Teleostei: Nemacheilidae) using molecules and morphology. Mol. Phylogenet. Evol. 2020, 151, 106894. [Google Scholar] [CrossRef]


| Limnonectes Species | pseudodoriae sp. nov. | doriae | limborgi | hascheanus | macrognathus | plicatellus | kohchangae | coffeatus | lauhachindai | gyldenstolpei | dabanus | savan |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pseudodoriae sp. nov. (n = 11) | 0.6 (0.0–1.3) | |||||||||||
| doriae (n = 17) | 11.8 (10.6–13.8) | 2.3 (0.0–5.9) | ||||||||||
| limborgi (n = 3) | 8.8 (8.6–9.4) | 6.6 (6.0–8.1) | 0.4 (0.0–0.6) | |||||||||
| hascheanus (n = 3) | 10.3 (10.1–10.8) | 7.6 (7.1–8.6) | 7.6 (7.5–7.7) | 0.0 (0.0) | ||||||||
| macrognathus (n = 3) | 12.6 (11.9–13.3) | 7.8 (7.2–9.7) | 4.9 (4.7–5.3) | 6.4 (6.2–6.7) | 0.5 (0.0–0.8) | |||||||
| plicatellus (n = 3) | 12.8 (12.6–13.3) | 8.7 (8.1–10.6) | 8.7 (8.0–9.8) | 9.7 (9.1–10.2) | 9.7 (8.6–10.5) | 3.8 (1.5–5.1) | ||||||
| kohchangae (n = 3) | 11.6 (10.8–13.1) | 8.7 (7.4–10.4) | 7.1 (6.8–7.7) | 8.3 (8.0–8.6) | 9.3 (8.7–10.4) | 9.0 (8.4–10.2) | 2.4 (0.1–3.7) | |||||
| coffeatus (n = 3) | 14.9 (14.7–15.1) | 11.7 (10.7–12.9) | 10.5 (10.4–10.6) | 10.6 (10.6) | 12.2 (11.6–12.7) | 10.7 (9.6–11.4) | 10.6 (10.2–11.5) | 0.0 (0.0) | ||||
| lauhachindai (n = 3) | 12.6 (12.6) | 11.4 (11.0–13.7) | 12.3 (12.3) | 13.9 (13.9) | 11.7 (11.3–12.4) | 12.2 (11.4–13.0) | 10.6 (10.4–10.8) | 13.5 (13.5) | 0.0 (0.0) | |||
| gyldenstolpei (n = 3) | 16.6 (16.2–17.1) | 14.7 (13.6–16.6) | 12.7 (12.6–13.0) | 12.8 (12.8) | 15.3 (14.7–15.8) | 14.9 (13.9–15.5) | 13.8 (13.4–14.4) | 16.4 (16.2–16.5) | 7.2 (7.1–7.5) | 1.0 (0.4–1.5) | ||
| dabanus (n = 3) | 15.9 (15.3–16.5) | 13.4 (12.3–15.1) | 12.3 (11.3–13.2) | 12.0 (11.3–12.8) | 14.1 (13.4–14.8) | 13.7 (13.2–14.7) | 12.3 (11.8–13.2) | 15.2 (14.9–15.6) | 6.6 (6.1–7.1) | 8.5 (8.2–9.0) | 0.3 (0.0–0.5) | |
| savan (n = 3) | 17.0 (16.6–17.1) | 13.1 (12.0–14.8) | 11.9 (11.7–12.1) | 12.2 (12.0–12.2) | 14.1 (13.3–14.7) | 13.7 (12.6–14.9) | 13.4 (13.1–13.8) | 15.6 (15.5–15.7) | 6.7 (6.6–6.7) | 8.3 (8.1–8.4) | 6.6 (6.1–7.1) | 0.2 (0.1–0.3) |
| Limnonectes Species | pseudodoriae sp. nov. | doriae | limborgi | hascheanus | kohchangae | gyldenstolpei | dabanus | fragilis | fujiangensis | banaensis | jarujini | blythii |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pseudodoriae sp. nov. (n = 11) | 0.9 (0.0–2.0) | |||||||||||
| doriae (n = 15) | 18.7 (17.4–19.3) | 3.8 (0.0–8.8) | ||||||||||
| limborgi (n = 3) | 19.9 (19.6–20.0) | 15.0 (14.2–15.8) | 0.3 (0.0–0.5) | |||||||||
| hascheanus (n = 3) | 20.2 (19.6–20.6) | 16.7 (15.6–17.2) | 15.9 (15.8–15.9) | 0.3 (0.3–0.4) | ||||||||
| kohchangae (n = 1) | 19.6 (19.1–19.9) | 14.5 (14.3–15.2) | 16.1 (16.1–16.2) | 17.1 (17.1–17.2) | - | |||||||
| gyldenstolpei (n = 1) | 22.2 (22.2–22.3) | 17.3 (16.3–18.5) | 20.0 (20.0–20.1) | 20.4 (20.3–20.5) | 19.7 (19.7) | - | ||||||
| dabanus (n = 1) | 22.6 (22.5–22.6) | 18.5 (18.1–18.9) | 20.6 (20.4–20.7) | 19.6 (19.6–19.7) | 19.1 (19.1) | 15.8 (15.8) | - | |||||
| fragilis (n = 1) | 24.0 (23.9–24.1) | 19.7 (19.4–20.3) | 22.4 (22.4) | 21.5 (21.5–21.6) | 19.7 (19.7) | 20.0 (20.0) | 19.6 (19.6) | - | ||||
| fujiangensis (n = 1) | 23.3 (23.1–23.5) | 20.7 (20.3–21.4) | 22.1 (22.0–22.2) | 21.7 (21.6–21.8) | 20.9 (20.9) | 20.5 (20.5) | 21.6 (21.6) | 20.6 (20.6) | - | |||
| banaensis (n = 1) | 24.1 (23.9–24.2) | 20.9 (20.7–21.3) | 22.0 (21.8–22.2) | 22.2 (22.1–22.3) | 21.3 (21.3) | 20.6 (20.6) | 20.2 (20.2) | 20.5 (20.5) | 16.1 (16.1) | - | ||
| jarujini (n = 1) | 25.2 (24.9–25.3) | 21.0 (20.7–21.8) | 22.2 (22.1–22.3) | 21.4 (21.4–21.5) | 19.7 (19.7) | 20.5 (20.5) | 21.0 (21.0) | 19.7 (19.7) | 17.2 (17.2) | 16.2 (16.2) | - | |
| blythii (n = 1) | 23.5 (23.1–23.6) | 20.5 (20.2–21.2) | 22.7 (22.6–22.7) | 21.5 (21.4–21.6) | 20.7 (20.7) | 21.4 (21.4) | 21.2 (21.2) | 20.7 (20.7) | 22.4 (22.4) | 21.9 (21.9) | 21.5 (21.5) | - |
| Character | Male | Female | ||||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 1 | PC 2 | PC 3 | |
| SVLadj | 0.424 | 0.183 | −0.121 | 0.651 | −0.049 | −0.190 |
| HDLadj | −0.122 | 0.776 | 0.309 | 0.933 | 0.201 | −0.112 |
| HWLadj | −0.324 | 0.875 | 0.066 | 0.947 | −0.046 | 0.151 |
| SNTadj | −0.031 | 0.716 | 0.143 | 0.917 | 0.043 | 0.155 |
| EYEadj | 0.704 | −0.075 | 0.017 | 0.770 | −0.475 | 0.259 |
| IODadj | 0.219 | 0.699 | −0.003 | 0.866 | −0.105 | −0.102 |
| INDadj | 0.778 | 0.463 | −0.107 | 0.862 | −0.316 | 0.025 |
| SHKadj | 0.858 | 0.195 | 0.061 | 0.969 | 0.038 | −0.007 |
| TGHadj | 0.914 | 0.048 | 0.180 | 0.907 | 0.243 | −0.104 |
| LALadj | 0.008 | −0.273 | 0.878 | 0.729 | 0.336 | −0.058 |
| HNDadj | 0.653 | −0.261 | 0.240 | 0.725 | 0.541 | −0.290 |
| FTLadj | 0.899 | 0.166 | 0.270 | 0.955 | 0.113 | −0.102 |
| IMLadj | 0.860 | 0.198 | −0.054 | 0.939 | 0.071 | −0.077 |
| IMWadj | 0.433 | 0.624 | −0.351 | 0.788 | −0.199 | 0.439 |
| TMPadj | −0.773 | 0.517 | 0.162 | 0.079 | 0.865 | 0.416 |
| TMP/EYE | −0.850 | 0.431 | 0.133 | −0.471 | 0.855 | 0.093 |
| Eigenvalue | 6.503 | 3.716 | 1.255 | 10.578 | 2.387 | 0.663 |
| Percentage of variance | 40.643 | 23.228 | 7.844 | 66.110 | 14.920 | 4.142 |
| Cumulative proportion | 40.643 | 63.871 | 71.715 | 66.110 | 81.030 | 85.172 |
| Characters | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| pseudodoriae sp. nov. | doriae | t | p | pseudodoriae sp. nov. | doriae | t | p | |
| n = 18 | n = 16 | n = 14 | n =14 | |||||
| SVL | 45.2 ± 1.7 (42.6–48.2) | 47.8 ± 3.7 (41.4–55.0) | 2.513 a | 0.021 * | 40.0 ± 2.8 (36.0–44.1) | 45.00± 3.3 (39.4–50.3) | 4.282 | <0.001 * |
| HDLadj | 21.56 ± 0.78 (19.91–22.61) | 21.92 ± 1.27 (18.53–23.42) | 185.000 b | 0.162 | 16.69 ± 0.33 (16.17–17.29) | 18.38 ± 0.46 (17.71–19.12) | 11.136 | <0.001 * |
| HDWadj | 21.61 ± 1.12 (18.91–22.93) | 21.61 ± 1.02 (19.78–23.36) | 0.019 | 0.985 | 15.91 ± 0.51 (15.10–16.73) | 17.84 ± 0.47 (16.85–18.67) | 196.000 b | <0.001 * |
| SNTadj | 8.23 ± 0.40 (7.27–8.91) | 8.38 ± 0.30 (7.92–8.99) | 1.211 | 0.235 | 6.52 ± 0.20 (6.16–6.85) | 7.41 ± 0.24 (7.18–8.14) | 196.000 b | <0.001 * |
| EYEadj | 4.70 ± 0.22 (4.28–5.09) | 5.03 ± 0.28 (4.58–5.63) | 3.770 | 0.001 * | 4.34 ± 0.20 (3.98–4.70) | 4.89 ± 0.31 (4.25–5.40) | 5.641 | <0.001 * |
| IODadj | 4.76 ± 0.42 (3.88–5.23) | 5.11 ± 0.49 (4.42–6.23) | 201.000 b | 0.051 | 3.34 ± 0.20 (3.00–3.62) | 3.94 ± 0.25 (3.40–4.47) | 7.088 | <0.001 * |
| INDadj | 3.76 ± 0.21 (3.49–4.24) | 4.81 ± 0.32 (4.10–5.32) | 11.433 | <0.001 * | 3.17 ± 0.28 (2.69–3.65) | 4.32 ± 0.41 (3.65–5.17) | 8.691 | <0.001 * |
| SHKadj | 22.72 ± 0.76 (21.13–24.02) | 25.15 ± 1.23 (23.16–27.32) | 7.026 | <0.001 * | 20.04 ± 1.02 (18.55–21.96) | 23.86 ± 0.50 (23.28–25.12) | 12.588 a | <0.001 * |
| TGHadj | 23.86 ± 0.49 (22.89–24.71) | 26.03 ± 1.02 (24.40–28.16) | 7.776 a | <0.001 * | 21.59 ± 0.82 (20.41–23.07) | 24.82 ± 1.21 (22.22–26.51) | 8.283 | <0.001 * |
| LALadj | 9.87 ± 0.44 (9.06–10.59) | 9.67 ± 0.60 (8.77–10.81) | 107.500 b | 0.214 | 8.99 ± 0.29 (8.72–9.84) | 9.40 ± 0.27 (9.07–9.90) | 175.000 b | <0.001 * |
| HNDadj | 11.18 ± 0.49 (10.30–12.06) | 11.62 ± 0.44 (10.91–12.60) | 2.748 | 0.010 * | 9.87 ± 0.36 (9.29–10.52) | 10.76 ± 0.63 (9.57–11.70) | 4.543 | <0.001 * |
| FTLadj | 22.56 ± 0.45 (21.86–23.42) | 24.87 ± 1.00 (23.03–25.91) | 281.500 b | <0.001 * | 20.08 ± 0.73 (18.87–21.54) | 23.96 ± 0.90 (22.78–25.93) | 12.556 | <0.001 * |
| IMLadj | 2.94 ± 0.17 (2.65–3.24) | 3.69 ± 0.26 (3.08–4.01) | 9.991 | <0.001 * | 2.44 ± 0.17 (2.24–2.74) | 3.42 ± 0.24 (3.06–3.92) | 12.651 | <0.001 * |
| IMWadj | 1.21 ± 0.15 (0.90–1.51) | 1.45 ± 0.18 (1.18–1.76) | 4.204 | <0.001 * | 1.04 ± 0.08 (0.90–1.21) | 1.24 ± 0.11 (1.07–1.38) | 5.516 | <0.001 * |
| TMPadj | 5.82 ± 0.67 (4.16–6.50) | 5.06 ± 0.54 (4.18–6.14) | 53.000 b | 0.002 * | 3.44 ± 0.22 (3.09–3.88) | 3.44 ± 0.30 (2.88–3.91) | 0.058 | 0.058 |
| TMP/EYE | 1.24 ± 0.17 (0.82–1.43) | 1.01 ± 0.13 (0.76–1.20) | 39.500 b | <0.001 * | 0.79 ± 0.06 (0.72–0.94) | 0.71 ± 0.09 (0.52–0.84) | −2.901 | 0.007 * |
| Call Parameters | L. pseudodoriae sp. nov. nindividual = 3 | L. doriae nndividual = 1 | |
|---|---|---|---|
| Call type | ncall = 180 | Type A ncall = 6 | Type B ncall = 3 |
| Note description | two-pulsed note | non-pulsed note | |
| Call duration (ms) | 335.66 ± 333.62 (124.56–1965.07) | 403.08 ± 249.40 (151.28–820.60) | 7253.86 ± 398.90 (7012.09–7714.28) |
| Intercall interval (s) | 1.28 ± 1.52 (0.38–10.84) | 108.22 ± 189.88 (0.41–451.06) | |
| Call rate (call/min) | 37.65 ± 7.62 (32.46–46.39) | 0.56 | |
| Number of note (note) | 1.98 ± 1.62 (1–10) | 5.17 ± 3.43 (2–11) | 84.67 ± 5.51 (81–91) |
| Note duration (ms) | 139.94 ± 12.69 (117.65–183.86) | 75.83 ± 21.13 (60.88–113.15) | 75.58 ± 2.67 (72.56–77.62) |
| Internote interval (ms) | 41.42 ± 11.94 (7.97–75.05) | 11.05 ± 2.82 (7.54–14.76) | 11.44 ± 3.55 (8.48–15.37) |
| Note rate (notes/s) | 5.60 ± 0.47 (4.55–7.11) | 11.32 ± 2.02 (7.82–13.23) | 11.65 ± 0.11 (11.54–11.77) |
| Dominant frequency (kHz) | 0.89 ± 0.13 (0.52–1.03) | 0.92 ± 0.25 (0.60–1.12) | 0.98 ± 0.25 (0.69–1.12) |
| Temperature (°C) | 26.0–26.1 | 24.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yodthong, S.; Rujirawan, A.; Stuart, B.L.; Aowphol, A. A New Limnonectes (Anura: Dicroglossidae) from Southern Thailand. Animals 2021, 11, 566. https://doi.org/10.3390/ani11020566
Yodthong S, Rujirawan A, Stuart BL, Aowphol A. A New Limnonectes (Anura: Dicroglossidae) from Southern Thailand. Animals. 2021; 11(2):566. https://doi.org/10.3390/ani11020566
Chicago/Turabian StyleYodthong, Siriporn, Attapol Rujirawan, Bryan L. Stuart, and Anchalee Aowphol. 2021. "A New Limnonectes (Anura: Dicroglossidae) from Southern Thailand" Animals 11, no. 2: 566. https://doi.org/10.3390/ani11020566
APA StyleYodthong, S., Rujirawan, A., Stuart, B. L., & Aowphol, A. (2021). A New Limnonectes (Anura: Dicroglossidae) from Southern Thailand. Animals, 11(2), 566. https://doi.org/10.3390/ani11020566






