Chemically-Induced Inflammation Changes the Number of Nitrergic Nervous Structures in the Muscular Layer of the Porcine Descending Colon
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
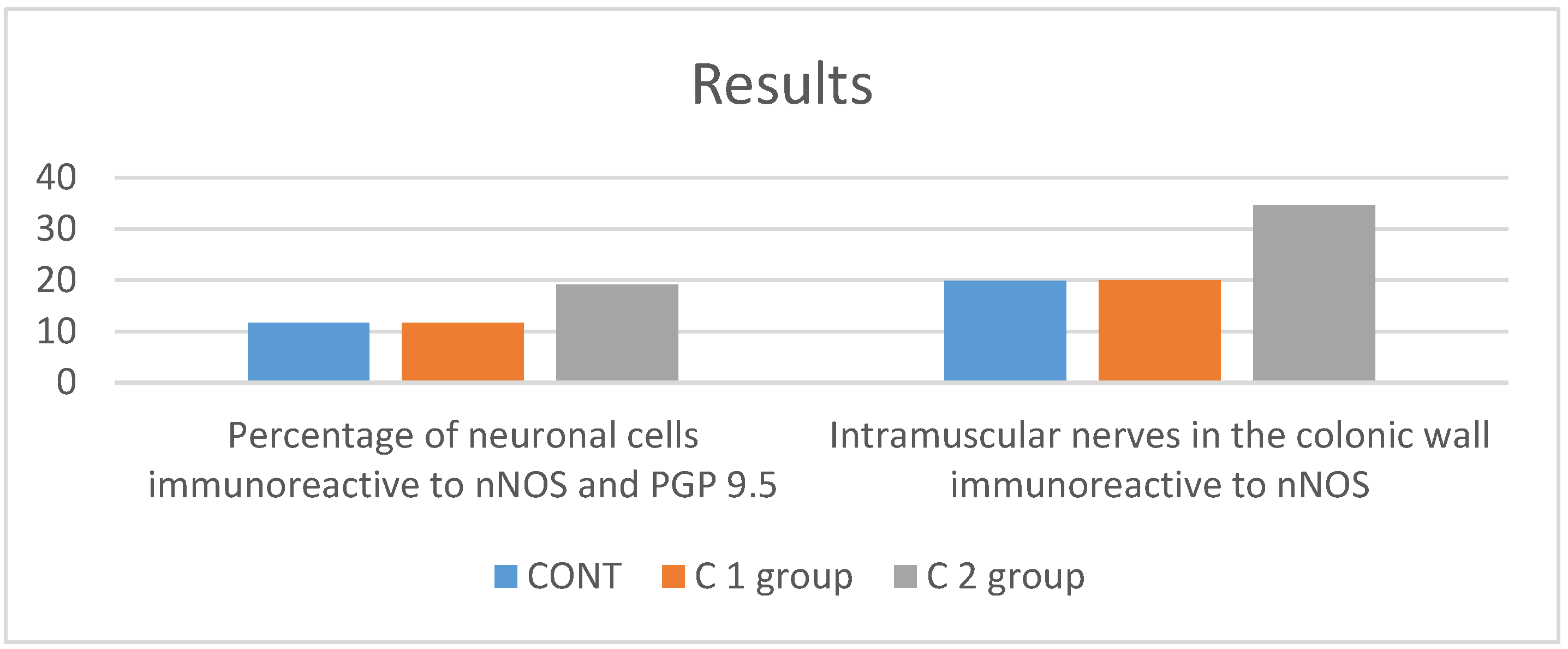
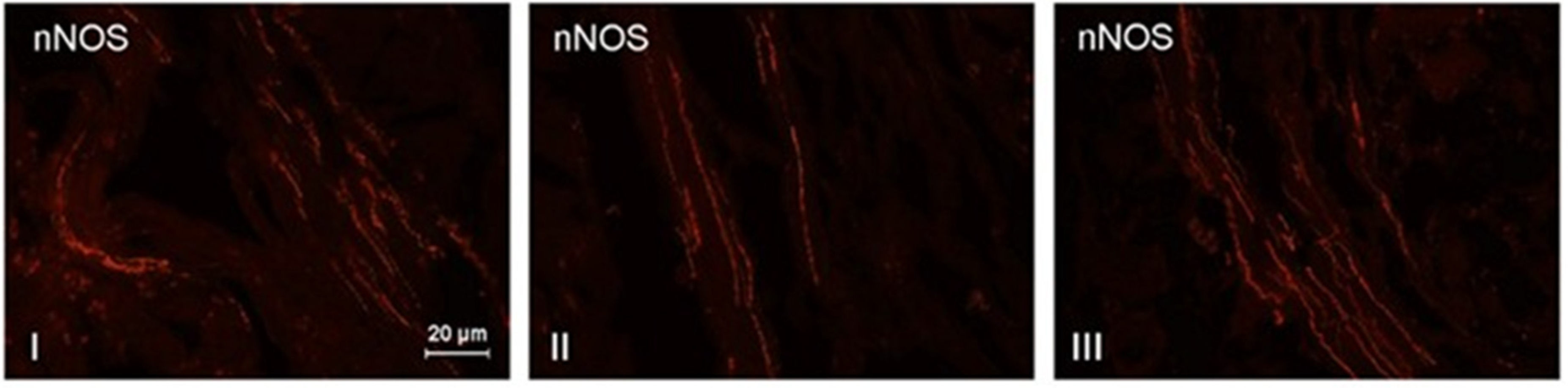
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ENS | enteric nervous system |
| NO | nitric oxide |
| GI | gastrointestinal |
| MP | myenteric plexus |
| OSP | outer submucous plexus |
| nNOS | neuronal isoform of nitric oxide synthase |
| PGP 9.5 | protein gene product 9.5 |
| IR | immunoreactive |
Appendix A
| Antigen | Species of Origin | Code | Supplier | Country | Working Dilutions |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| PGP-9.5 | Mouse | 7863-2004 | Biogenesis | USA | 1:1000 |
| nNOS | Rabbit | AB5380 | Merck Millipore | Poland | 1:2000 |
| Secondary antibodies | |||||
| Alexa fluor 488 | Anti Mouse | A21206 | Invitrogen | USA | 1:1000 |
| Alexa fluor 546 | Anti Rabbit | A10036 | Invitrogen | USA | 1:1000 |
References
- Grundy, D.; Schemann, M. Enteric nervous system. Curr. Opin. Gastroenterol. 2006, 22, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Schemann, M. Control of gastrointestinal motility by the “gut brain”—The enteric nervous system. J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. 1), S4–S6. [Google Scholar] [CrossRef]
- Furness, J.B.; Stebbing, M.J. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil. 2018, 30, e13234. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B. The enteric nervous system I: Organisation and classification. Pharmacol. Toxicol. 2003, 92, 105–113. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 toxin-induced changes in cocaine- and amphetamine-regulated transcript (CART)-like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2017, 1, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Zacharko-Siembida, A.; Valverde Piedra, J.L.; Szymańczyk, S.; Arciszewski, M.B. Immunolocalization of NOS, VIP, galanin and SP in the small intestine of suckling pigs treated with red kidney bean (Phaseolus vulgaris) lectin. Acta Histochem. 2013, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- McCann, C.J.; Cooper, J.E.; Natarajan, D.; Jevans, B.; Burnett, L.E.; Burns, A.J.; Thapar, N. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat. Commun. 2017, 8, 1593–1597. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Rao, Y.M.; Chaudhury, A.; Goyal, R.K. Active and inactive pools of nNOS in the nerve terminals in mouse gut: Implications for nitrergic neurotransmission. Am. J. Physiol. Gastrointest. Liver. Physiol. 2008, 294, G627–G634. [Google Scholar] [CrossRef][Green Version]
- Cossenza, M.; Socodato, R.; Portugal, C.C.; Domith, I.C.; Gladulich, L.F.; Encarnação, T.G.; Calaza, K.C.; Mendonça, H.R.; Campello-Costa, P.; Paes-de-Carvalho, R. Nitric oxide in the nervous system: Biochemical, developmental, and neurobiological aspects. Vitam. Horm. 2014, 96, 79–125. [Google Scholar]
- Stojanović, M.; Šćepanović, L.; Hrnčić, D.; Rašić-Marković, A.; Djuric, D.; Stanojlović, O. Multidisciplinary approach to nitric oxide signaling: Focus on the gastrointestinal and the central nervous system. Vojnosanit. Pregl. 2015, 72, 619–624. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, D.Y. Hippocampus and nitric oxide. Vitam. Horm. 2014, 96, 127–160. [Google Scholar]
- Savidge, T.C. Importance of NO and its related compounds in enteric nervous system regulation of gut homeostasis and disease susceptibility. Curr. Opin. Pharmacol. 2014, 19, 54–60. [Google Scholar] [CrossRef]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Corsani, L.; Bani, D.; Faussone-Pellegrini, M.S. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci. Lett. 2002, 326, 191–195. [Google Scholar] [CrossRef]
- Kochar, N.I.; Chandewal, A.V.; Bakal, R.L.; Kochar, P.N. Nitric oxide and the gastrointestinal tract. Int. J. Pharmacol. 2011, 7, 31–39. [Google Scholar] [CrossRef]
- Maake, C.; Kaufmann, C.; Reinecke, M. Ontogeny of neurohormonal peptides, serotonin, and nitric oxide synthase in the gastrointestinal neuroendocrine system of the axolotl (Ambystoma mexicanum): An immunohistochemical analysis. Gen. Comp. Endocrinol. 2001, 121, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Kita, K.; Hayashi, S.; Aihara, E. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol. Ther. 2011, 130, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 8, 1397–1406. [Google Scholar] [CrossRef]
- Grider, J.R.; Murthy, K.S. Autoinhibition of endothelial nitric oxide synthase (eNOS) in gut smooth muscle by nitric oxide. Regul. Pept. 2008, 151, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Wittmeyer, V.; Merrot, T.; Mazet, B. Tonic inhibition of human small intestinal motility by nitric oxide in children but not in adults. Neurogastroenterol. Motil. 2010, 22, 1078-e282. [Google Scholar] [CrossRef] [PubMed]
- Meile, T.; Glatzle, J.; Habermann, F.M.; Kreis, M.E.; Zittel, T.T. Nitric oxide synthase inhibition results in immediate postoperative recovery of gastric, small intestinal and colonic motility in awake rats. Int. J. Colorectal. Dis. 2006, 21, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int. J. Mol. Sci. 2019, 20, 1024. [Google Scholar] [CrossRef]
- Rychlik, A.; Gonkowski, S.; Kaczmar, E.; Obremski, K.; Calka, J.; Makowska, K. The T2 toxin produced by Fusarium spp. impacts porcine duodenal nitric oxide synthase (nNOS)-positive nervous structures-the preliminary study. Int. J. Mol. Sci. 2020, 21, 5118. [Google Scholar] [CrossRef]
- Schumacher, U.; Mitchell, B.S.; Kaiserling, E. The neuronal marker protein gene product 9.5 (PGP 9.5) is phenotypically expressed in human breast epithelium, in milk, and in benign and malignant breast tumors. DNA Cell Biol. 1994, 13, 839–843. [Google Scholar] [CrossRef]
- Day, I.N.M.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar] [CrossRef]
- D’Andrea, V.; Malinovsky, L.; Berni, A.; Biancari, F.; Biassoni, L.; Di Matteo, F.M.; Corbellini, L.; Falvo, L. The immunolocalization of PGP 9.5 in normal human kidney and renal cell carcinoma. G Chir. 1997, 18, 521–524. [Google Scholar]
- Olerud, J.E.; Chiu, D.S.; Usui, M.L.; Gibran, N.S.; Ansel, J.C. Protein gene product 9.5 is expressed by fibroblasts in human cutaneous wounds. J. Invest. Dermatol. 1998, 111, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, L.; Martin, R.; Paniagua, R.; Fraile, B.; Nistal, M.; Terenghi, G.; Polak, J.M. Protein gene product 9.5 and ubiquitin immunoreactivities in rat epididymis epithelium. Histochemistry 1993, 100, 131–138. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. The influence of inflammation and nerve damage on the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) neurons in the enteric nervous system of the porcine descending colon. Int. J. Mol. Sci. 2018, 19, 548. [Google Scholar] [CrossRef]
- Beck, K.; Friebe, A.; Voussen, B. Nitrergic signaling via interstitial cells of Cajal and smooth muscle cells influences circular smooth muscle contractility in murine colon. Neurogastroenterol. Motil. 2018, 30, e13300. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach-the study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef]
- Bruno, C.J.; Greco, T.M.; Ischiropoulos, H. Nitric oxide counteracts the hyperoxia-induced proliferation and proinflammatory responses of mouse astrocytes. Free Radic. Biol. Med. 2011, 51, 474–479. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Liu, X.; Guo, P.; Liu, A.; Wu, Q.; Xue, X.; Dai, M.; Hao, H.; Qu, W.; Xie, S.; Wang, X.; et al. Nitric oxide (NO)-mediated mitochondrial damage plays a critical role in T-2 toxin-induced apoptosis and growth hormone deficiency in rat anterior pituitary GH3 cells. Food Chem. Toxicol. 2017, 102, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Mayasi, Y.; Hannoun, A.; Eslami, S.M.; Carandang, R. Nitric oxide and mitochondrial function in neurological diseases. Neuroscience 2018, 376, 48–71. [Google Scholar] [CrossRef] [PubMed]
- Karaçay, B.; Bonthius, D.J. The neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicity. Cell. Mol. Neurobiol. 2015, 35, 449–461. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Terry, M.H.; Schroeder, H.; Wilson, S.M.; Power, G.G.; Li, Q.; Tipple, T.E.; Borchardt, D.; Blood, A.B. Nitrite potentiates the vasodilatory signaling of S-nitrosothiols. Nitric Oxide 2018, 75, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Belzer, V.; Hanani, M. Nitric oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia 2019, 67, 1296–1307. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rytel, L.; Gonkowski, I.; Grzegorzewski, W.; Wojtkiewicz, J. Chemically-Induced Inflammation Changes the Number of Nitrergic Nervous Structures in the Muscular Layer of the Porcine Descending Colon. Animals 2021, 11, 394. https://doi.org/10.3390/ani11020394
Rytel L, Gonkowski I, Grzegorzewski W, Wojtkiewicz J. Chemically-Induced Inflammation Changes the Number of Nitrergic Nervous Structures in the Muscular Layer of the Porcine Descending Colon. Animals. 2021; 11(2):394. https://doi.org/10.3390/ani11020394
Chicago/Turabian StyleRytel, Liliana, Ignacy Gonkowski, Waldemar Grzegorzewski, and Joanna Wojtkiewicz. 2021. "Chemically-Induced Inflammation Changes the Number of Nitrergic Nervous Structures in the Muscular Layer of the Porcine Descending Colon" Animals 11, no. 2: 394. https://doi.org/10.3390/ani11020394
APA StyleRytel, L., Gonkowski, I., Grzegorzewski, W., & Wojtkiewicz, J. (2021). Chemically-Induced Inflammation Changes the Number of Nitrergic Nervous Structures in the Muscular Layer of the Porcine Descending Colon. Animals, 11(2), 394. https://doi.org/10.3390/ani11020394






