Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling and Tissue Preparation
2.3. Conventional Histochemistry
2.4. Lectin Histochemistry
2.5. Sialidase Treatment
2.6. Morphometry and Statistical Analysis
3. Results
3.1. Animals
3.2. Morphometry
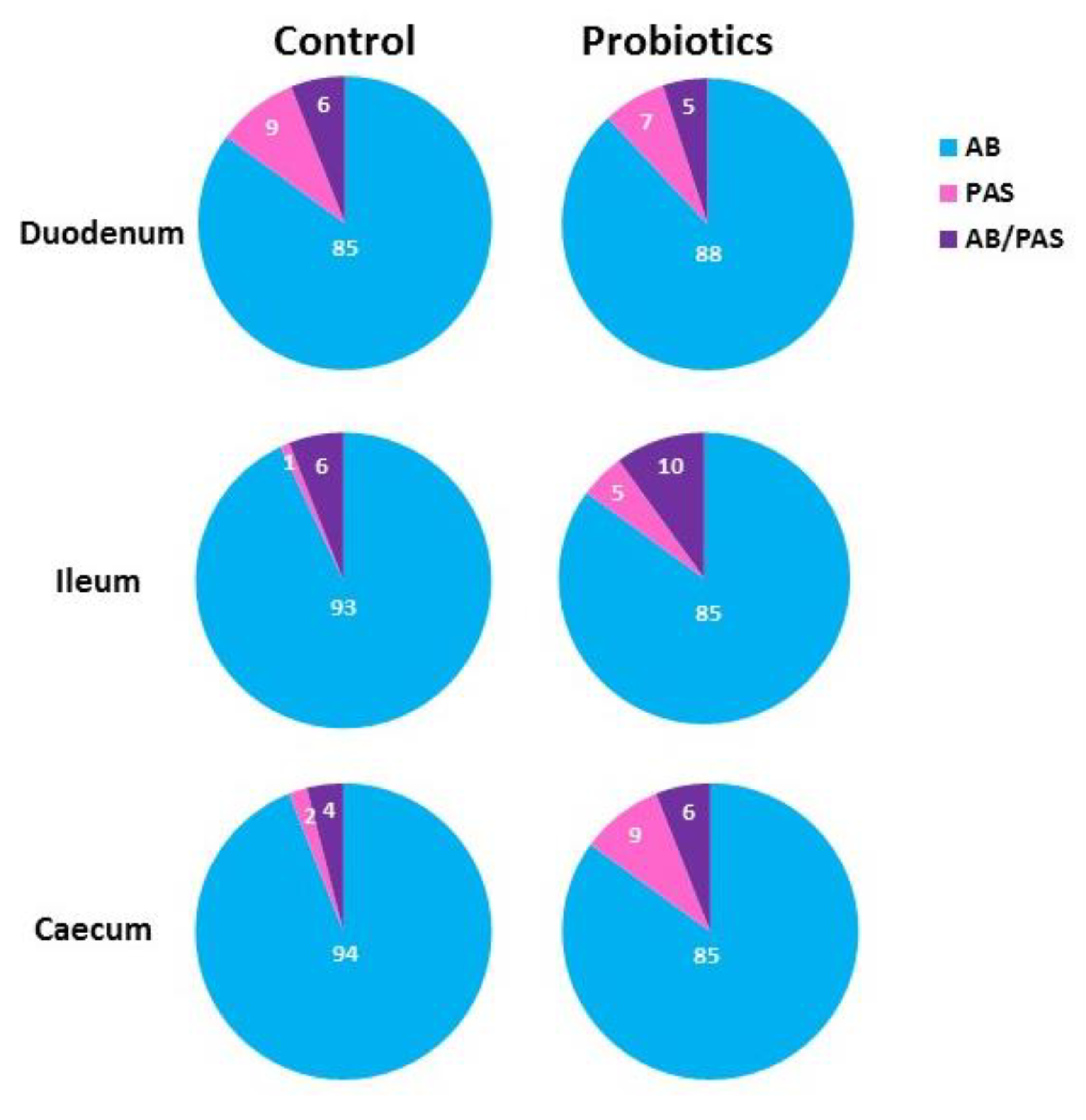
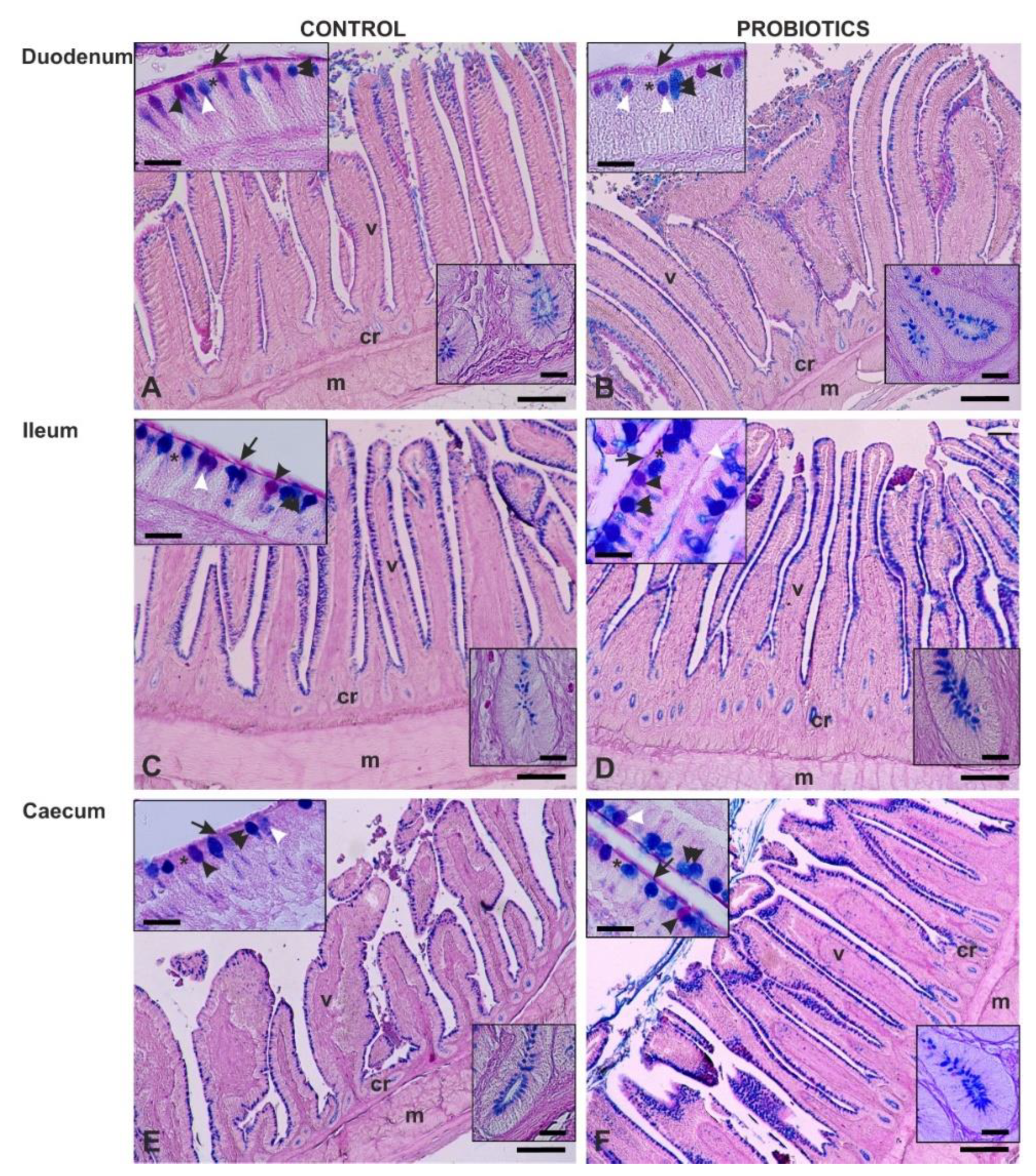
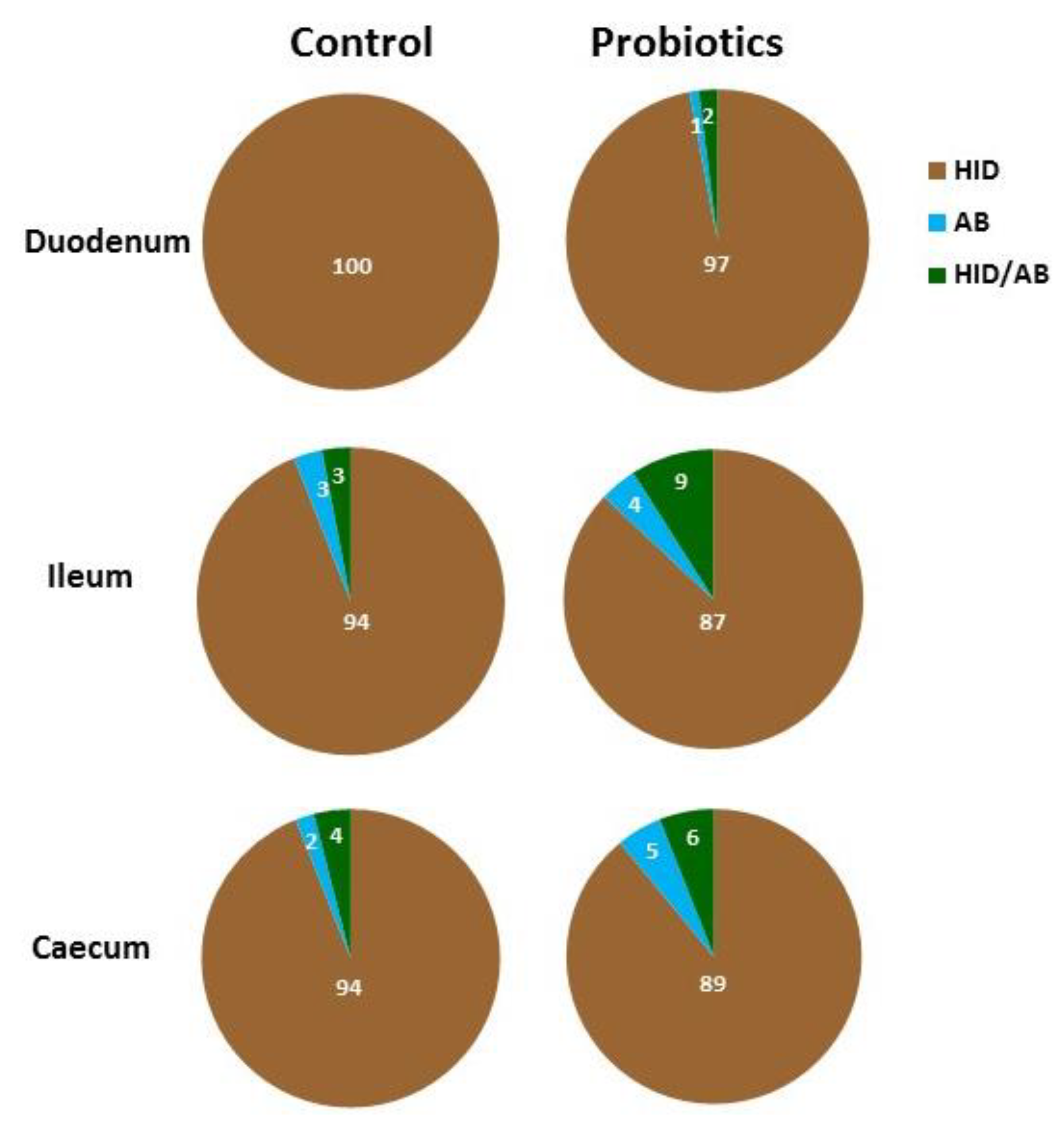
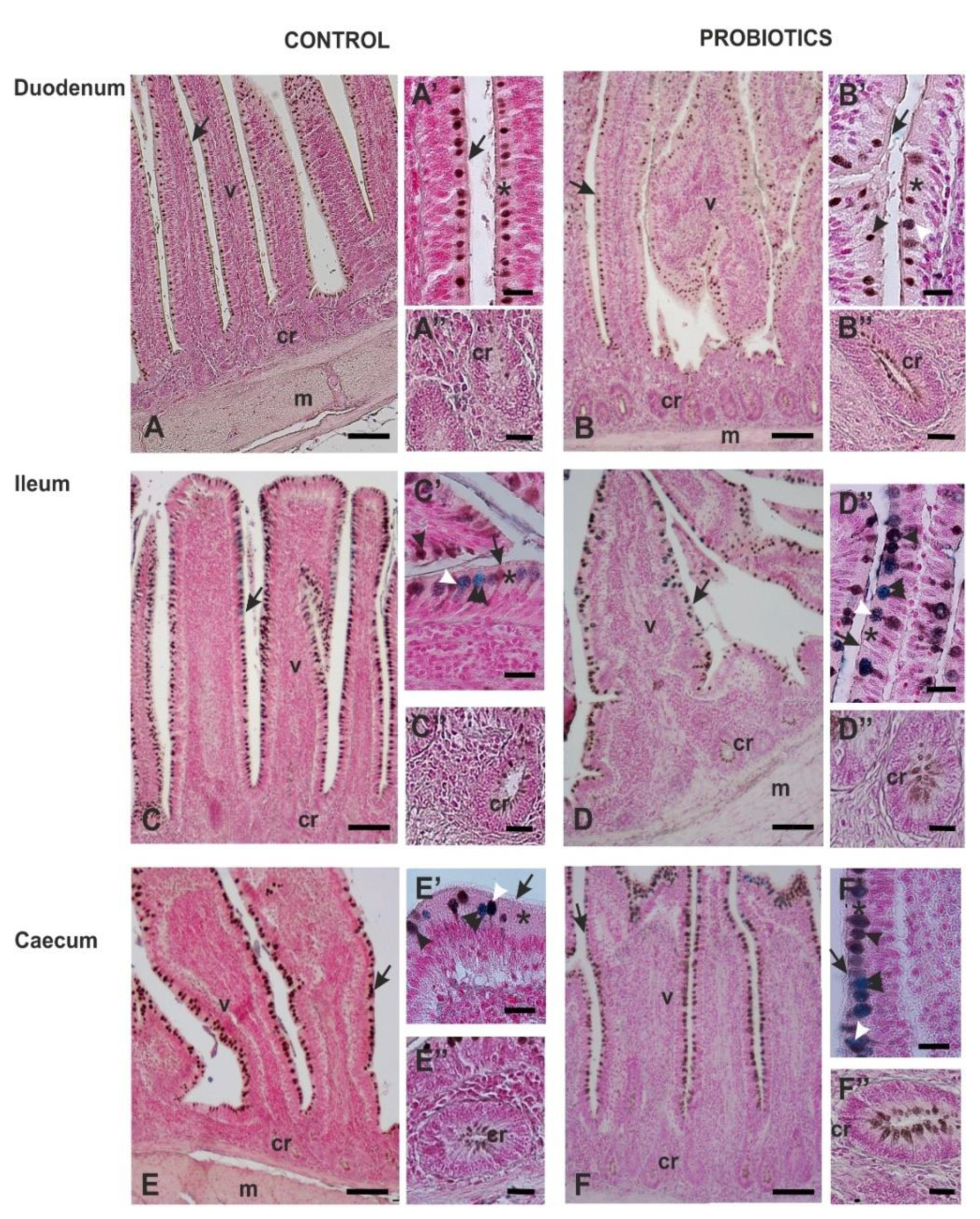
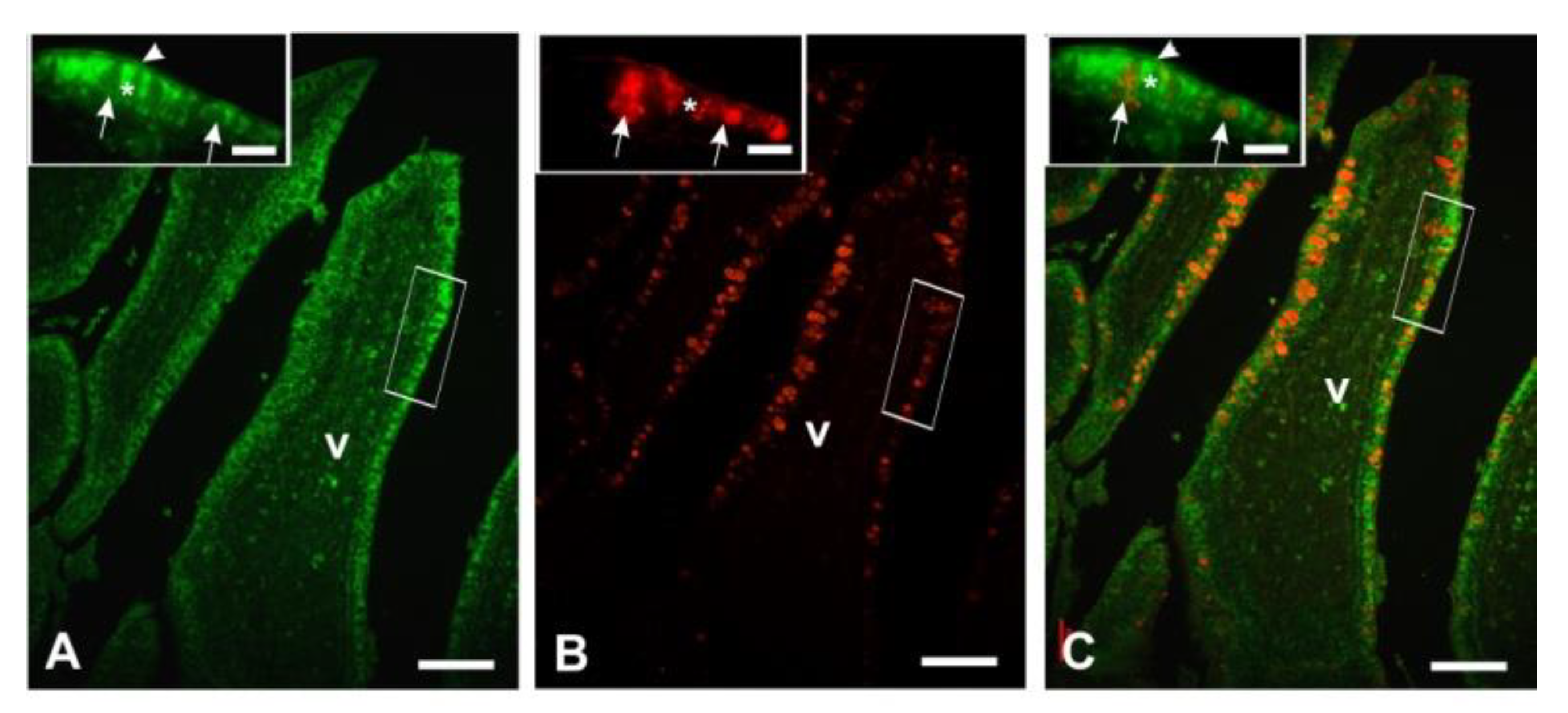
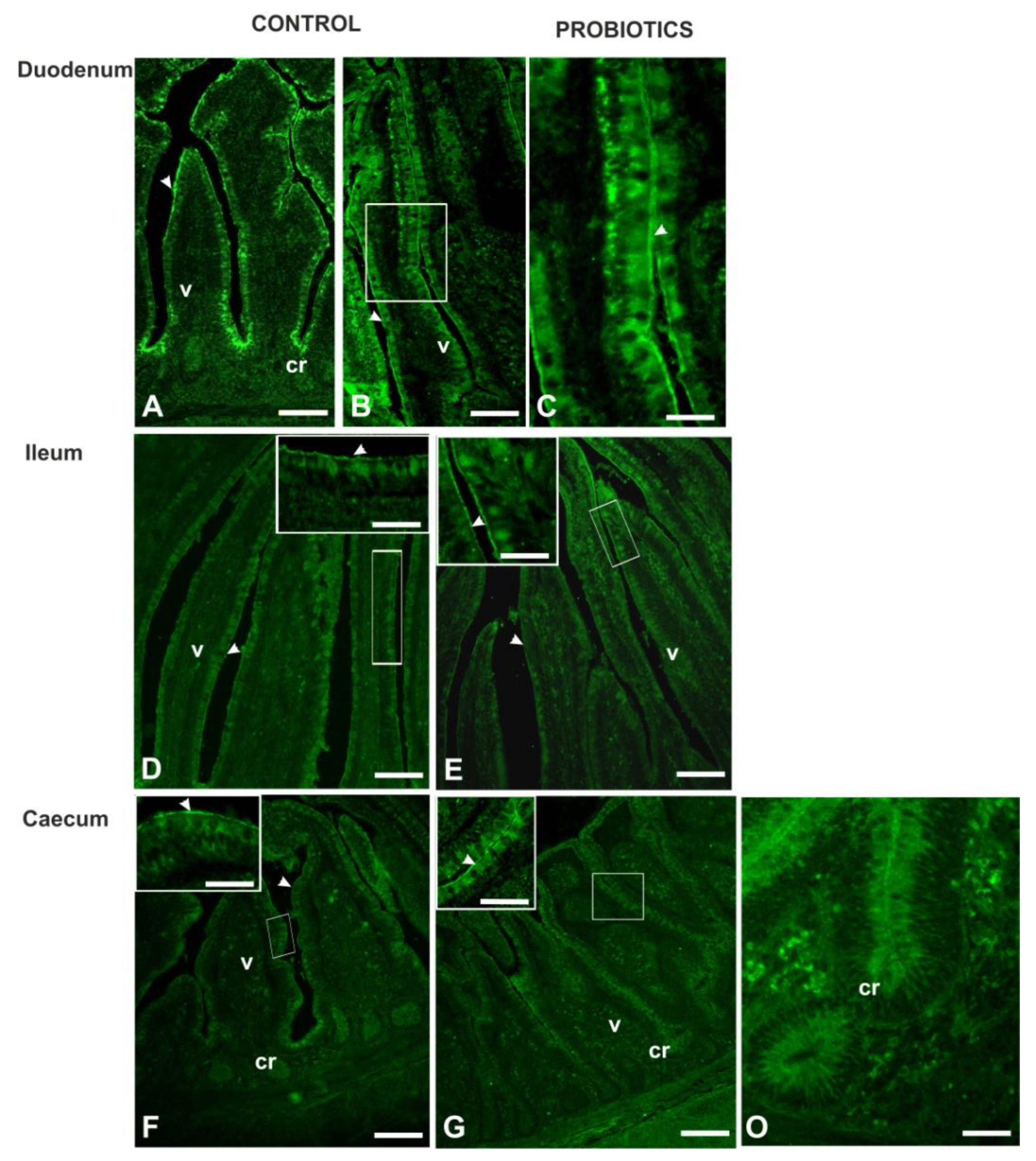
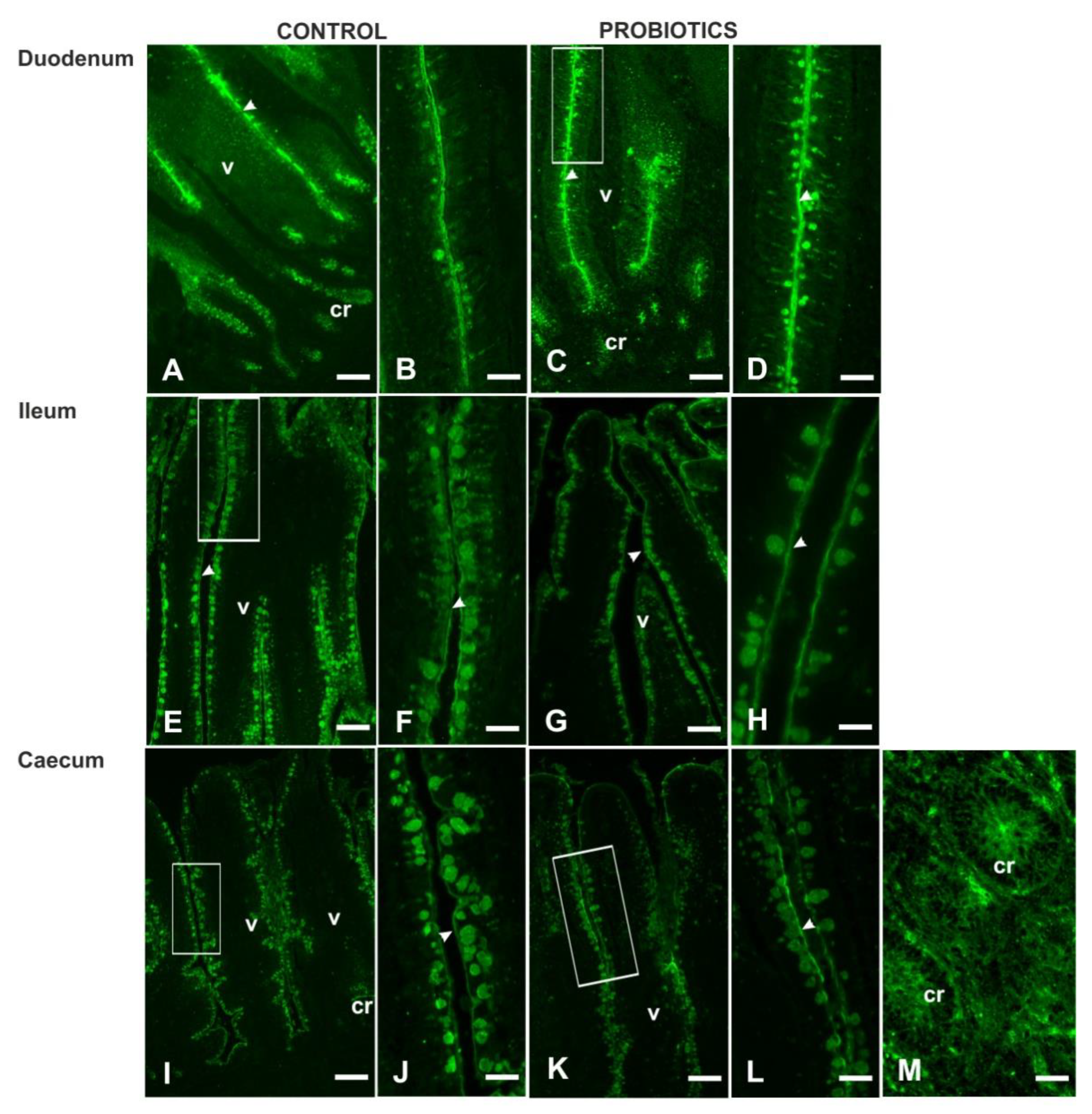
3.3. Histochemistry
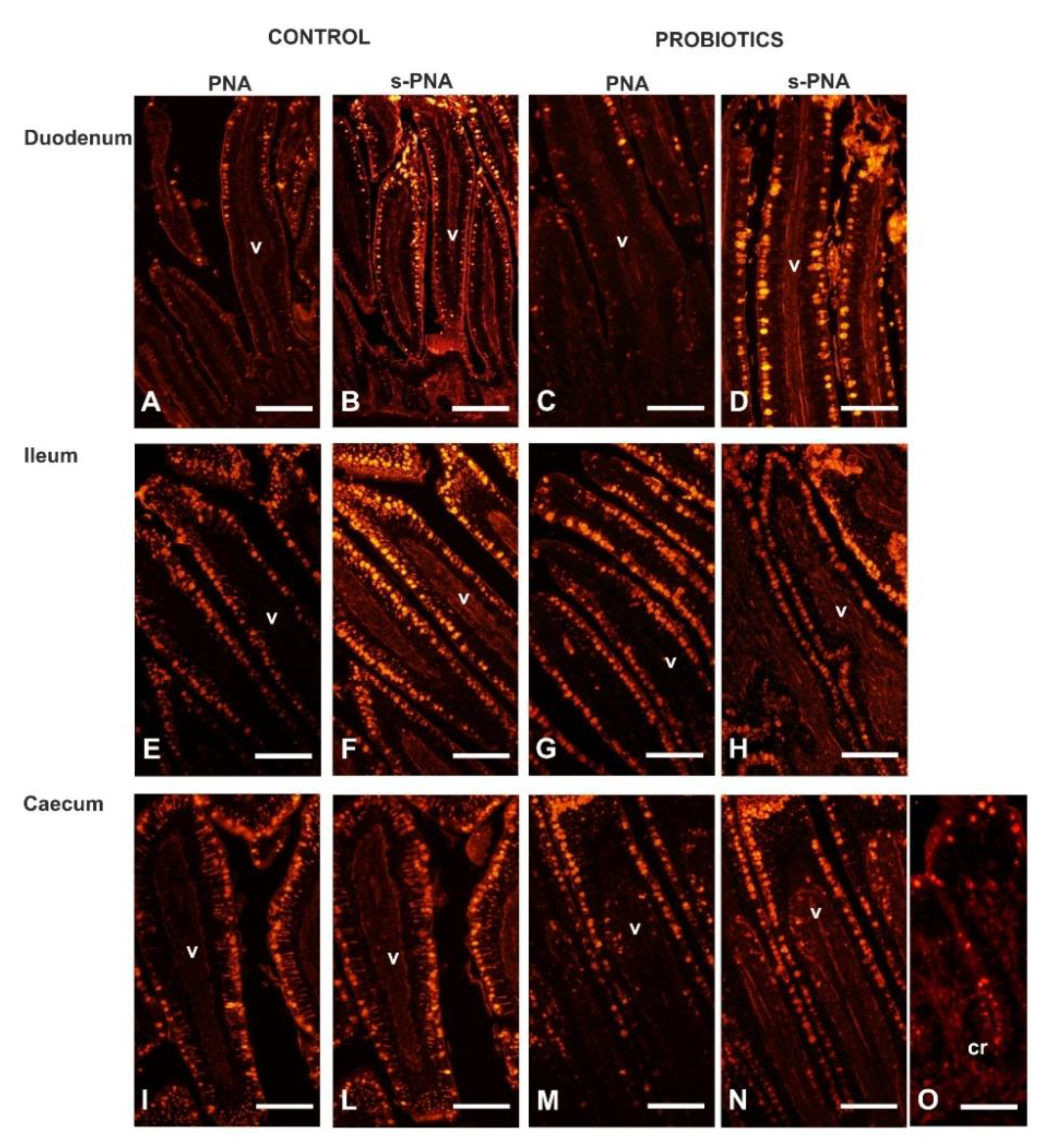
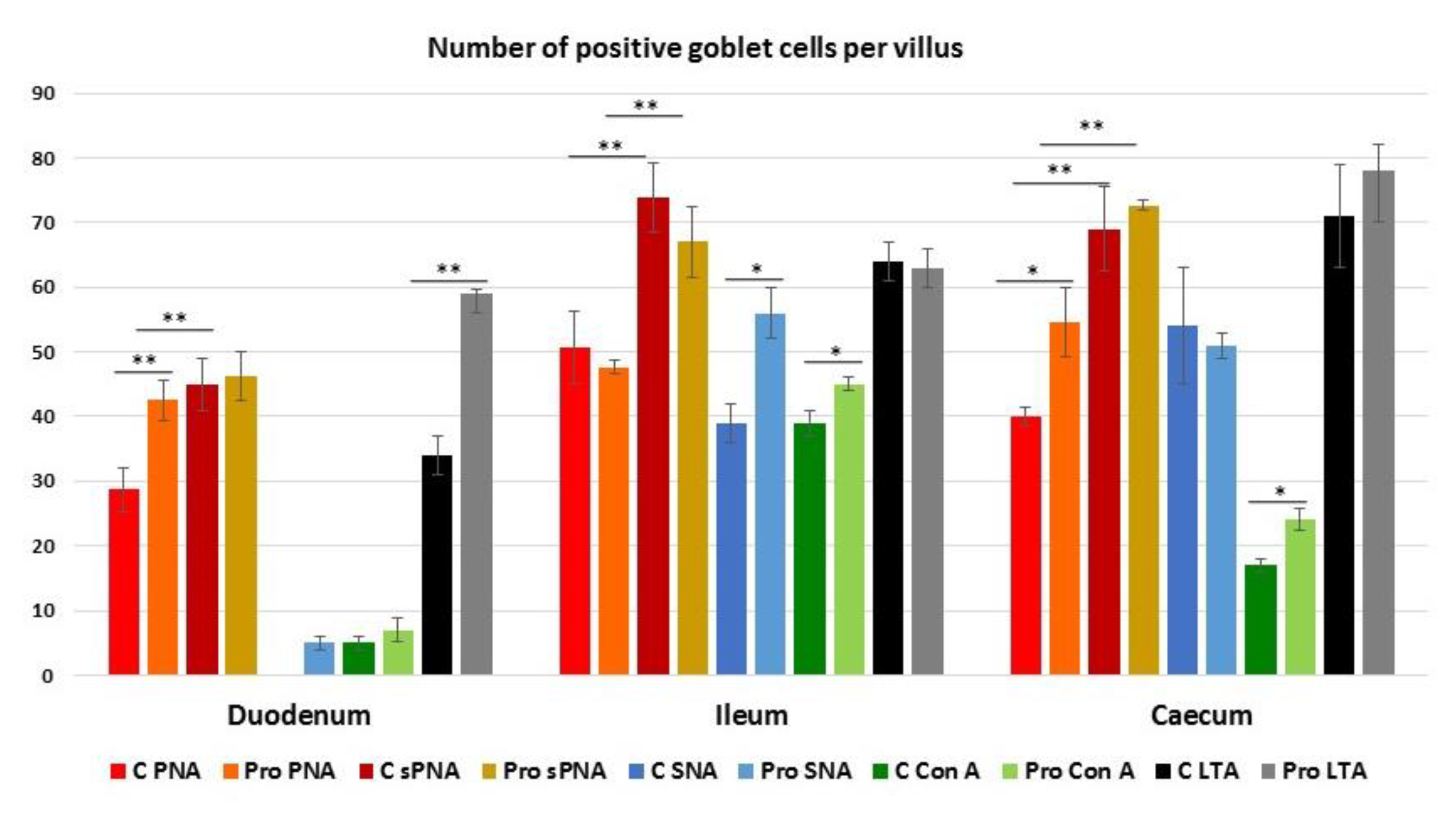
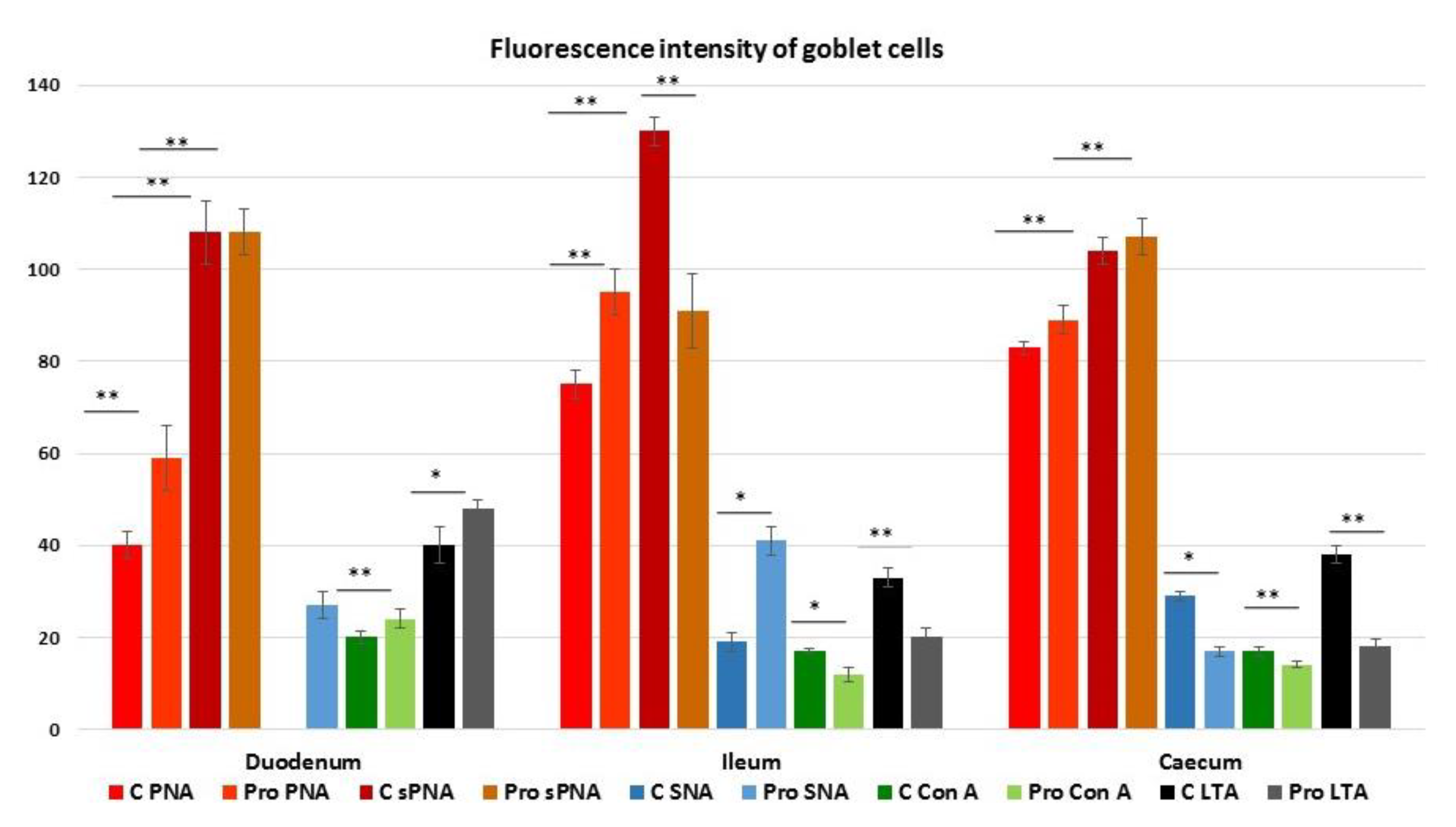
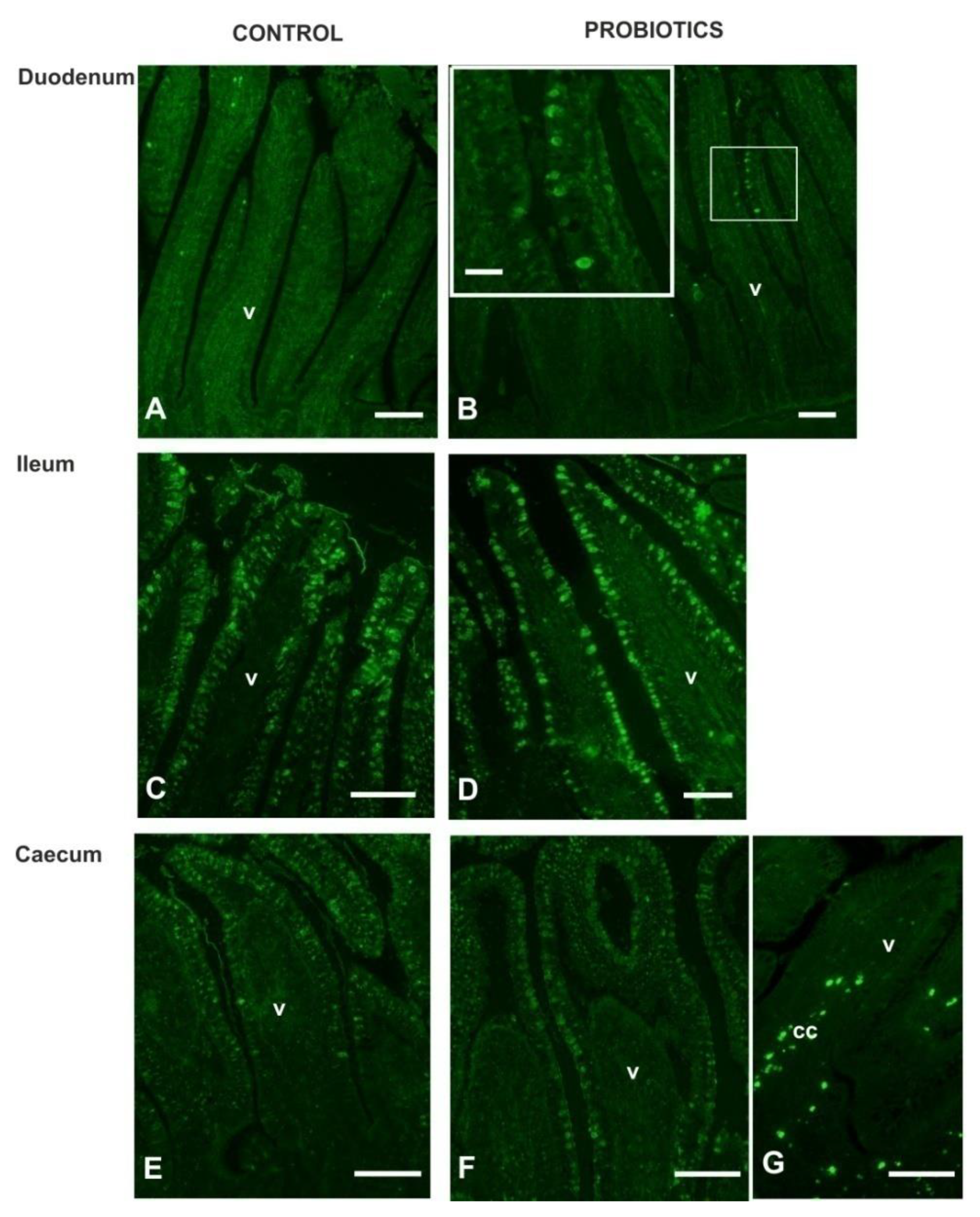
3.4. Lectin Histochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Pearson, P. Mucus glycoproteins of the normal gastrointestinal tract. Eur. J. Gastroen. Hepat. 1993, 5, 193–199. [Google Scholar] [CrossRef]
- Looft, T.; Cai, G.; Choudhury, B.; Lai, L.X.; Lippolis, J.D.; Reinhardt, T.A.; Sylte, M.J.; Casey, T.A. Avian intestinal mucus modulates Campylobacter jejune gene expression in a host-specific manner. Front. Microbiol. 2019, 9, 3215. [Google Scholar] [CrossRef]
- Varki, A.; Cornefeld, S. Historical background and overview. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 8–12. [Google Scholar]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Garner, B.; Merry, A.H.; Royle, L.; Harvey, D.J.; Rudd, P.M.; Thillet, J. Structural elucidation of the N- and O-glycans of human apolipoprotein(a): Role of O-glycans in conferring protease resistance. J. Biol. Chem. 2001, 276, 22200–22208. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boettcher, K.; Geonnotti, A.R.; Kavanaugh, N.L.; Hirsch, J.B.; Ribbeck, K.; Lieleg, O. Modulating mucin hydration and lubrication by deglycosylation and polyethylene glycol binding. Adv. Mater. Interfaces 2015, 2, 1500308. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Day, C.J.; Tiralongo, J.; Hartnell, R.D.; Logue, C.A.; Wilson, J.C.; von Itzstein, M.; Korolik, V. Differential carbohydrate recognition by Campylobacter jejuni strain 11168: Influences of temperature and growth conditions. PLoS ONE 2009, 4, e4927. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Hayashi, M.P.; Padra, M.; Padra, J.T.; Benktander, J.; Lindén, S.K. Mucus-pathogen interactions in the gastrointestinal tract of farmed animals. Microorganisms 2018, 6, 55. [Google Scholar] [CrossRef]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: A systematic review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Grzeskowiak, L.; Salminen, S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Galosi, L.; Berardi, S.; Tambella, A.M.; Attili, A.R.; Gavazza, A.; Cerquetella, M.; Jergens, A.E.; Guard, B.C.; et al. Effects of the probiotic mixture Slab51® (SivoMixx®) as food supplement in healthy dogs: Evaluation of fecal microbiota, clinical parameters and immune function. Front. Vet. Sci. 2020, 7, 613. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.-M.; Verlha, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Forstner, G.; Forstner, J.F. Gastrointestinal mucus. In Physiology of the Gastrointestinal Tract; Jonson, L.R., Ed.; Raven Press: New York, NY, USA, 1994; pp. 1245–1283. [Google Scholar]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Galosi, L.; Desantis, S.; Roncarati, A.; Robino, P.; Bellato, A.; Ferrocino, I.; Santamaria, N.; Biagini, L.; Filoni, L.; Attili, A.-R.; et al. Effects of a multistrain probiotic on the intestinal morphology and microbiota of guinea fowls (Numida meleagris). Poult. Sc. 2021. under revision. [Google Scholar]
- Krunt, O.; Zita, L.; Kraus, A.; Okrouhlá, M.; Chodová, D.; Stupka, R. Guinea fowl (Numida meleagris) eggs and free range housing: A convenient alternative to laying hens’ eggs in terms of food safety? Poult. Sci. 2021. [Google Scholar] [CrossRef]
- Accogli, G.; Crovace, A.; Mastrodonato, M.; Rossi, G.; Francioso, E.G.; Desantis, S. Probiotic supplementation affects the glycan composition of mucins secreted by Brunner’s glands of the pig duodenum. Ann. Anat. 2018, 218, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Desantis, S.; Mastrodonato, M.; Accogli, G.; Rossi, G.; Crovace, A.M. Effects of a probiotic on the morphology and mucin composition of pig intestine. Histol. Histopath. 2019, 34, 1037–1050. [Google Scholar]
- McManus, J.F.A. Histological and histochemical uses of periodic acid. Stain. Technol. 1948, 23, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.G.E. Histochemistry Theoretical and Applied, 3rd ed.; Churchill: London, UK, 1968. [Google Scholar]
- Spicer, S.S. Diamine methods for differentialingmucosubstanceshistochemically. J. Histochem. Cytochem. 1965, 13, 211–234. [Google Scholar] [CrossRef]
- Reid, P.E.; Culling, C.F.A.; Dunn, W.L. A histochemical method for the demonstration of 9-O-acyl sialic acids. An investigation of bovine submaxillary mucin and intestinal mucins. J. Histochem. Cytochem. 1978, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Nahashon, S.N.; Aggrey, S.E.; Adefope, N.A.; Amenyenu, A. Modeling growth characteristics of meat-type guinea fowl. Poult. Sci. 2006, 85, 943–946. [Google Scholar] [CrossRef]
- Vignal, A.; Boitard, S.; Thébault, N.; Dayo, G.K.; Yapi-Gnaore, V.; YoussaoAbdou Karim, I.; Berthouly-Salazar, C.; Pálinkás-Bodzsár, N.; Guémené, D.; Thibaud-Nissen, F.; et al. A guinea fowl genome assembly provides new evidence on evolution following domestication and selection in galliformes. Mol. Ecol. 2019, 19, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Crovace, A.M.; Rossi, G.; Laudadio, V. Effect of a dietary probiotic blend on performance, blood characteristics, meat quality and faecal microbial shedding in growing-finishing pigs. S. Afr. J. Anim. Sci. 2017, 47, 875–882. [Google Scholar] [CrossRef]
- Pearson, J.P.; Brownlee, I.A. The interaction of large bowel microflora with the colonic mucus barrier. Int. J. Inflam. 2010, 321426. [Google Scholar] [CrossRef][Green Version]
- Le, M.H.; Galle, S.; Yang, Y.; Landero, J.L.; Beltranena, E.; Gänzle, M.G.; Zijlstra, R.T. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J. Anim. Sci. 2016, 94, 4677–4687. [Google Scholar] [CrossRef]
- Yi, H.; Wang, L.; Xiong, Y.; Wen, X.; Wang, Z.; Yang, X.; Gao, K.; Jiang, Z. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 2018, 96, 2342–2351. [Google Scholar] [CrossRef]
- Rezaei, M.; KarimiTorshizi, M.A.; Wall, H.; Ivarsson, E. Bodygrowth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fibre. Br. Poult. Sci. 2018, 59, 422–429. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroidesthetaiotaomicron and Faecalibacteriumprausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 1–13. [Google Scholar] [CrossRef]
- Martens, E.C.; Chiang, H.C.; Gordon, J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 2008, 4, 447–457. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Solis de los Santos, F.; Donoghue, A.M.; Farnell, M.B.; Huff, G.R.; Huff, W.E.; Donoghue, D.J. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (alphamune). Poult. Sci. 2007, 86, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Yang, C.L.; Tong, J.; Nishimaki, H.; Masuda, K.; Takeo, T.; Kasai, K.; Itoh, G. Rat small intestinal goblet cell kinetics in the process of restitution of surface epithelium subjected to ischemia-reperfusion injury. Dig. Dis. Sci. 2002, 47, 590–600. [Google Scholar] [CrossRef]
- Ashraf, S.; Zaneb, H.; Yousaf, M.S.; Ijaz, A.; Sohail, M.U.; Muti, S.; Usman, M.M.; Ijaz, S.; Rehman, H. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. J. Anim. Physiol. Anim. Nutr. 2013, 97, 68–73. [Google Scholar] [CrossRef]
- Shah, M.; Zanerb, H.; Masood, S.; Khan, R.U.; Ashraf, S.; Slkandar, A.; Rehman, H.F.U.; Rehman, H.U. Effect of dietary supplementation of zinc and multi-microbe probiotic on growth traits and alteration of intestinal architecture in broiler. Probiotics Antimicrob. 2019, 11, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Forder, R.E.; Howart, G.S.; Tivey, D.R.; Hughes, R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early post hatch development of poultry. Poult. Sci. 2007, 86, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Perez, R.; Amit-Romach, E.; Sklan, D.; Uni, Z. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 2005, 135, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Georgiades, P.; Pudney, P.D.; Thornton, D.J.; Waigh, T.A. Particle tracking micro rheology of purified gastrointestinal mucins. Biopolymers 2014, 101, 366–377. [Google Scholar] [CrossRef]
- Runnels, P.L.; Moon, H.W.; Schneider, R.A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect. Immun. 1980, 28, 298–300. [Google Scholar] [CrossRef]
- Dean-Nystrom, E.A.; Samuel, J.E. Age-related resistance to 987P fimbria-mediated colonization correlates with specific glycolipid receptors in intestinal mucus in swine. Infect. Immun. 1994, 62, 4789–4794. [Google Scholar] [CrossRef]
- Struwe, W.B.; Gough, R.; Gallagher, M.E.; Kenny, D.T.; Carrington, S.D.; Karlsson, N.G.; Rudd, P.M. Identification of O-glycan structures from chicken intestinal mucins provides insight into Campylobacter jejuni pathogenicity. Mol. Cell. Proteom. 2015, 14, 1464–1477. [Google Scholar] [CrossRef]
- Kawashima, H. Roles of sulfated glycans in lymphocyte homing. Biol. Pharm. Bull. 2006, 29, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, J.P.; de Vos, W.M.; Belzer, C. Glycobiome: Bacteria and mucus at the epithelial interface. Best. Pract. Res. Clin. Gastroenterol. 2013, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, C.; Thornton, D.J.; Grencis, R.K. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 2018, 40, e12517. [Google Scholar] [CrossRef]
- Corfield, A.P.; Wagner, S.A.; Clamp, J.R.; Kriaris, M.S.; Hoskins, L.C. Mucin degradation in the human colon: Production of sialidase, sialateO-acetylesterase, N-acetylneuraminatelyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 1992, 60, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Kamisago, S.; Iwamori, M.; Tai, T.; Mitamura, K.; Yazaki, Y.; Sugano, K. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect. Immun. 1996, 64, 624–628. [Google Scholar] [CrossRef]
- Veerman, E.C.; Bank, C.M.; Namavar, F.; Appelmelk, B.J.; Bolscher, J.G.; NieuwAmerongen, A.V. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology 1997, 7, 737–743. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015, 2, 75. [Google Scholar] [CrossRef]
- Jung, T.H.; Park, J.H.; Jeon, W.M.; Han, K.S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pr. 2015, 9, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrateenhances the intestinalbarrier by facilitating tight junctionassembly via activation of amp-activatedproteinkinase in caco-2 cellmonolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Rinttila, T.; Apajalahti, J. Intestinal microbiota and metabolites-implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonicregulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.B.; Matthews, E.; Carrington, S.D.; Mulcahy, G. Interaction of Eimeriatenella with intestinal mucin in vitro. J. Parasitol. 2007, 93, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Schnaar, R.L.; Schauer, R. Sialic acids and other nonulosonic acids. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 179–195. [Google Scholar]
- Guo, C.T.; Takahashi, N.; Yagi, H.; Kato, K.; Takahashi, T.; Yi, S.Q.; Chen, Y.; Ito, T.; Otsuki, K.; Kida, H.; et al. The quail and chicken intestine have sialyl-Gal sugar chains responsible for the binding of influenza A viruses to human type receptors. Glycobiology 2007, 17, 713–724. [Google Scholar] [CrossRef][Green Version]
- Liu, P.; Pieper, R.; Tedin, L.; Martin, L.; Meyer, W.; Rieger, J.; Plendl, J.; Vahjen, W.; Zentek, J. Effect of dietary zinc oxide onjejunal morphological and immunological characteristics in weanedpiglets. J. Anim. Sci. 2014, 92, 5009–5018. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, J.L.; Vicaretti, S.D.; Noyovitz, B.; Xing, X.; Low, K.E.; Inglis, G.D.; Zaytsoff, S.J.M.; Boraston, A.B.; Smith, S.P.; Uwiera, R.R.E.; et al. Structural analysis of broiler chicken small intestinal mucin O-glycan modification by Clostridium perfringens. Poult. Sci. 2019, 98, 5074–5088. [Google Scholar] [CrossRef]
- Brockhausen, I.; Stanley, P. O-GalNAcglycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 113–123. [Google Scholar]
- Stanley, P.; Taniguchi, N.; Aebi, N. N-glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 99–111. [Google Scholar]
- Rahimi, S.; Kathariou, S.; Fletcher, O.; Grimes, J.M. The effectiveness of a dietary direct-fed microbial and mannan oligosaccharide on ultrastructural changes of intestinal mucosa of turkey poults infected with Salmonella and Campylobacter. Poult. Sci. 2020, 99, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Falk, P.G.; Gordon, J.I. Analyzing the molecular foundations of commensalism in the mouse intestine. Curr. Opin. Microbiol. 2000, 3, 79–85. [Google Scholar] [CrossRef]
- Stahl, M.; Friis, L.M.; Nothaft, H.; Liu, X.; Li, J.; Szymanski, C.M.; Stintzi, A. L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc. Natl. Acad. Sci. USA 2011, 108, 7194–7199. [Google Scholar] [CrossRef]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in animals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef]
- Rahimi, S.; Grimes, J.L.; Fletcher, O.; Oviedo, E.; Sheldon, B.W. Effect of a direct-fed microbial (Primalac) on structure and ultrastructure of small intestine in turkey poults. Poult. Sci. 2009, 88, 491–503. [Google Scholar] [CrossRef]
- Arrighi, S.; Bosi, G.; Accogli, G.; Desantis, S. Seasonal and ageing-depending changes of Aquaporins 1 and 9 expression in the genital tract of buffalo bulls (Bubalusbubalis). Reprod. Domest. Anim. 2016, 51, 515–523. [Google Scholar] [CrossRef]
- Schauer, R. Achievements and challenges of sialic acid research. Glycoconj. J. 2000, 17, 485–499. [Google Scholar] [CrossRef]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Aust. J. Anim. Sci. 2012, 9, 1285–1293. [Google Scholar] [CrossRef]
| Chemical Composition (%) | I Period (10–40 Day) | II Period (41–120 Day) |
|---|---|---|
| Protein | 22 | 21 |
| Lipids | 5 | 5.8 |
| Ash | 7.4 | 7.5 |
| Cellulose | 4.4 | 4.5 |
| Ca | 1 | 1.04 |
| P | 1 | 0.85 |
| Na | 0.2 | 0.19 |
| Lysine | 1.2 | 1.2 |
| Methionine | 0.6 | 0.58 |
| Specific phytase promoter | 1500 | 750 |
| Digestion promoterEndo-1.4-ß-Xylanasi | 200 | 0 |
| Vitamin A E672 (UI/kg) | 0 | 4000 |
| Vitamin D3 E671 (UI/kg) | 2000 | 1250 |
| Vitamin E (alpha toc. 91%) (mg/kg) | 40 | 20 |
| Cu E4 (mg/kg) | 16 | 10 |
| Se E8 (mg/kg) | 0.16 | 0.2 |
| Luteine E161b (g/kg) | 0 | 41 |
| Zeaxanthine E161 (g/kg) | 0 | 8.4 |
| Lectin Abbreviation | Source of Lectin | µg/mL | Sugar Specificity | Inhibitory Sugar |
|---|---|---|---|---|
| MAL II | Maackia amurensis | 25 | NeuNAcα2-3Galβ1-3(± NeuNAcα2,6)GalNAc | NeuNAc |
| SNA | Sambucus nigra | 25 | Neu5Acα2,6Gal/GalNAc | NeuNAc |
| Con A | Canavalia ensiformis | 25 | Terminal/internal αMan > αGlc | Mannose |
| PNA * | Arachis hypogaea | 25 | Terminal Galβl,3GalNAc | Galactose |
| LTA | Lotus tetragonolobus | 30 | Terminal a L-Fuc | Fucose |
| UEA I | Ulex europaeus | 30 | Terminal L-Fucαl,2Galβl,4GlcNAcβ | Fucose |
| Item | Control | Probiotics | p-Value | |
|---|---|---|---|---|
| Duodenum | ||||
| Villus height (µm) | 649.11 ± 22.71 | 895.71 ± 16.46 | <0.001 | |
| Crypt depth (µm) | 101.58 ± 27.62 | 143.38 ± 8.72 | <0.001 | |
| Goblet cell numbers per villus | 122.92 ± 3.4 | 169.11 ± 6.4 | <0.001 | |
| Ileum | ||||
| Villus height (µm) | 671.88 ± 15.41 | 747.52 ± 20.18 | 0.010 | |
| Crypt depth (µm) | 110.97 ± 5.31 | 126.58 ± 7.93 | 0.002 | |
| Goblet cell numbers per villus | 139.24 ± 5.35 | 155.73 ± 1.46 | <0.001 | |
| Caecum | ||||
| Villus height (µm) | 464.81 ± 60.02 | 594.97 ± 68.31 | 0.043 | |
| Crypt depth (µm) | 84.82 ± 6.81 | 119.10 ± 9.44 | <0.001 | |
| Goblet cell numbers per villus | 94.09 ± 2.12 | 141.15 ± 6.71 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desantis, S.; Galosi, L.; Santamaria, N.; Roncarati, A.; Biagini, L.; Rossi, G. Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®. Animals 2021, 11, 495. https://doi.org/10.3390/ani11020495
Desantis S, Galosi L, Santamaria N, Roncarati A, Biagini L, Rossi G. Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®. Animals. 2021; 11(2):495. https://doi.org/10.3390/ani11020495
Chicago/Turabian StyleDesantis, Salvatore, Livio Galosi, Nicoletta Santamaria, Alessandra Roncarati, Lucia Biagini, and Giacomo Rossi. 2021. "Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®" Animals 11, no. 2: 495. https://doi.org/10.3390/ani11020495
APA StyleDesantis, S., Galosi, L., Santamaria, N., Roncarati, A., Biagini, L., & Rossi, G. (2021). Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®. Animals, 11(2), 495. https://doi.org/10.3390/ani11020495







