Thermal Mechanisms Preventing or Favoring Multiple Ovulations in Dairy Cattle
Abstract
Simple Summary
Abstract
1. Introduction
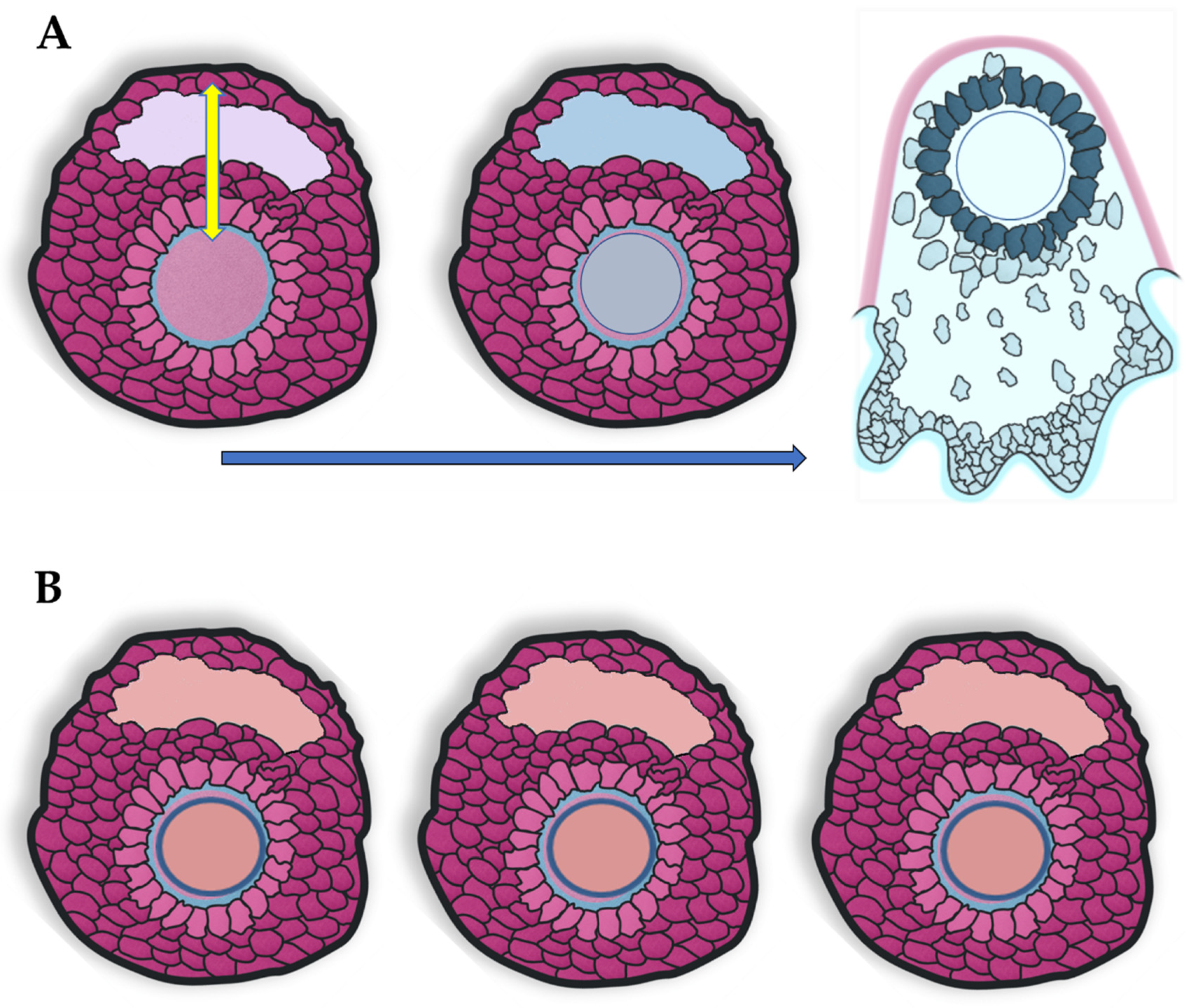
2. Temperature Gradients in Mammalian Female Reproductive Organs
3. Single versus Double Ovulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mee, J.F. Factors affecting the spontaneous twinning rate and the effect of twinning on calving problems in nine Irish dairy herds. Irish Vet. J. 1991, 44, 14–20. [Google Scholar]
- Kinsel, M.L.; Marsh, W.E.; Ruegg, P.L.; Etherington, W.G. Risk factors for twinning in dairy cows. J. Dairy Sci. 1998, 81, 989–993. [Google Scholar] [CrossRef]
- Andreu-Vázquez, C.; Garcia-Ispierto, I.; Ganau, S.; Fricke, P.M.; López-Gatius, F. Effects of twinning on the subsequent reproductive performance and productive lifespan of high-producing dairy cows. Theriogenology 2012, 78, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Andreu-Vázquez, C.; Mur-Novales, R.; Cabrera, V.E.; Hunter, R.H.F. The dilemma of twin pregnancies in dairy cattle. A review of practical prospects. Livest. Sci. 2017, 197, 121–126. [Google Scholar] [CrossRef]
- Johanson, J.M.; Berger, P.J.; Kirkpatrick, B.W.; Dentines, M.R. Twinning rates for North American Holstein sires. J. Dairy Sci. 2001, 84, 2081–2088. [Google Scholar] [CrossRef]
- Fricke, P.M.; Wiltbank, M.C. Effect of milk production on the incidence of double ovulation in dairy cows. Theriogenology 1999, 52, 1133–1143. [Google Scholar] [CrossRef]
- Lopez, H.; Caraviello, D.Z.; Satter, L.D.; Fricke, P.M.; Wiltbank, M.C. Relationship between level of milk production and multiple ovulations in lactating dairy cows. J. Dairy Sci. 2005, 88, 2783–2793. [Google Scholar] [CrossRef]
- López-Gatius, F.; López-Béjar, M.; Fenech, M.; Hunter, R.H.F. Ovulation failure and double ovulation in dairy cattle: Risk factors and effects. Theriogenology 2005, 63, 1298–1307. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; López-Gatius, F. A three-day PGF2α plus eCG-based fixed-time AI protocol improves fertility compared with spontaneous estrus in dairy cows with silent ovulation. J. Reprod. Dev. 2013, 59, 393–397. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; Roselló, M.A.; De Rensis, F.; López-Gatius, F. A five-day progesterone plus eCG-based fixed-time AI protocol improves fertility over spontaneous estrus in high-producing dairy cows under heat stress. J. Reprod. Dev. 2013, 59, 544–548. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Fricke, P.M.; Sangritasvong, S.; Sartori, R.; Ginther, O.J. Mechanisms that prevent and produce double ovulations in dairy cattle. J. Dairy Sci. 2000, 83, 2998–3007. [Google Scholar] [CrossRef]
- Andreu-Vázquez, C.; Garcia-Ispierto, I.; López-Gatius, F. Photoperiod length and the estrus synchronization protocol used before AI affect the twin pregnancy rate in dairy cattle. Theriogenology 2012, 78, 1209–1216. [Google Scholar] [CrossRef]
- Labhsetwar, A.P.; Tyler, W.J.; Casida, L.E. Analysis of variation in some factors affecting multiple ovulations in Holstein cattle. J. Dairy Sci. 1963, 46, 840–842. [Google Scholar] [CrossRef]
- Macmillan, K.; Kastelic, J.P.; Colazo, M.G. Update on multiple ovulations in dairy cattle. Animals 2018, 8, 62. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Einer-Jensen, N.; Greve, T. Presence and significance of temperature gradients among different ovarian tissues. Microsc. Res. Tech. 2006, 69, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F. Temperature gradients in female reproductive tissues. Reprod. BioMed. Online 2012, 24, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F.; López-Gatius, F.; López-Albors, O. Temperature gradients in vivo influence maturing male and female gametes in mammals: Evidence from the cow. Reprod. Fertil. Dev. 2017, 29, 2301–2304. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; López-Gatius, F. Temperature gradients in the mammalian ovary and genital tract: A clinical perspective. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 382–386. [Google Scholar] [CrossRef]
- Ali, H.E.; Kitahara, G.; Tamura, Y.; Kobayashi, I.; Hemmi, K.; Torisu, S.; Sameshima, H.; Horii, Y.; Zaabel, S.; Kamimura, S. Presence of a temperature gradient among genital tract portions and the thermal changes within these portions over the estrous cycle in beef cows. J. Reprod. Dev. 2013, 59, 59–65. [Google Scholar]
- Hunter, R.H.F.; Nichol, R.; Crabtree, S.M. Transport of spermatozoa in the ewe: Timing of the establishment of a functional population in the oviduct. Reprod. Nutr. Dev. 1980, 20, 869–875. [Google Scholar] [CrossRef]
- Hunter, R.H.F. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J. Reprod. Fertil. 1981, 63, 109–117. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Wilmut, I. Sperm transport in the cow: Periovulatory redistribution of viable cells within the oviduct. Reprod. Nutr. Dev. 1984, 24, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F.; Nichol, R.A. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J. Reprod. Fertil. 1986, 77, 599–606. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; López-Gatius, F. Evolutionary sequences in mammalian reproductive biology. J. Exp. Zool. A 2020, 333, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Rutllant, J.; López-Béjar, M.; López-Gatius, F. Ultrastructural and rheological properties of bovine vaginal fluid and its relation to sperm motility and fertilization: A review. Reprod. Domest. Anim. 2005, 40, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F.; López-Gatius, F. Intra-follicular temperature acts to regulate mammalian ovulation. Acta Obstet. Gynecol. Scand. 2020, 99, 301–302. [Google Scholar] [CrossRef]
- Benoit, H.J.; Borth, R.; Ellicott, A.R.; Woolever, C.A. Periovulatory changes in ovarian temperature in ewes. Am. J. Obstet. Gynecol. 1976, 124, 356–360. [Google Scholar] [CrossRef]
- Grinsted, J.; Glendstrup, K.; Andreasen, M.P.; Byskov, A.G. Temperature measurements of rabbit antral follicles. J. Reprod. Fertil. 1980, 60, 149–155. [Google Scholar] [CrossRef]
- Grinsted, J.; Kjer, J.J.; Blendstrup, K.; Pedersen, J.F. Is low temperature of the follicular fluid prior to ovulation necessary for normal oocyte development? Fertil. Steril. 1985, 43, 34–39. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Grondahl, C.; Greve, T.; Schmidt, M. Graafian follicles are cooler than neighbouring ovarian tissues and deep rectal temperatures. Hum. Reprod. 1997, 12, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunter, R.H.F.; Bogh, I.B.; Einer-Jensen, N.; Müller, S.; Greve, T. Pre-ovulatory Graafian follicles are cooler than neighbouring stroma in pig ovaries. Hum. Reprod. 2000, 15, 273–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greve, T.; Grøndahl, C.; Schmidt, M.; Hunter, R.H.F.; Avery, B. Bovine preovulatory follicular temperature: Implications for in vitro production of embryos. Archiv. Tierzucht. 1996, 39, 7–14. [Google Scholar]
- López-Gatius, F.; Hunter, R.H.F. Clinical relevance of pre-ovulatory follicular temperature in heat-stressed lactating dairy cows. Reprod. Domest. Anim. 2017, 52, 366–370. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R.H.F. Pre-ovulatory follicular temperature in bi-ovular cows. J. Reprod. Dev. 2019, 65, 191–194. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R.H.F. Pre-ovulatory follicular cooling correlates positively with the potential for pregnancy in dairy cows: Implications for human IVF. J. Gyn. Obstet. Hum. Reprod. 2019, 48, 419–422. [Google Scholar] [CrossRef]
- Morita, Y.; Ozaki, R.; Mukaiyama, A.; Sasaki, T.; Tatebyashi, R.; Morishima, A.; Kitagawa, Y.; Suzumura, R.; Abe, R.; Tsukamura, H.; et al. Establishment of long-term chronic recording technique of in vivo ovarian parenchymal temperature in Japanese Black cows. J. Reprod. Dev. 2020, 66, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Einer-Jensen, N.; Hunter, R.H.F. Physiological and pharmacological aspects of local transfer of substances in the ovarian adnexa in women. Hum. Reprod. Update 2000, 6, 132–138. [Google Scholar] [CrossRef]
- Cicinelli, E.; Einer-Jensen, N.; Barba, B.; Luisi, D.; Alfonso, R.; Tartagni, M. Blood to the corneal area of uterus is mainly supplied from the ovarian artery in the follicular phase and from the uterine artery in the luteal phase. Hum. Reprod. 2004, 19, 1003–1008. [Google Scholar] [CrossRef][Green Version]
- Einer-Jensen, N.; Hunter, R.H.F. Counter-current transfer in reproductive biology. Reproduction 2005, 129, 9–18. [Google Scholar] [CrossRef]
- Roth, Z. Effect of heat stress on reproduction in dairy cows: Insights into the cellular and molecular responses of the oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef]
- Hansen, P.J. Reproductive physiology of the heat-stressed dairy cow: Implications for fertility and assisted reproduction. Anim. Reprod. 2019, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Hunter, R.H.F. Local cooling of the ovary and its implications for heat stress effects on reproduction. Theriogenology 2020, 149, 98–103. [Google Scholar] [CrossRef]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ispierto, I.; López-Gatius, F. Effects of five-day progesterone-based fixed-time AI protocols on follicular/luteal dynamics and fertility in dairy cows. J. Reprod. Dev. 2014, 60, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ispierto, I.; De Rensis, F.; Pérez-Salas, J.A.; Nunes, J.M.; Pradés, B.; Serrano-Pérez, B.; López-Gatius, F. The GnRH analogue dephereline given in a fixed-time AI protocol improves ovulation and embryo survival in dairy cows. Res. Vet. Sci. 2019, 122, 170–174. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Garcia-Ispierto, I.; Serrano-Pérez, B.; Hunter, R.H.F. The presence of two ovulatory follicles at timed artificial insemination influences the ovulatory response to GnRH in high-producing dairy cows. Theriogenology. 2018, 120, 91–97. [Google Scholar] [CrossRef]
- Ginther, O.J.; Wiltbank, M.C.; Fricke, P.M.; Gibbons, J.R.; Kot, K. Selection of the dominant follicle in cattle. Biol. Reprod. 1996, 55, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ispierto, I.; De Rensis, F.; Casas, X.; Caballero, F.; Mur-Novales, R.; López-Gatius, F. Reproductive performance of lactating dairy cows after inducing ovulation using hCG in a five-day progesterone-based fixed-time AI protocol. Theriogenology 2018, 107, 175–179. [Google Scholar] [CrossRef]
- Bleach, E.C.; Glencross, R.G.; Knight, P.G. Association between ovarian follicle development and pregnancy rates in dairy cows undergoing spontaneous oestrous cycles. Reproduction 2004, 127, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, H.; Miura, H.; Kikuchi, M.; Sakaguchi, M. Incidence of double ovulation during the early postpartum period in lactating dairy cows. Theriogenology 2017, 91, 98–103. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Garcia-Ispierto, I.; Hunter, R.H.F. Twin pregnancies in dairy cattle: Observations in a large herd of Holstein-Friesian dairy cows. Animals 2020, 10, 2165. [Google Scholar] [CrossRef]
- Echternkamp, S.E. Fetal development in cattle with multiple ovulations. J. Anim. Sci. 1992, 70, 2309–2321. [Google Scholar] [CrossRef]
- Echternkamp, S.E.; Roberts, A.J.; Lunstra, D.D.; Wise, T.; Spicer, L.J. Ovarian follicular development in cattle selected for twin ovulations and births. J. Anim. Sci. 2004, 82, 459–471. [Google Scholar] [CrossRef]
- Acosta, T.J.; Beg, M.A.; Ginther, O.J. Effects of modified FSH surges on follicle selection and codominance in heifers. Anim. Reprod. 2005, 2, 28–40. [Google Scholar]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.-A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Vinet, A.; Drouilhet, L. Genetic control of multiple births in low ovulating mammalian species. Mamm. Genome 2012, 23, 727–740. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R.H.F. Preventing twin pregnancies in dairy cattle, turning the odds into reality. Livest. Sci. 2019, 229, 1–3. [Google Scholar] [CrossRef]
- López-Gatius, F.; Garcia-Ispierto, I. Transfer of a single embryo versus drainage of subordinate follicles to prevent twin pregnancies in dairy cows. Why not both? J. Reprod. Dev. 2020, 66, 287–289. [Google Scholar] [CrossRef]
- López-Gatius, F. Twins in dairy herds. Is it better to maintain or reduce a pregnancy? Animals 2020, 10, 2006. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rensis, F.; Morini, G.; Garcia-Ispierto, I.; López-Gatius, F. Thermal Mechanisms Preventing or Favoring Multiple Ovulations in Dairy Cattle. Animals 2021, 11, 435. https://doi.org/10.3390/ani11020435
De Rensis F, Morini G, Garcia-Ispierto I, López-Gatius F. Thermal Mechanisms Preventing or Favoring Multiple Ovulations in Dairy Cattle. Animals. 2021; 11(2):435. https://doi.org/10.3390/ani11020435
Chicago/Turabian StyleDe Rensis, Fabio, Giorgio Morini, Irina Garcia-Ispierto, and Fernando López-Gatius. 2021. "Thermal Mechanisms Preventing or Favoring Multiple Ovulations in Dairy Cattle" Animals 11, no. 2: 435. https://doi.org/10.3390/ani11020435
APA StyleDe Rensis, F., Morini, G., Garcia-Ispierto, I., & López-Gatius, F. (2021). Thermal Mechanisms Preventing or Favoring Multiple Ovulations in Dairy Cattle. Animals, 11(2), 435. https://doi.org/10.3390/ani11020435





