Expression of Apoptosis-Related Genes in Cat Testicular Tissue in Relation to Sperm Morphology and Seasonality—A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Preparation
2.2. Study Design
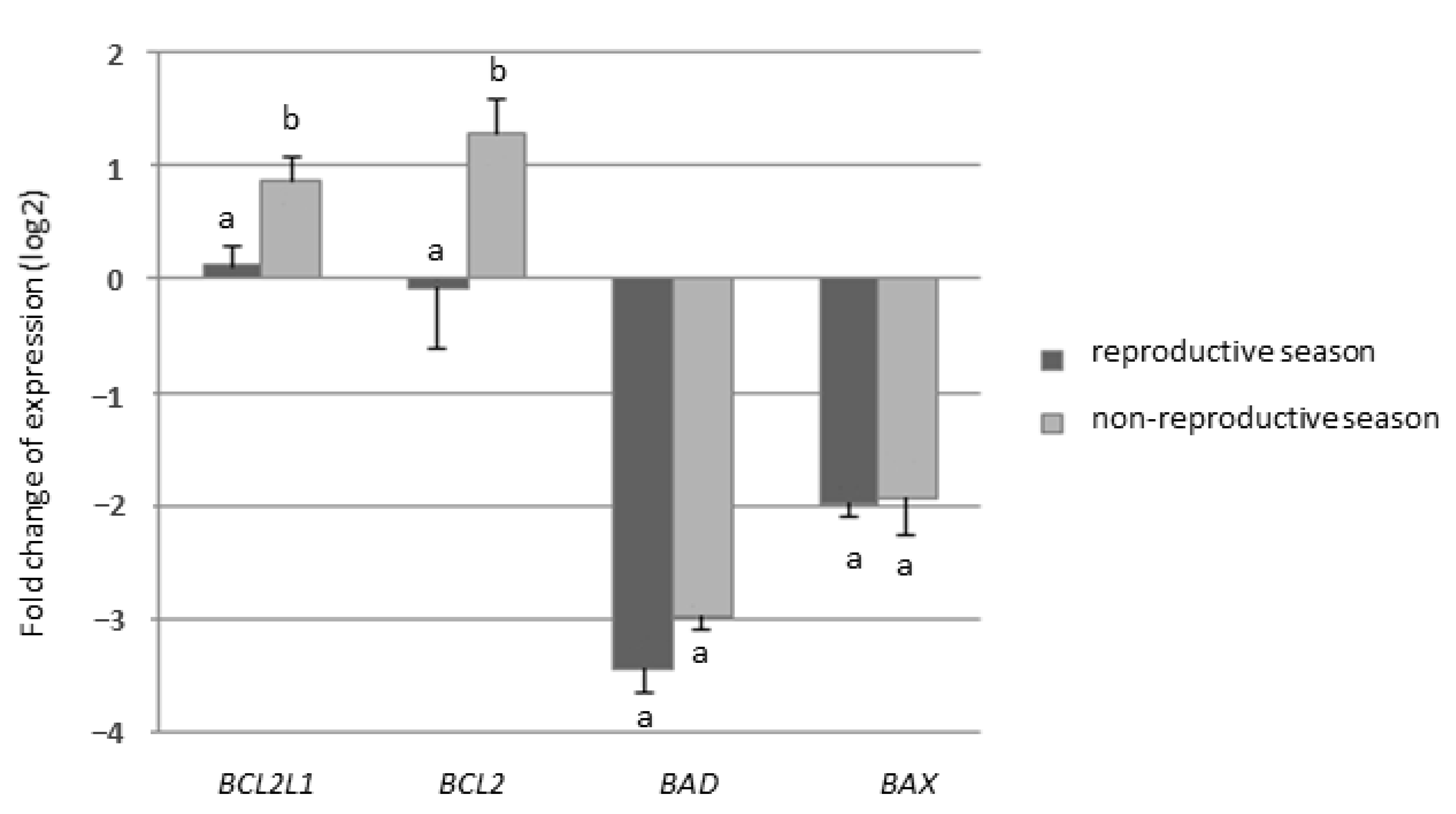
2.2.1. Experiment I—Expression of Genes from BCL-2 Family
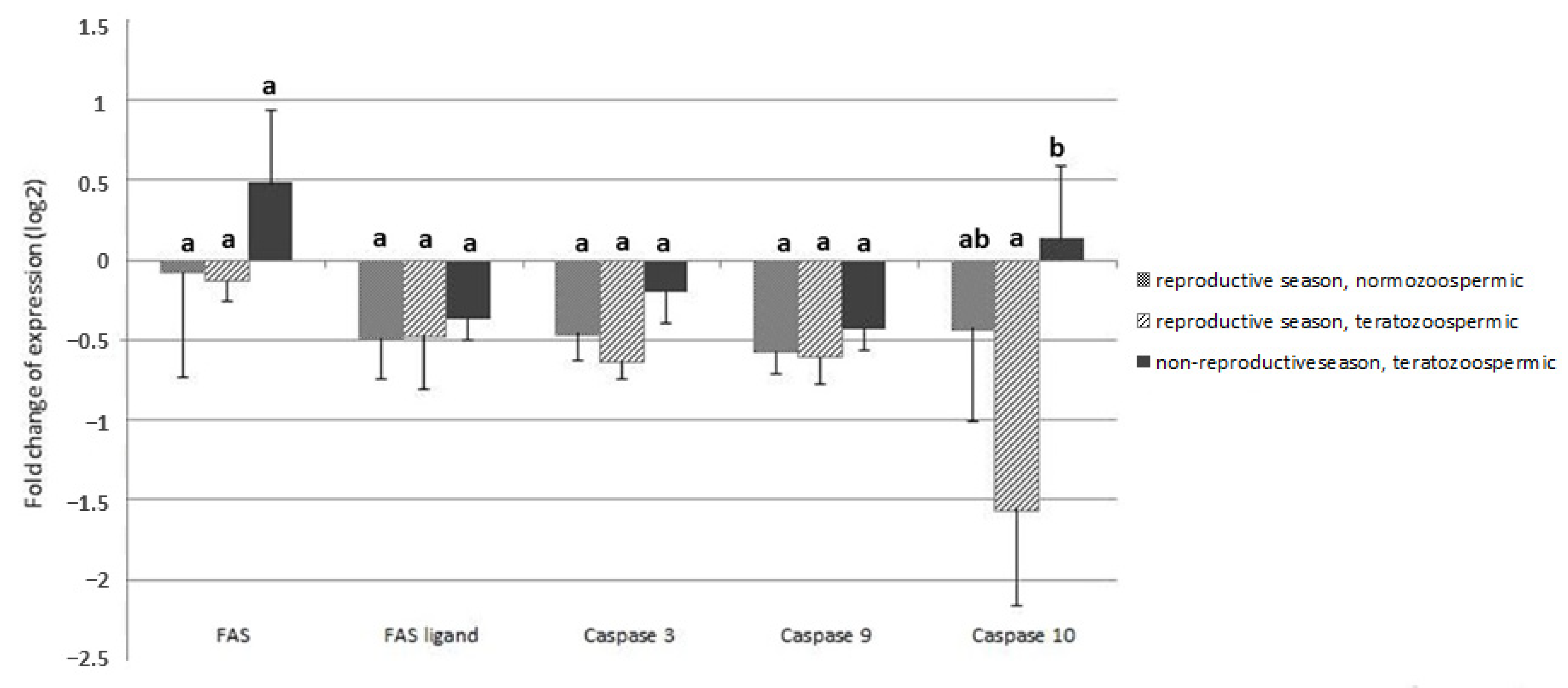
2.2.2. Experiment II—Expression of Genes of FAS/FAS-Ligand and Caspases
2.3. Gene Expression Analysis
2.3.1. Isolation of RNA and Reverse Transcription
2.3.2. RT-qPCR Technique
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Print, C.G.; Loveland, K.L. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays 2000, 22, 423–430. [Google Scholar] [CrossRef]
- Blanco-Rodríguez, J. A matter of death and life: The significance of germ cell death during spermatogenesis. Int. J. Androl. 1998, 21, 236–248. [Google Scholar] [CrossRef]
- Odorisio, T.; Rodriguez, T.A.; Evans, E.P.; Clarke, A.R.; Burgoyne, P.S. The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat. Genet. 1998, 18, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodríguez, J.; Martínez-García, C. Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol. Reprod. 1999, 61, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Nelson, R.J. Mediation of seasonal testicular regression by apoptosis. Reproduction 2001, 122, 677–685. [Google Scholar] [CrossRef]
- Lue, Y.H.; Hikim, A.P.; Swerdloff, R.S.; Im, P.; Taing, K.S.; Bui, T.; Leung, A.; Wang, C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: Role of intratesticular testosterone on stage specificity. Endocrinology 1999, 140, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Mitsumori, K.; Yasuhara, K.; Uneyama, C.; Onodera, H.; Takegawa, K.; Takahashi, M.; Umemura, T. Involvement of apoptosis in the rat germ cell degeneration induced by nitrobenzene. Arch. Toxicol. 1998, 72, 296–302. [Google Scholar] [CrossRef]
- Cai, L.; Hales, B.F.; Robaire, B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol. Reprod. 1997, 56, 1490–1497. [Google Scholar] [CrossRef][Green Version]
- Henriksén, K.; Kulmala, J.; Toppari, J.; Mehrotra, K.; Parvinen, M. Stage-specific apoptosis in the rat seminiferous epithelium: Quantification of irradiation effects. J. Androl. 1996, 17, 394–402. [Google Scholar]
- Sinha Hikim, A.P.; Rajavashisth, T.B.; Hikim, I.S.; Lue, Y.; Bonavera, J.J.; Leung, A.; Wang, C.; Swerdloff, R.S. Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol. Reprod. 1997, 57, 1193–1201. [Google Scholar] [CrossRef]
- Tapanainen, J.S.; Tilly, J.L.; Vihko, K.K.; Hsueh, A.J. Hormonal control of apoptotic cell death in the testis: Gonadotropins and androgens as testicular cell survival factors. Mol. Endocrinol. 1993, 7, 643–650. [Google Scholar] [CrossRef]
- Siemieniuch, M.J. Apoptotic changes in the epithelium germinativum of the cat (Felis catus s. domestica, L. 1758) at different ages and breeding seasons. Reprod. Domest. Anim. 2008, 43, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Blottner, S.; Jewgenow, K. Moderate seasonality in testis function of domestic cat. Reprod. Domest. Anim. 2007, 42, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Onodera, F.; Oba, H.; Mizutani, T.; Hori, T. Plasma hormone levels and semen quality in male cats during non–breeding and breeding seasons. Reprod. Domest. Anim. 2009, 44, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Spindler, R.E.; Wildt, D.E. Circannual variations in intraovarian oocyte but not epididymal sperm quality in the domestic cat. Biol. Reprod. 1999, 61, 188–194. [Google Scholar] [CrossRef]
- Prochowska, S.; Niżański, W.; Ochota, M.; Partyka, A. Characteristics of urethral and epididymal semen collected from domestic cats––A retrospective study of 214 cases. Theriogenology 2015, 84, 1565–1571. [Google Scholar] [CrossRef]
- Howard, J.G.; Brown, J.L.; Bush, M.; Wildt, D.E. Teratospermic and normospermic domestic cats: Ejaculate traits, pituitary–gonadal hormones, and improvement of spermatozoal motility and morphology after swim–up processing. J. Androl. 1990, 11, 204–215. [Google Scholar]
- Jewgenow, K.; Neubauer, K.; Blottner, S.; Schön, J.; Wildt, D.E.; Pukazhenthi, B.S. Reduced germ cell apoptosis during spermatogenesis in the teratospermic domestic cat. J. Androl. 2009, 30, 460–468. [Google Scholar] [CrossRef]
- Neubauer, K.; Jewgenow, K.; Blottner, S.; Wildt, D.E.; Pukazhenthi, B.S. Quantity rather than quality in teratospermic males: A histomorphometric and flow cytometric evaluation of spermatogenesis in the domestic cat (Felis catus). Biol. Reprod. 2004, 71, 1517–1524. [Google Scholar] [CrossRef]
- Pukazhenthi, B.S.; Neubauer, K.; Jewgenow, K.; Howard, J.; Wildt, D.E. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology 2006, 66, 112–121. [Google Scholar] [CrossRef]
- Jewgenow, K.; Pukazhenthi, B.S.; Schoen, J. Analysis of Sertoli cell efficiency allows the differentiation between two fundamentally different forms of feline teratospermia. Theriogenology 2013, 79, 261–266. [Google Scholar] [CrossRef]
- Zambelli, D.; Prati, F.; Cunto, M.; Iacono, E.; Merlo, B. Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 2008, 69, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper––Excel–based tool using pair–wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Martino–Andrade, A.J.; Santos, A.S.; Reghelin, A.L.; Garcia, D.M.; Sant’Ana, G.R.; Spercoski, K.M.; Meyer, K.B.; Torres, S.M.; Júnior, V.S.; et al. Testicular testosterone: Estradiol ratio in domestic cats and its relationship to spermatogenesis and epididymal sperm morphology. Theriogenology 2012, 78, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.; Johnson, W.; Cambre, R.C.; Citino, S.; Quigley, K.B.; Brousset, D.; Morais, R.; Moreira, N.; O’Brien, S.J.; Wildt, D.E. Reproductive status of endemic felid species in Latin American Zoos and implications for ex situ conservation. Zoo Biology 2003, 22, 421–441. [Google Scholar] [CrossRef]
- Axnér, E.; Linde Forsberg, C. Sperm morphology in the domestic cat, and its relation with fertility: A retrospective study. Reprod. Domest. Anim. 2007, 42, 282–291. [Google Scholar] [CrossRef]
- Sinha Hikim, A.P.; Lue, Y.; Diaz–Romero, M.; Yen, P.H.; Wang, C.; Swerdloff, R.S. Deciphering the pathways of germ cell apoptosis in the testis. J. Steroid Biochem. Mol. Biol. 2003, 85, 175–182. [Google Scholar] [CrossRef]
- Murphy, C.J.; Richburg, J.H. Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis 2015, 4, e979110. [Google Scholar] [CrossRef]
- Kirkpatrick, J. Seasonal testosterone levels, testosterone clearance, and testicular weights in male domestic cats. Can. J. Zool. 2011, 63, 1285–1287. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef]

| Name | Accession No. | Primers Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|---|
| RPS7 | NM_001009832.1 | F: GGGCAAGAGAATCCGTGTGA R: CCTTGCCCGTGAGCTTCTTA | 131 |
| GAPDH | NM_001009307.1 | F: GGAGAAAGCTGCCAAATATG R: CAGGAAATGAGCTTGACAAAGTGG | 192 |
| BCL2L1 | NM_001009228.1 | F: GCTTGGATGGCCACTTACCT R: TGCTGCATTGTTCCCGTAGA | 99 |
| BCL2 | NM_001009340.1 | F: GGAGGATTGTGGCCTTCT R: GTTATCCTGGATCCAGGTGT | 143 |
| BAX | NM_001009282.2 | F: GCTCTGAGCAGATCATGAAGACA R: CATTCGCCCTGCTCGATCTT | 71 |
| BAD | XM_003993558.3 | F: GGGCTCCTTCAAGGGACTTC R: TCCTCTCCCCAAGTTCCGAT | 118 |
| FAS | NM_001009314.1 | F: GCTCCTGATTCTACCGTCCG R: CCGGAGCAGTTGGACTTTCT | 283 |
| FASLG | NM_001009352.1 | F: TCCACCAGCCAAAAGCATGT R: TTGAGTTGGGCTTGCCTGTT | 118 |
| CASP3 | NM_001009338.1 | F: CCGGCAAACCCAAACTCTTC R: AACCAGGGGCTGTGGAATAC | 153 |
| CASP8 | XM_006935474.2 | F: CGCTTCTTTGGTAAGGCTACA R: GGATGTAGTCCAGGCTCAGG | 140 |
| CASP9 | XM_011284592.1 | F: CTAGTTTGCCCACACCCAGT R: ACAGCATTAGCGACCCTGAG | 175 |
| CASP10 | XM_006935472.2 | F: CCGAGCATTCACCTCCTACC R: TCAGTCCGGGGAAAACCAAC | 435 |
| Study Group | According to Morphology | According to Season | ||
|---|---|---|---|---|
| Normospermic (n = 5) | Teratospermic (n = 7) | Reproductive (n = 5) | Non-Reproductive (n = 7) | |
| Subjective motility [%] | 80.0 ± 7.1 | 65.7 ± 15.1 | 74.3 ± 11.3 | 68.0 ± 17.9 |
| MORPHOLOGY | ||||
| Normal [%] | 66.2 ± 5.8 | 31.1 ± 13.5 | 50.9 ± 20.4 | 38.6 ± 21.7 |
| Distal droplet [%] | 11.6 ± 7.7 | 11.2 ± 9.3 | 14.1 ± 8.0 | 7.6 ± 7.9 |
| Bent tail [%] | 5.7 ± 5.8 | 18.3 ± 19.4 | 8.2 ± 9.4 | 19.8 ± 22.0 |
| Detached head [%] | 1.8 ± 0.7 | 1.6 ± 1.3 | 1.6 ± 0.9 | 1.7 ± 1.3 |
| Coiled tail [%] | 0.1 ± 0.2 | 0.3 ± 0.5 | 0.1 ± 0.2 | 0.4 ± 0.5 |
| Proximal droplet [%] | 3.0 ± 2.2 | 4.8 ± 7.5 | 5.6 ± 7.2 | 1.9 ± 1.5 |
| Head abnormalities [%] | 1.8 ± 0.4 | 3.5 ± 3.0 | 2.0 ± 1.9 | 3.9 ± 2.8 |
| Acrosome abnormalities [%] | 6.1 ± 6.1 | 7.1 ± 3.7 | 6.1 ± 5.3 | 7.4 ± 4.0 |
| Midpiece defects [%] | 3.4 ± 3.1 | 19.5 ± 8.1 | 10.2 ± 9.5 | 16.4 ± 11.6 |
| Dag-like defect [%] | 0.3 ± 0.7 | 2.6 ± 1.8 | 1.2 ± 1.7 | 2.3 ± 2.1 |
| Study Group: | Normospermic in Reproductive Season (n = 6) | Teratospermic in Reproductive Season (n = 6) | Teratospermic in Non- Reproductive Season (n = 6) |
|---|---|---|---|
| Subjective motility [%] | 81.7 ± 7.5 | 77.5 ± 11.7 | 53.3±19.7 |
| MORPHOLOGY Normal [%] | 65.6 ± 5.6 | 38.6 ± 9.7 | 21.5 ± 7.6 |
| Distal droplet [%] | 8.8 ± 6.6 | 14.5 ± 9.2 | 11.8 ± 14.3 |
| Bent tail [%] | 4.8 ± 5.6 | 6.3 ± 4.2 | 20.3 ± 18.9 |
| Detached head [%] | 2.0 ± 1.0 | 2.1 ± 3.0 | 4.0 ± 7.5 |
| Coiled tail [%] | 0.1 ± 0.2 | 0.3 ± 0.6 | 0.9 ± 0.9 |
| Proximal droplet [%] | 2.8 ± 2.0 | 11.0 ± 8.0 | 4.4 ± 5.7 |
| Head abnormalities [%] | 1.5 ± 0.5 | 4.6 ± 4.6 | 5.8 ± 3.8 |
| Acrosome abnormalities [%] | 6.2 ± 5.6 | 6.4 ± 3.0 | 6.1 ± 3.9 |
| Midpiece defects [%] | 6.6 ± 5.6 | 13.8 ± 10.5 | 19.8 ± 8.4 |
| Dag-like defect [%] | 1.7 ± 3.2 | 2.5 ± 1.4 | 5.2 ± 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochowska, S.; Partyka, A.; Niżański, W. Expression of Apoptosis-Related Genes in Cat Testicular Tissue in Relation to Sperm Morphology and Seasonality—A Preliminary Study. Animals 2021, 11, 489. https://doi.org/10.3390/ani11020489
Prochowska S, Partyka A, Niżański W. Expression of Apoptosis-Related Genes in Cat Testicular Tissue in Relation to Sperm Morphology and Seasonality—A Preliminary Study. Animals. 2021; 11(2):489. https://doi.org/10.3390/ani11020489
Chicago/Turabian StyleProchowska, Sylwia, Agnieszka Partyka, and Wojciech Niżański. 2021. "Expression of Apoptosis-Related Genes in Cat Testicular Tissue in Relation to Sperm Morphology and Seasonality—A Preliminary Study" Animals 11, no. 2: 489. https://doi.org/10.3390/ani11020489
APA StyleProchowska, S., Partyka, A., & Niżański, W. (2021). Expression of Apoptosis-Related Genes in Cat Testicular Tissue in Relation to Sperm Morphology and Seasonality—A Preliminary Study. Animals, 11(2), 489. https://doi.org/10.3390/ani11020489







