Nutrient Incorporation in First Feeding Seahorses Evidenced by Stable Carbon Isotopes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock
2.2. Experimental Design
2.2.1. Experiment 1: Artemia vs. Copepods Feeding
2.2.2. Experiment 2: Shifting of Feeding Regime
2.3. Sampling and Data Analysis
- Prey: δ13CN = δ13CF/1.016
- Seahorse juveniles: δ13CN = (δ13CF + 1.166)/1.024, where δ13CF corresponded to the isotopic values in frozen samples and δ13CN was the normalised (lipid free) isotopic value.
- Prey: CNN = CNF/1.201
- Seahorse juveniles: CNN = CNF/1.226,
2.4. Bioethics
3. Results
3.1. Growth and Survival
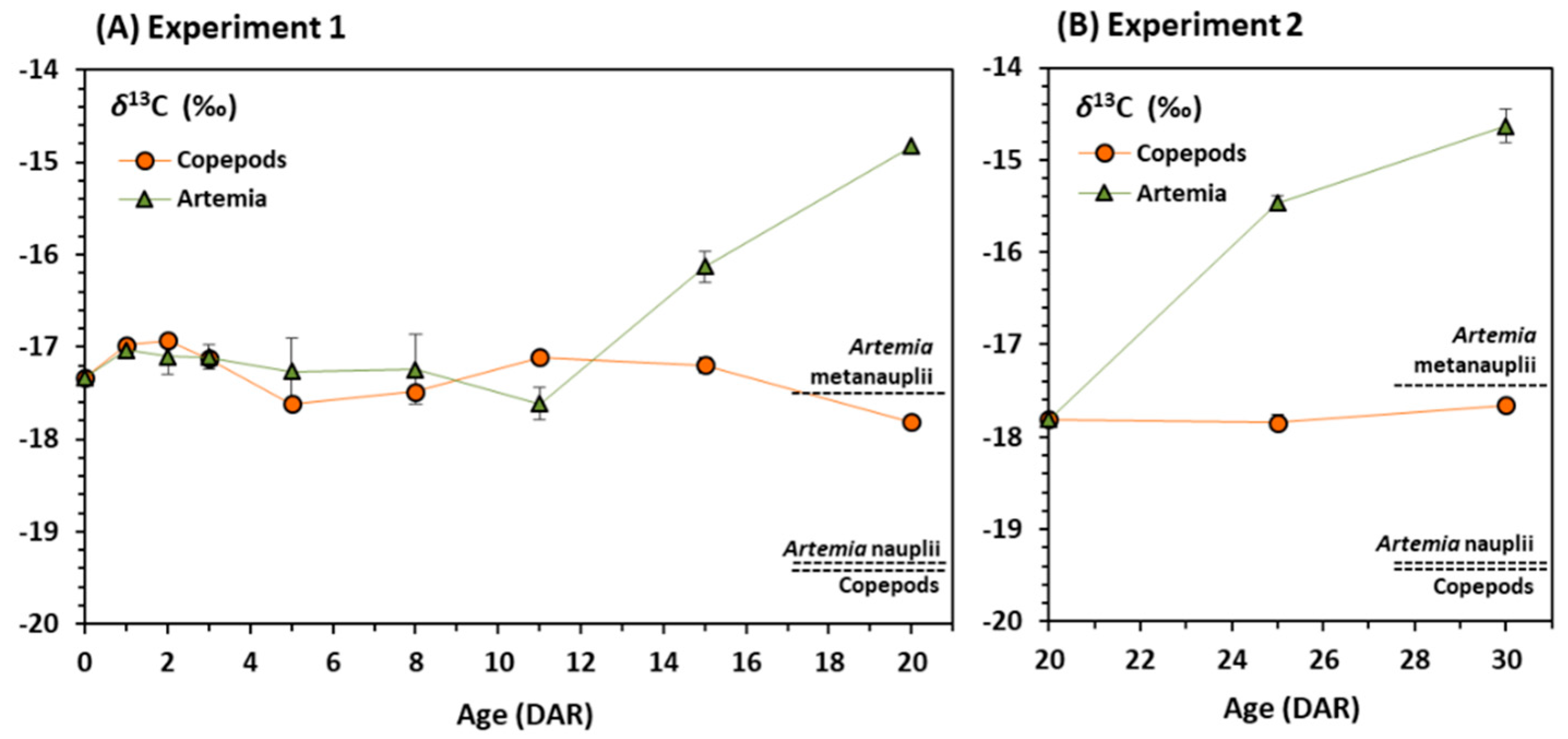
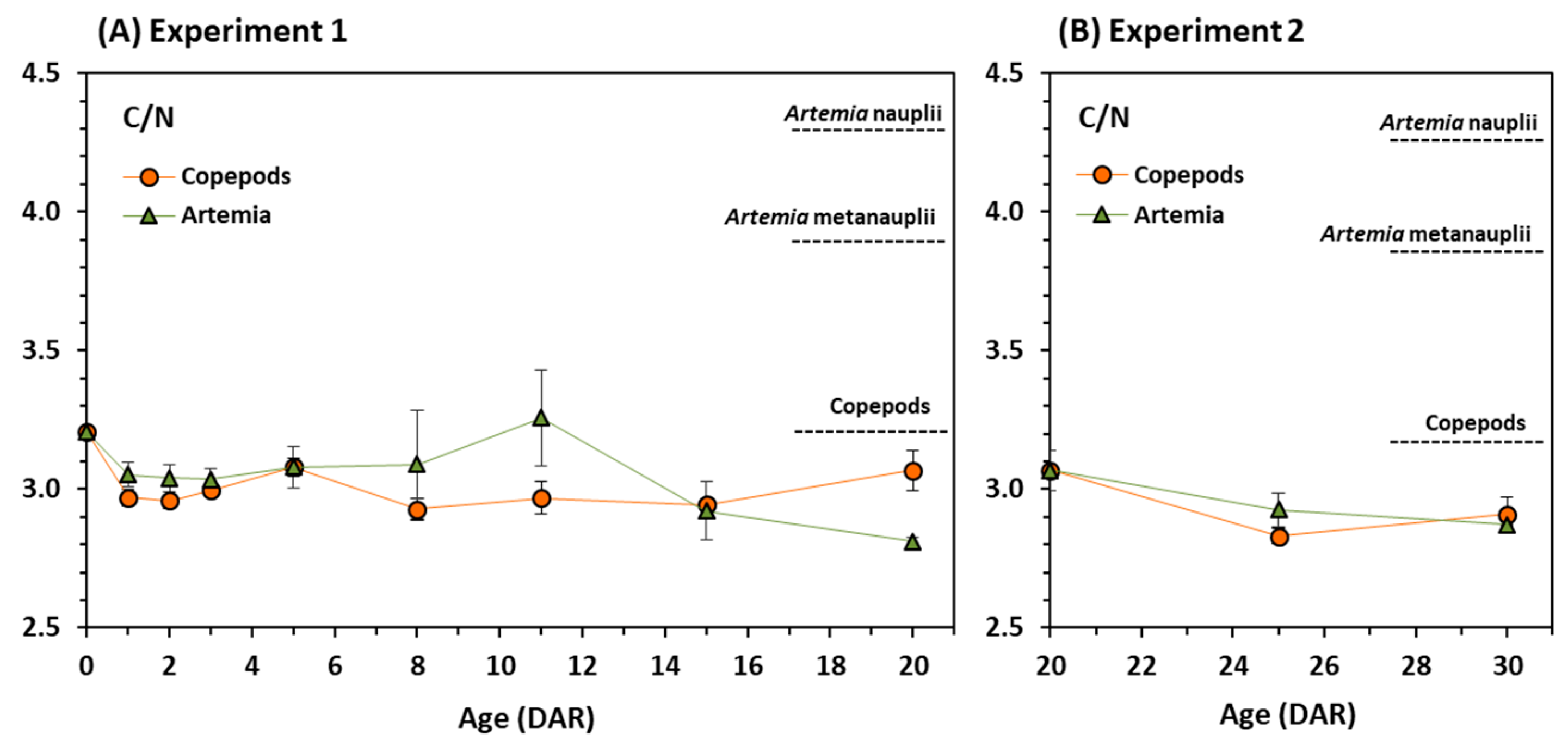
3.2. Stable Isotopes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, S.J.; Vincent, A.C.J. Life history and ecology of seahorses: Implications for conservation and management. J. Fish. Biol. 2004, 65, 1–61. [Google Scholar] [CrossRef]
- Koldewey, H.J.; Martin-Smith, K.M. A global review of seahorse aquaculture. Aquaculture 2010, 302, 131–152. [Google Scholar] [CrossRef]
- Vincent, A.C.J. The International Trade in Seahorses; TRAFFIC International: Cambridge, UK, 1996; p. 163. [Google Scholar]
- Lourie, S.A.; Vincent, A.C.J.; Hall, H.J. Seahorses: An Identification Guide to the World’s Species and Their Conservation; Project Seahorse: London, UK, 1999. [Google Scholar]
- Olivotto, I.; Planas, M.; Simoes, N.; Holt, G.J.; Avella, A.M.; Calado, R. Advances in breeding and rearing marine ornamentals. J. World Aquac. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Cohen, P.A.F.; Valenti, W.C.; Planas, M.; Calado, R. Seahorse Aquaculture, Biology and Conservation: Knowledge Gaps and Research Opportunities. Rev. Fish. Sci. Aquac. 2016, 25, 100–111. [Google Scholar] [CrossRef]
- Wilson, M.J.; Vincent, A.C.J. Preliminary success in closing the life cycle of exploited seahorse species, Hippocampus spp., in captivity. Aquar. Sci. Conserv. 1998, 2, 179–196. [Google Scholar] [CrossRef]
- Job, S.D.; Dob, H.H.; Meeuwig, J.J.; Hall, H.J. Culturing the oceanic seahorse, (Hippocampus kuda). Aquaculture 2002, 214, 333–341. [Google Scholar] [CrossRef]
- Woods, C.M.C. Effects of varying enrichment on growth and survival of juvenile seahorses, Hippocampus abdominalis. Aquaculture 2003, 220, 537–548. [Google Scholar] [CrossRef]
- Murugan, A.; Dhanya, S.; Sreepada, R.A.; Rajagopal, S.; Balasubramanian, T. Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 2009, 290, 87–96. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Molina, L.; Socorro, J.; Herrera, R.; Fernández-Palacios, H.; Izquierdo, M. Live prey first feeding regimes for short-snouted seahorse Hippocampus hippocampus (Linnaeus, 1758) juveniles. Aquac. Res. 2010, 41, e8–e19. [Google Scholar] [CrossRef]
- Planas, M.; Blanco, A.; Chamorro, A.; Valladares, S.; Pintado, J. Temperature-induced changes of growth and survival in the early development of the seahorse Hippocampus guttulatus. J. Exp. Mar. Biol. Ecol. 2012, 438, 154–162. [Google Scholar] [CrossRef]
- Payne, M.F.; Rippingale, R.J. Rearing West Australian seahorse, Hippocampus subelongatus, juveniles on copepod nauplii and enriched Artemia. Aquaculture 2000, 188, 353–361. [Google Scholar] [CrossRef]
- Olivotto, I.; Avella, M.A.; Sampaolesi, G.; Piccinetti, C.C.; Navarro-Ruiz, P.; Carnevali, O. Breeding and rearing the longsnout seahorse Hippocampus reidi: Rearing and feeding studies. Aquaculture 2008, 283, 92–96. [Google Scholar] [CrossRef]
- Blanco, A.; Chamorro, A.; Planas, M. Implications of physical key factors in the early rearing of the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2014, 433, 214–222. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M.; Moyano, F.J. Ontogeny of digestive enzymatic capacities in juvenile seahorses Hippocampus guttulatus fed on different live diets. Aquac. Res. 2015, 47, 3558–3569. [Google Scholar] [CrossRef]
- Cunha, I.; Planas, M. Optimal prey size for early turbot larvae (Scophthalmus maximus L.) based on mouth and ingested prey size. Aquaculture 1999, 175, 103–110. [Google Scholar] [CrossRef]
- Tudela, S.; Palomera, I.; Quílez, G. Feeding of anchovy Engraulis encrasicolus larvae in the north-west Mediterranean. J. Mar. Biolog. Associ. UK 2002, 82, 349–350. [Google Scholar] [CrossRef]
- Koven, W.M.; Parra, G.; Kolkovski, S.; Tandler, A. The effect of dietary phosphatidylcholine and its constituent fatty acids on microdiet ingestion and fatty acid absorption rate in gilthead seabream, Sparus auratus, larvae. Aquac. Nutr. 1998, 4, 39–45. [Google Scholar] [CrossRef]
- Morais, S.; Conceição, L.E.C.; Dinis, M.T.; Rønnestad, I. A method for radiolabeling Artemia with applications in studies of food intake, digestibility, protein and amino acid metabolism in larval fish. Aquaculture 2004, 231, 469–487. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Skjermo, J.; Skjåk-Bræk, G.; Verreth, J.A.J. Effect of an immunostimulating alginate on protein turnover of turbot (Scophthalmus maximus L) larvae. Fish. Physiol. Biochem. 2001, 24, 207–212. [Google Scholar] [CrossRef]
- Rønnestad, I.; Rojas-García, C.R.; Tonheim, S.K.; Conceição, L.E.C. In vivo studies of digestion and nutrient assimilation in marine fish larvae. Aquaculture 2001, 201, 161–175. [Google Scholar] [CrossRef]
- Verschoor, A.M.; Boonstra, H.; Meijer, T. Application of stable isotope tracers to studies of zooplankton feeding, using the rotifer Brachionus calyciflorus as an example. Hydrobiologia 2005, 546, 535–549. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Focken, U.; Becker, K. Stable isotopes as a tool for nutrient assimilation studies on larval fish feeding on live food. Aquat. Ecol. 2004, 38, 93–100. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Le Vay, L.; Gamboa-Delgado, J. Naturally-occurring stable isotopes as direct measures of larval feeding efficiency, nutrient incorporation and turnover. Aquaculture 2001, 315, 95–103. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Palma, J.; Stockdale, J.; Correia, M.; Andrade, J.P. Growth and survival of adult long snout seahorse (Hippocampus guttulatus) using frozen diets. Aquaculture 2008, 278, 55–59. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef]
- Valladares, S.; Planas, M. Application of effective day degrees in the assessment of stable isotope patterns in developing seahorses under different temperatures. Animals 2020, 10, 1571. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P. Maturation of Hippocampus guttulatus and Hippocampus hippocampus females by manipulation of temperature and photoperiod regimes. Aquaculture 2013, 391, 147–152. [Google Scholar] [CrossRef]
- Planas, M.; Paltrinieri, A.; Carneiro, M.D.D.; Hernández-Urcera, J. Effects of tissue preservation on carbon and nitrogen stable isotope signatures in syngnathid fishes and prey. Animals 2020, 10, 2301. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, B.; Rolla, L.; Ofelio, C.; Planas, M.; Gioacchini, G.; Vargas Abúndez, J.A.; Giorgini, E.; Olivotto, I. The influence of diet on the early development of two seahorse species (H. guttulatus and H. reidi): Traditional and innovative approaches. Aquaculture 2018, 490, 75–90. [Google Scholar] [CrossRef]
- Shields, R.J.; Bell, J.G.; Luizi, F.S.; Gara, B.; Bromage, N.R.; Sargent, J.R. Natural copepods are superior to enriched Artemia nauplii as feed for halibut larvae (Hippoglossus hippoglossus) in terms of survival, pigmentation and retinal morphology: Relation to dietary essential fatty acids. J. Nutr. 1999, 129, 1186–1194. [Google Scholar] [CrossRef]
- Ofelio, C.; Díaz, A.O.; Radaelli, G.; Planas, M. Histological characterization of early developmental stages in the seahorse Hippocampus guttulatus. J. Fish. Biol. 2018, 93, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Jomori, R.K.; Ducatti, C.; Carneiro, D.J.; Portella, M. Stable carbon (δ13C) and nitrogen (δ15N) isotopes as natural indicators of live and dry food in Piaractus mesopotamicus (Holmberg, 1887) larval tissue. Aquac. Res. 2008, 39, 370–381. [Google Scholar] [CrossRef]
- Bouligand, Y.; Neville, A.C. Filaments, fibrilles et paracristaux dans la cuticle des arthropodes. J. Microsc. 1973, 17, 198–217. [Google Scholar]
- Bresciani, J. The fine structure of the integument of free-living and parasitic copepods. A review. Acta Zool. 1986, 67, 125–145. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M. Mouth growth and prey selection in juveniles of the European long-snouted seahorse, Hippocampus guttulatus. J. World Aquac. Soc. 2015, 46, 596–607. [Google Scholar] [CrossRef]
- Willadino, L.; Souza-Santos, L.; Mélo, R.; Brito, A.; Barros, N.; Araújo-Castro, C.; Galvão, D.; Gouveia, A.; Regis, C.; Cavalli, R. Ingestion rate, survival and growth of newly released seahorse Hippocampus reidi fed exclusively on cultured live food items. Aquaculture 2012, 360–361, 10–16. [Google Scholar] [CrossRef]
- Tipton, K.; Bell, S.S. Foraging patterns of two syngnathid fishes: Importance of harpacticoid copepods. Mar. Ecol. Prog. Ser. 1988, 47, 31–43. [Google Scholar] [CrossRef]
- Teixeira, R.L.; Musick, J.A. Reproduction and food habits of the lined seahorse, Hippocampus erectus (Teleostei: Syngnathidae) of Chesapeake Bay, Virginia. Rev. Bras. Biol. 2001, 61, 79–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manning, C.G.; Foster, S.J.; Vincent, A.C.J. A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (Family Syngnathidae). Rev. Fish Biol. Fish. 2019, 29, 197–221. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (δ15N and δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
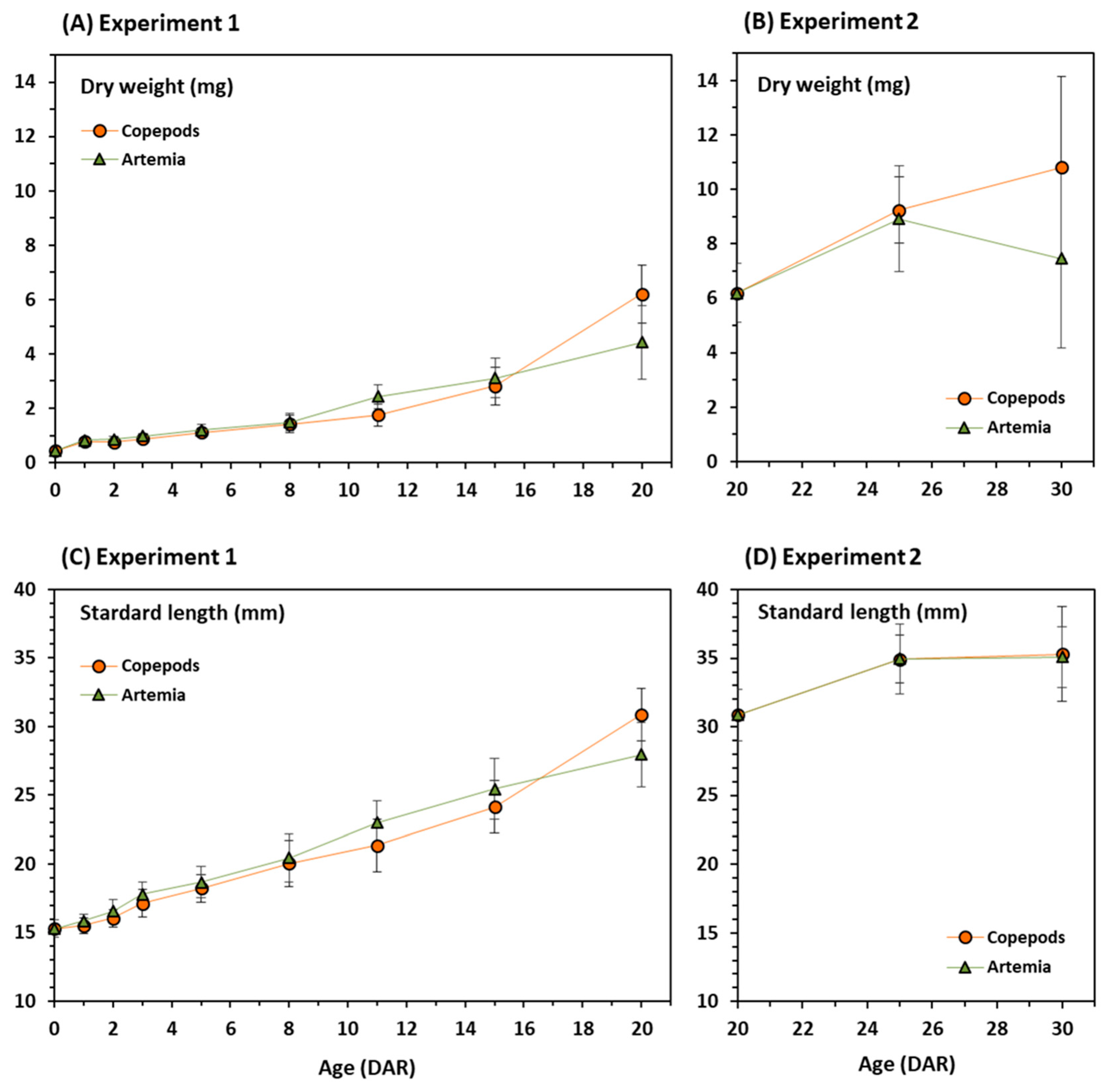

| Diet | Day | Survival (%) | DW (mg) | SL (mm) | WG (%) | SG (%) |
|---|---|---|---|---|---|---|
| Initial | 0 | 100 ± 0 | 0.44 ± 0.03 | 15.30 ± 0.62 | ||
| Experiment 1: Days 0–20 | ||||||
| Copepods | 20 | 98.33 ± 2.36 | 6.20 ± 1.07 | 30.87 ± 1.89 | 1409 | 202 |
| Artemia | 20 | 7.87 ± 1.12 | 4.42 ± 1.36 | 27.96 ± 2.35 | 1005 | 183 |
| Experiment 2: Days 20–30 | ||||||
| Copepods | 30 | 100 ± 0.00 | 10.80 ± 3.34 | 35.29 ± 3.44 | 2455 | 231 |
| Artemia | 30 | 91.19 ± 1.48 | 7.46 ± 3.27 | 35.08 ± 2.19 | 1695 | 229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladares, S.; Planas, M. Nutrient Incorporation in First Feeding Seahorses Evidenced by Stable Carbon Isotopes. Animals 2021, 11, 470. https://doi.org/10.3390/ani11020470
Valladares S, Planas M. Nutrient Incorporation in First Feeding Seahorses Evidenced by Stable Carbon Isotopes. Animals. 2021; 11(2):470. https://doi.org/10.3390/ani11020470
Chicago/Turabian StyleValladares, Sonia, and Miquel Planas. 2021. "Nutrient Incorporation in First Feeding Seahorses Evidenced by Stable Carbon Isotopes" Animals 11, no. 2: 470. https://doi.org/10.3390/ani11020470
APA StyleValladares, S., & Planas, M. (2021). Nutrient Incorporation in First Feeding Seahorses Evidenced by Stable Carbon Isotopes. Animals, 11(2), 470. https://doi.org/10.3390/ani11020470







