An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors
Simple Summary
Abstract
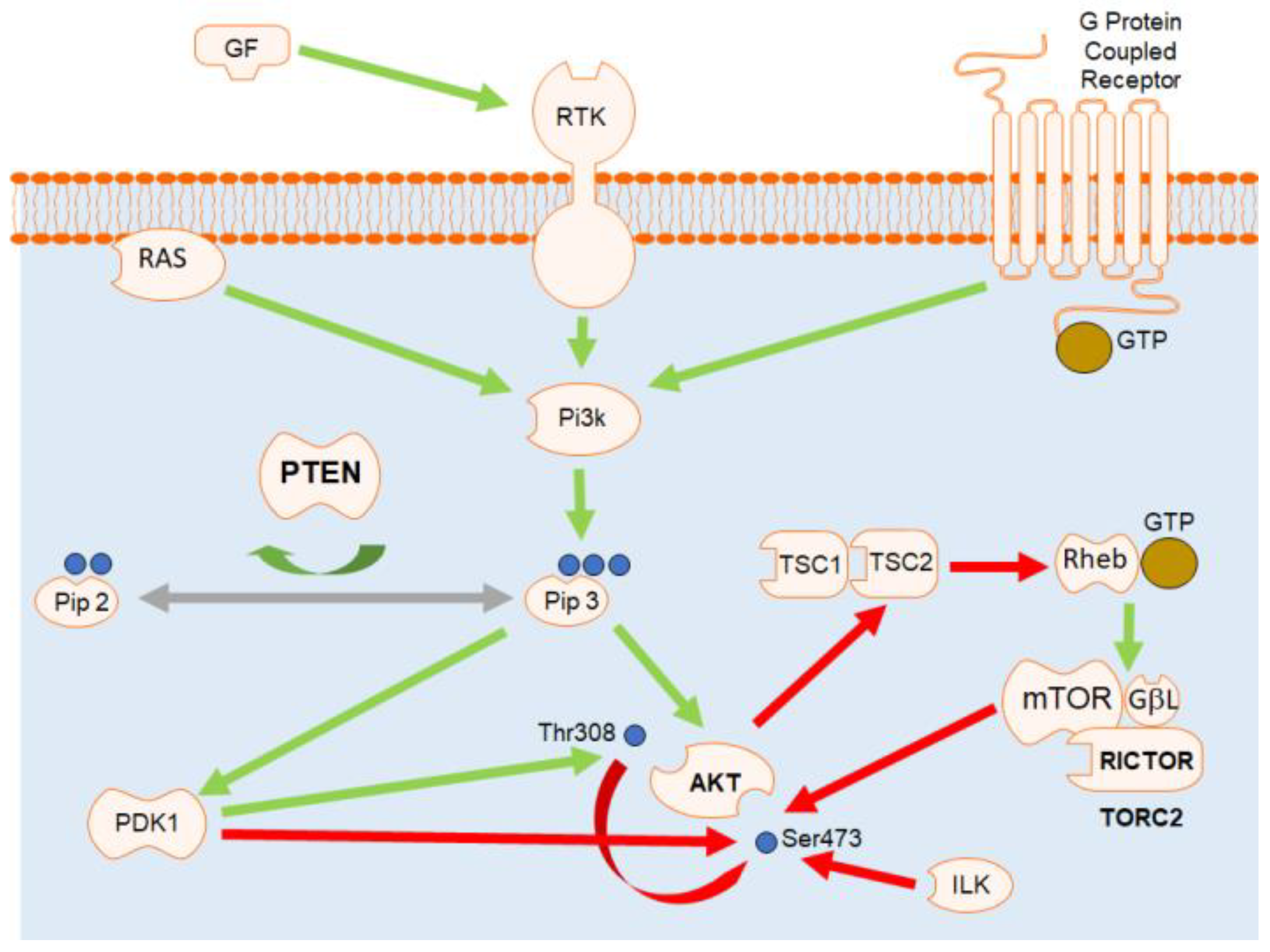
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
2.2. Tissue Processing and Histopathology
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. General Data
3.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carnero, A.; Paramio, J.M. The PTEN/PI3K/AKT Pathway in vivo, Cancer Mouse Models. Front. Oncol. 2014, 4, 252. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Bioenerg. 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, L. Chapter 2 PI3K/PTEN Signaling in Angiogenesis and Tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [PubMed]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shtilbans, V.; Wu, M.; Burstein, D.E. Current overview of the role of Akt in cancer studies via applied immunohistochemistry. Ann. Diagn. Pathol. 2008, 12, 153–160. [Google Scholar] [CrossRef]
- Skinner, H.D.; Zheng, J.Z.; Fang, J.; Agani, F.; Jiang, B.-H. Vascular Endothelial Growth Factor Transcriptional Activation Is Mediated by Hypoxia-inducible Factor 1α, HDM2, and p70S6K1 in Response to Phosphatidylinositol 3-Kinase/AKT Signaling. J. Biol. Chem. 2004, 279, 45643–45651. [Google Scholar] [CrossRef]
- Pal, S.K.; Reckamp, K.; Yu, H.; Figlin, R.A. Akt inhibitors in clinical development for the treatment of cancer. Exp. Opin. Investig. Drugs 2010, 19, 1355–1366. [Google Scholar]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Giles, K.M.; Rosenbaum, B.E.; Berger, M.; Izsak, A.; Li, Y.; Illa-Bochaca, I.; De Miera, E.V.-S.; Wang, J.; Darvishian, F.; Zhong, H.; et al. Revisiting the Clinical and Biologic Relevance of Partial PTEN Loss in Melanoma. J. Investig. Dermatol. 2019, 139, 430–438. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, J.; Cao, H.; Pilo, M.G.; Cigliano, A.; Shao, Z.; Xu, M.; Ribback, S.; Dombrowski, F.; Calvisi, D.F.; et al. Loss of Pten synergizes with c-Met to promote hepatocellular carcinoma development via mTORC2 pathway. Exp. Mol. Med. 2018, 50, e417. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Sun, R.; Tang, C.; Tzankov, A.; Zhang, J.; Manyam, G.C.; Xiao, M.; Miao, Y.; Jabbar, K.; et al. Clinical Significance of PTEN Deletion, Mutation, and Loss of PTEN Expression in De Novo Diffuse Large B-Cell Lymphoma. Neoplasia 2018, 20, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Blazes, M.S.; Wu, R.; Cho, K.R.; Bose, S.; Wang, S.I.; Li, J.; Parsons, R.; Ellenson, L.H. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997, 57, 3935–3940. [Google Scholar] [PubMed]
- Bruni, P.; Boccia, A.; Baldassarre, G.; Trapasso, F.; Santoro, M.; Chiappetta, G.; Fusco, A.; Viglietto, G. PTEN expression is reduced in a subset of sporadic thyroid carcinomas: Evidence that PTEN-growth suppressing activity in thyroid cancer cells is mediated by p27kip. Oncogene 2000, 19, 3146–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chi, J.; Gao, M.; Zhi, J.; Li, Y.; Zheng, X. Loss of PTEN Expression Is Associated with High MicroRNA 24 Level and Poor Prognosis in Patients with Tongue Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2017, 75, 1449.e1–1449.e8. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Bianco, S.R.; Fosmire, S.; Wojcieszyn, J.; Modiano, J.F. Expression and significance of p53, rb, p21/waf-1, p16/ink-4a, and PTEN tumor suppressors in canine melanoma. Veter. Pathol. 2002, 39, 458–472. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Thomas, R.; Fosmire, S.P.; Lamerato-Kozicki, A.R.; Bianco, S.R.; Wojcieszyn, J.W.; Breen, M.; Helfand, S.C.; Modiano, J.F. Mutations of Phosphatase and Tensin Homolog Deleted from Chromosome 10 in Canine Hemangiosarcoma. Veter. Pathol. 2005, 42, 618–632. [Google Scholar] [CrossRef]
- Levine, R.A.; Forest, T.; Smith, C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Veter. Pathol. 2002, 39, 372–378. [Google Scholar] [CrossRef]
- Russell, D.S.; Jaworski, L.; Kisseberth, W.C. Immunohistochemical detection of p53, PTEN, Rb, and p16 in canine osteosarcoma using tissue microarray. J. Veter. Diagn. Investig. 2018, 30, 504–509. [Google Scholar] [CrossRef]
- Calderón, L.G.R.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; Vasconcelos, R.D.O.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Veter. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef]
- Ressel, L.; Millanta, F.; Caleri, E.; Innocenti, V.M.; Poli, A. Reduced PTEN Protein Expression and Its Prognostic Implications in Canine and Feline Mammary Tumors. Veter. Pathol. 2009, 46, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, L.; Iussich, S.; Mulas, J.M.D.L.; Millán, Y.; Biolatti, B.; Sasaki, N.; Nakagawa, T.; De Maria, R. Activation of AKT in feline mammary carcinoma: A new prognostic factor for feline mammary tumours. Veter. J. 2012, 191, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Lin, D.; Wang, J.; Wang, L. Expression and significance of PTEN in canine mammary gland tumours. Res. Vet. Sci. 2008, 85, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Borge, K.S.; Nord, S.; Van Loo, P.; Lingjærde, O.C.; Gunnes, G.; Alnæs, G.I.G.; Solvang, H.K.; Lüders, T.; Kristensen, V.N.; Børresen-Dale, A.-L.; et al. Canine Mammary Tumours Are Affected by Frequent Copy Number Aberrations, including Amplification of MYC and Loss of PTEN. PLoS ONE 2015, 10, e0126371. [Google Scholar] [CrossRef] [PubMed]
- Wickenden, J.A.; Watson, C.J. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: A play in 3 Akts. Breast Cancer Res. 2010, 12, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Ouyang, F.; Liu, X.; Wu, S.; Wu, H.-M.; Xu, Y.; Wang, B.; Zhu, J.; Xu, X.; Zhang, L. Overexpressed CISD2 has prognostic value in human gastric cancer and promotes gastric cancer cell proliferation and tumorigenesis via AKT signaling pathway. Oncotarget 2016, 7, 3791–3805. [Google Scholar] [CrossRef]
- Fenic, I.; Steger, K.; Gruber, C.; Arens, C.; Woenckhaus, J. Analysis of PIK3CA and Akt/protein kinase B in head and neck squamous cell carcinoma. Oncol. Rep. 2007, 18, 253–259. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Hui, Y.; Hua, Y.; Huang, L.; Wang, L.; Peng, F.; Tang, C.; Liu, D.; Song, J.; Wang, F. EG-VEGF silencing inhibits cell proliferation and promotes cell apoptosis in pancreatic carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2019, 109, 762–769. [Google Scholar] [CrossRef]
- De Marco, C.; Rinaldo, N.; Bruni, P.; Malzoni, C.; Zullo, F.; Fabiani, F.; Losito, N.S.; Scrima, M.; Marino, F.Z.; Franco, R.; et al. Multiple Genetic Alterations within the PI3K Pathway Are Responsible for AKT Activation in Patients with Ovarian Carcinoma. PLoS ONE 2013, 8, e55362. [Google Scholar] [CrossRef]
- Akcakanat, A.; Sahin, A.; Shaye, A.N.; Bs, M.A.V.; Meric-Bernstam, F. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer 2008, 112, 2352–2358. [Google Scholar] [CrossRef]
- Esteva, F.J.; Guo, H.; Zhang, S.; Santa-Maria, C.; Stone, S.; Lanchbury, J.S.; Sahin, A.A.; Hortobagyi, G.N.; Yu, D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: Association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am. J. Pathol. 2010, 177, 1647–1656. [Google Scholar] [CrossRef]
- Gallia, G.L.; Tyler, B.M.; Hann, C.L.; Siu, I.-M.; Giranda, V.L.; Vescovi, A.L.; Brem, H.; Riggins, G.J. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol. Cancer Ther. 2009, 8, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Arai, H.; Beppu, T.; Ogasawara, K. Detection of gene amplification and deletion in high-grade gliomas using a genome DNA microarray (GenoSensor Array 300). Brain Tumor Pathol. 2003, 20, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Murai, A.; Asa, S.A.; Kodama, A.; Hirata, A.; Yanai, T.; Sakai, H. Constitutive phosphorylation of the mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma cell lines. BMC Veter. Res. 2012, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Collins, C.J.; Ye, F. Activation of the AKT and mammalian target of rapamycin pathways and the inhibitory effects of rapamycin on those pathways in canine malignant melanoma cell lines. Am. J. Veter. Res. 2009, 70, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.S.; Denton, C.L.; Gustafson, D.L. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma. Veter. Comp. Oncol. 2013, 13, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Uchida, K.; Kikuta, F.; Suzuki, K.; Nakayama, H. Immunohistochemical Characterization of Canine Neuroepithelial Tumors. Veter. Pathol. 2010, 47, 741–750. [Google Scholar] [CrossRef]
- Kim, S.-H.; Seung, B.-J.; Cho, S.-H.; Lim, H.-Y.; Hwang, J.-H.; Sur, J.-H. Expression of Oestrogen Receptor, Progesterone Receptor and Akt in Canine Circumanal Gland Tumours. J. Comp. Pathol. 2018, 162, 59–65. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, H.; Ellis, A.E.; Northrup, N.C.; Dobbin, K.K.; Shin, D.M.; Zhao, S. Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level. PLoS Genet. 2015, 11, e1005277. [Google Scholar] [CrossRef]
- Ressel, B.S.; Massone, A.; Barbeito, C. Dysregulated Expression of Phosphorylated Epidermal Growth Factor Receptor and Phosphatase and Tensin Homologue in Canine Cutaneous Papillomas and Squamous Cell Carcinomas. J. Comp. Pathol. 2020, 174, 26–33. [Google Scholar] [CrossRef]
- Rodriguez, S.; Fadlalla, K.; Graham, T.; Tameru, B.; Fermin, C.D.; Samuel, T. Immunohistochemical evaluation of AKT protein activation in canine mast cell tumours. J. Comp. Pathol. 2012, 147, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Dumaz, N. The role of RICTOR downstream of receptor tyrosine kinase in cancers. Mol. Cancer 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 Is Necessary for Akt/PKB Phosphorylation, and Its Isoforms Define Three Distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef]
- Li, H.; Lin, J.; Wang, X.; Yao, G.; Wang, L.; Zheng, H.; Yang, C.; Jia, C.; Liu, A.; Bai, X.-C. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res. Treat. 2012, 134, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Wazir, U.; Newbold, R.F.; Jiang, W.G.; Sharma, A.K.; Mokbel, K. Prognostic and therapeutic implications of mTORC1 and Rictor expression in human breast cancer. Oncol. Rep. 2013, 29, 1969–1974. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, H.; Pan, T.; Liu, Y.; Su, Y.; Ren, C.; Huang, D.; Zha, X.; Liang, C. mTOR regulate EMT through RhoA and Rac1 pathway in prostate cancer. Mol. Carcinog. 2014, 54, 1086–1095. [Google Scholar] [CrossRef]
- Rogers-Broadway, K.R.; Kumar, J.; Sisu, C.; Wander, G.; Mazey, E.; Jeyaneethi, J.; Pados, G.; Tsolakidis, D.; Klonos, E.; Grunt, T.; et al. Differential expression of mTOR components in endometriosis and ovarian cancer: Effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 istochiometry. Inter. J. Mol. Med. 2019, 43, 47–56. [Google Scholar]
- Gulhati, P.; Bowen, K.A.; Liu, J.; Stevens, P.D.; Rychahou, P.G.; Chen, M.; Lee, E.Y.; Weiss, H.L.; O’Connor, K.L.; Gao, T.; et al. mTORC1 and mTORC2 Regulate EMT, Motility, and Metastasis of Colorectal Cancer via RhoA and Rac1 Signaling Pathways. Cancer Res. 2011, 71, 3246–3256. [Google Scholar] [CrossRef]
- Alvarenga, A.W.; Machado, L.E.; Rodrigues, B.R.; Lupinacci, F.C.S.; Sanemastu, P.; Matta, E.; Roffé, M.; Torres, L.F.B.; Da Cunha, I.W.; Martins, V.R.; et al. Evaluation of Akt and RICTOR Expression Levels in Astrocytomas of All Grades. J. Histochem. Cytochem. 2016, 65, 93–103. [Google Scholar] [CrossRef]
- Masri, J.; Bernath, A.; Martin, J.; Jo, O.D.; Vartanian, R.; Funk, A.; Gera, J. mTORC2 Activity Is Elevated in Gliomas and Promotes Growth and Cell Motility via Overexpression of Rictor. Cancer Res. 2007, 67, 11712–11720. [Google Scholar] [CrossRef]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.; Gama, A.; Scruggs, J.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animal; Kiupel, M., Ed.; Mammary Tumors; Charles Louis Davis, D.V.M. Foundation: Gurnee, IL, USA, 2008; Volume 2. [Google Scholar]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.W.; Musil, K.M.; Davies, J.L.; Hendrick, S.; Duncan, C.; Jackson, M.L.; Kidney, B.; Philibert, H.; Wobeser, B.K.; Simko, E. Prognostic value of histologic grading for feline mammary carcinoma: A retrospective survival analysis. Vet. Pathol. 2015, 52, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, Anatomy, Histology, Lymphatic Drainage, Clinical Features, and Cell Differentiation Markers of Canine Mammary Gland Neoplasms. Veter. Pathol. 2010, 48, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Calderón, L.G.R.; Felisbino, S.L.; Rinaldi, J.D.C.; Drigo, S.A.; Rogatto, S.R.; Laufer-Amorim, R. Immunohistochemical panel to characterize canine prostate carcinomas according to aberrant p63 expression. PLoS ONE 2018, 13, e0199173. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.-F.; Yip, W.K.; Loh, H.W.; Ithnin, H.; Por, P.; Rohaizak, M. Immunohistochemical Detection of Phospho-Akt, Phospho-BAD, HER2 and Oestrogen Receptors α and β in Malaysian Breast Cancer Patients. Pathol. Oncol. Res. 2009, 16, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, L.; Van Hengel, J.; Bruyneel, E.; Mareel, M.M.; Van Roy, F.; Chastre, E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2004, 19, 115–117. [Google Scholar] [CrossRef]
- Bose, S.; Crane, A.; Hibshoosh, H.; Mansukhani, M.; Sandweis, L.; Parsons, R. Reduced expression of PTEN correlates with breast cancer progression. Hum. Pathol. 2002, 33, 405–409. [Google Scholar] [CrossRef]
- Perren, A.; Weng, L.-P.; Boag, A.H.; Ziebold, U.; Thakore, K.; Dahia, P.L.M.; Komminoth, P.; Lees, J.A.; Mulligan, L.M.; Mutter, G.L.; et al. Immunohistochemical Evidence of Loss of PTEN Expression in Primary Ductal Adenocarcinomas of the Breast. Am. J. Pathol. 1999, 155, 1253–1260. [Google Scholar] [CrossRef]
- Tsutsui, S.; Inoue, H.; Yasuda, K.; Suzuki, K.; Higashi, H.; Era, S.; Mori, M. Reduced Expression of PTEN Protein and Its Prognostic Implications in Invasive Ductal Carcinoma of the Breast. Oncology 2005, 68, 398–404. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Ruggeri, B.; Klein, W.M.; Sonoda, G.; Altomare, D.A.; Watson, D.K.; Testa, J.R. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA 1996, 93, 3636–3641. [Google Scholar] [CrossRef]
- Staal, S.P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 1987, 84, 5034–5037. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tenorio, G.; Stål, O.; Members of the Southeast Sweden Breast Cancer Group. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br. J. Cancer 2002, 86, 540–545. [Google Scholar]
- Oh, W.J.; Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 2011, 10, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.; Li, M.; Chen, P.; Zhang, B.; Guo, H.; Cao, W.; Wei, X.; Cao, X.; Hao, H.; et al. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 2010, 70, 9360–9370. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Stevens, D.M.; Saitoh, M.; Kinkel, S.; Crosby, K.; Sheen, J.-H.; Mullholland, D.J.; Magnuson, M.A.; Wu, H.; Sabatini, D.M. mTOR Complex 2 Is Required for the Development of Prostate Cancer Induced by Pten Loss in Mice. Cancer Cell 2009, 15, 148–159. [Google Scholar] [CrossRef]
- Im-Aram, A.; Farrand, L.; Bae, S.-M.; Song, G.; Song, Y.S.; Han, J.Y.; Tsang, B.K. The mTORC2 Component Rictor Contributes to Cisplatin Resistance in Human Ovarian Cancer Cells. PLoS ONE 2013, 8, e75455. [Google Scholar] [CrossRef]
- Dahia, P.L.M.; Aguiar, R.C.T.; Alberta, J.; Kum, J.B.; Caron, S.; Sill, H.; Marsh, D.J.; Ritz, J.; Freedman, H.; Stiles, C.; et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum. Mol. Genet. 1999, 8, 185–193. [Google Scholar] [CrossRef]
- Dahia, P.L. PTEN, a unique tumor suppressor gene. Endocrine-Related Cancer 2000, 7, 115–129. [Google Scholar] [CrossRef]
- Di Cristofano, A.; Pandolfi, P.P. The Multiple Roles of PTEN in Tumor Suppression. Cell 2000, 100, 387–390. [Google Scholar] [CrossRef]
- Garcia, J.M.; Silva, J.M.; Dominguez, G.; Gonzalez, R.; Navarro, A.; Carretero, L.; Provencio, M.; España, P.; Bonilla, F. Allelic loss of the PTEN region (10q23) in breast carcinomas of poor pathophenotype. Breast Cancer Res. Treat. 1999, 57, 237–243. [Google Scholar] [CrossRef]
- Whang, Y.E.; Wu, X.; Suzuki, H.; Reiter, R.E.; Tran, C.; Vessella, R.L.; Said, J.W.; Isaacs, W.B.; Sawyers, C.L. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. USA 1998, 95, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Sandell, S.; Schuit, R.J.L.; Bunyan, D.J. An intronic polymorphic deletion in the PTEN gene: Implications for molecular diagnostic testing. Br. J. Cancer 2013, 108, 438–441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabinovsky, R.; Pochanard, P.; McNear, C.; Brachmann, S.M.; Duke-Cohan, J.S.; Garraway, L.A.; Sellers, W.R. p85 Associates with Unphosphorylated PTEN and the PTEN-Associated Complex. Mol. Cell. Biol. 2009, 29, 5377–5388. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, P.; Fostira, F. Hereditary breast cancer: The era of new susceptibility genes. Biomed. Res. Intr. 2013, 2013, 747318. [Google Scholar] [CrossRef]

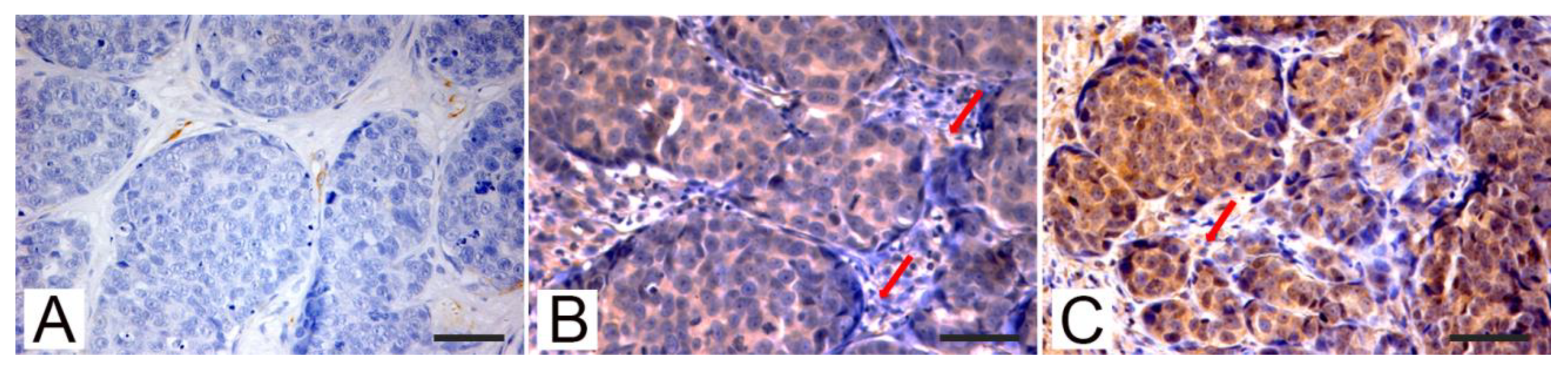

| Tumors | PTEN | p-AKT | Rictor | |||
|---|---|---|---|---|---|---|
| Positive | % | Positive | % | Positive | % | |
| DOG | ||||||
| Adenomas | 10/10 a | 100 | 0 | 0 | 1 | 10 |
| Carcinomas | 25/40 | 62.5 | 15/40 b | 37.5 | 24/40 c | 60 |
| CAT | ||||||
| Carcinomas | 7/30 | 23.3 | 24/30 | 80 | 20/30 | 66.7 |
| n = 30 | PTEN | p-AKT | Rictor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pos | Neg | p | Pos | Neg | p | Pos | Neg | p | |
| Histotype | |||||||||
| Tubular and tubulopapillary | 6 | 10 | 0.049 | 12 | 4 | 0.464 | 10 | 6 | 0.604 |
| Solid | 1 | 13 | 12 | 2 | 10 | 4 | |||
| Lymphatic invasion | |||||||||
| Negative | 4 | 4 | 0.037 | 5 | 3 | 0.148 | 4 | 4 | 0.243 |
| Positive | 3 | 19 | 19 | 3 | 16 | 6 | |||
| Mitotic index | |||||||||
| <Median | 6 | 9 | 0.031 | 11 | 4 | 0.361 | 9 | 6 | 0.439 |
| >Median | 1 | 14 | 13 | 2 | 11 | 4 | |||
| Mills grade | |||||||||
| I | 4 | 5 | 0.192 | 6 | 3 | 0.359 | 5 | 4 | 0.607 |
| II | 2 | 10 | 11 | 1 | 8 | 4 | |||
| III | 1 | 8 | 7 | 2 | 7 | 2 | |||
| Overall survival | 0.010 | 0.002 | 0.001 | ||||||
| Alive | 6 | 7 | 7 | 6 | 4 | 9 | |||
| Dead | 1 | 16 | 17 | 0 | 16 | 1 | |||
| n = 40 | PTEN | p-AKT | Rictor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pos | Neg | p | Pos | Neg | p | Pos | Neg | p | |
| Morphology | |||||||||
| Complex | 12 | 1 | 0.007 | 2 | 11 | 0.045 | 8 | 5 | 0.890 |
| Simple | 13 | 14 | 13 | 14 | 16 | 11 | |||
| Histotype | |||||||||
| Tubular and Tubulopapillary | 7 | 5 | 343 | 3 | 9 | 0.031 | 3 | 9 | 0.247 |
| Solid and Anaplastic | 6 | 9 | 0. | 10 | 5 | 7 | 8 | ||
| Lymphatic invasion | |||||||||
| Negative | 22 | 7 | 0.005 | 6 | 23 | 0.001 | 13 | 16 | 0.001 |
| Positive | 3 | 8 | 9 | 2 | 11 | 0 | |||
| Mitotic index | |||||||||
| <Median | 16 | 4 | 0.009 | 5 | 15 | 0.102 | 15 | 5 | 0.053 |
| >Median | 9 | 11 | 10 | 10 | 9 | 11 | |||
| Peña grade | |||||||||
| WDC | 14 | 2 | 0.029 | 3 | 13 | 0.124 | 8 | 8 | 0.218 |
| MDC | 6 | 7 | 6 | 7 | 7 | 6 | |||
| PDC | 5 | 6 | 6 | 5 | 9 | 2 | |||
| Overall survival | |||||||||
| Alive | 21 | 4 | 0.001 | 3 | 22 | 0.001 | 12 | 13 | 0.050 |
| Dead | 4 | 11 | 12 | 3 | 12 | 3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asproni, P.; Millanta, F.; Ressel, L.; Podestà, F.; Parisi, F.; Vannozzi, I.; Poli, A. An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors. Animals 2021, 11, 365. https://doi.org/10.3390/ani11020365
Asproni P, Millanta F, Ressel L, Podestà F, Parisi F, Vannozzi I, Poli A. An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors. Animals. 2021; 11(2):365. https://doi.org/10.3390/ani11020365
Chicago/Turabian StyleAsproni, Pietro, Francesca Millanta, Lorenzo Ressel, Fabio Podestà, Francesca Parisi, Iacopo Vannozzi, and Alessandro Poli. 2021. "An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors" Animals 11, no. 2: 365. https://doi.org/10.3390/ani11020365
APA StyleAsproni, P., Millanta, F., Ressel, L., Podestà, F., Parisi, F., Vannozzi, I., & Poli, A. (2021). An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors. Animals, 11(2), 365. https://doi.org/10.3390/ani11020365






