Early Immunocastration of Pigs: From Farming to Meat Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Housing and Feeding
2.3. Fattening and Slaughtering Performance
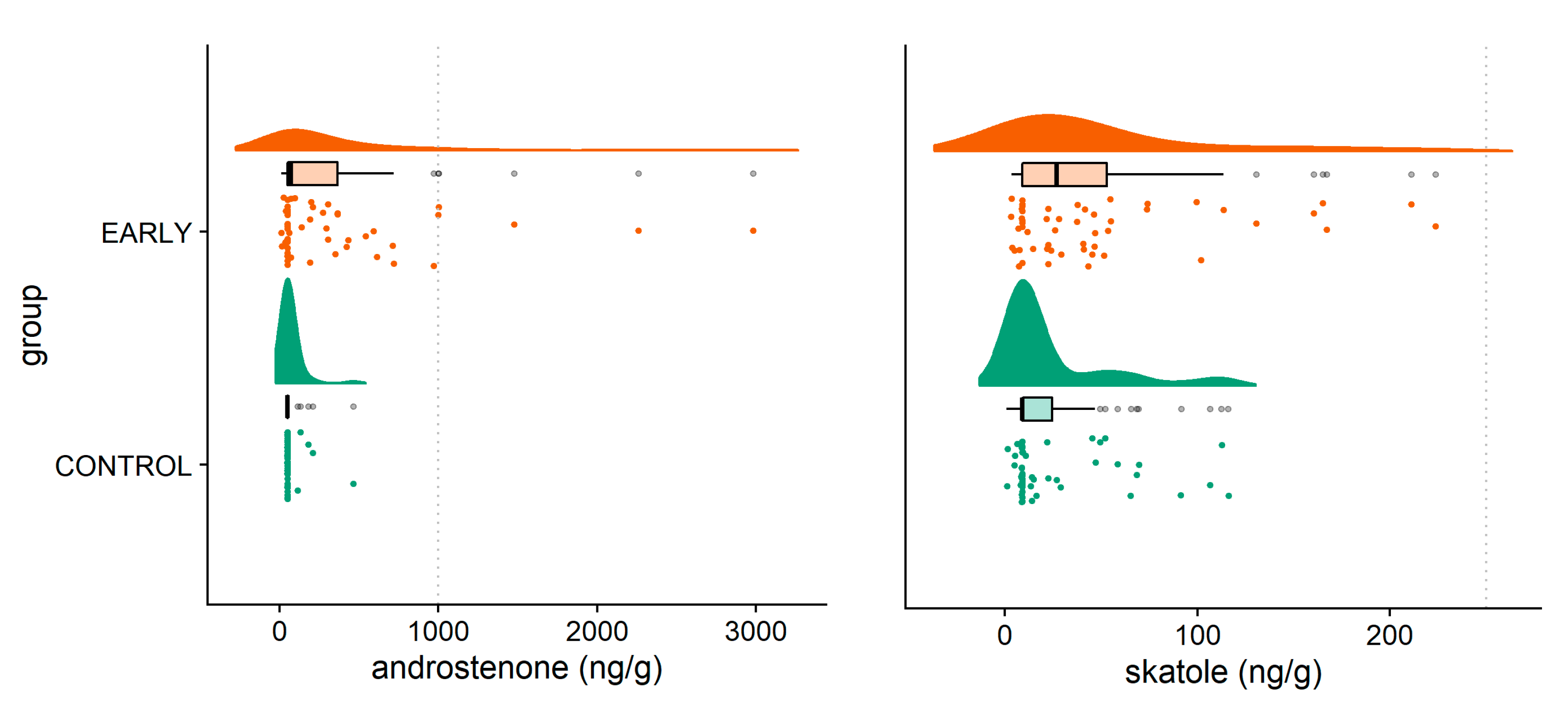
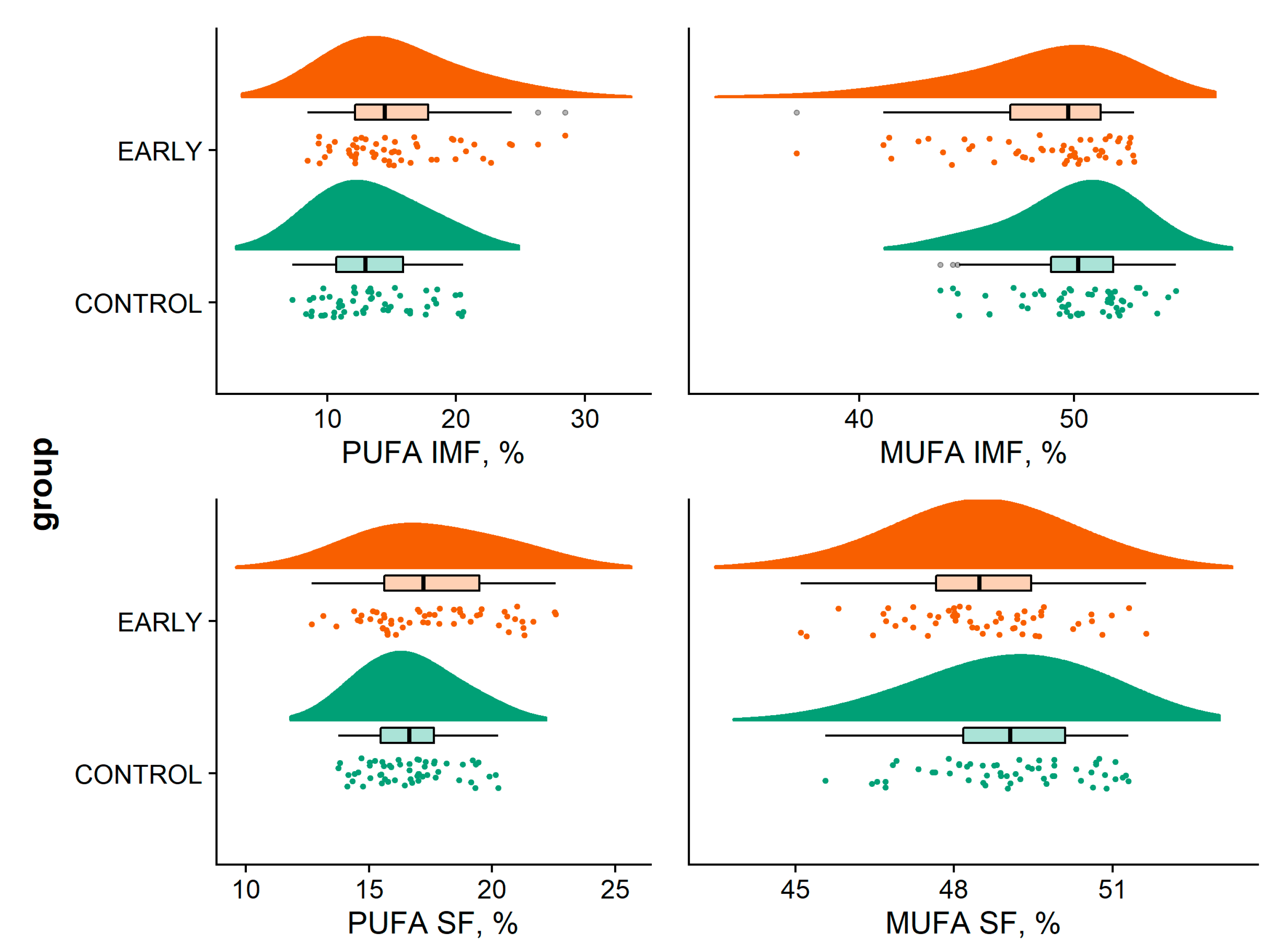
2.4. Boar Taint and Fatty Acid Composition
2.4.1. Androstenone and Skatole
2.4.2. Determination of the Fatty Acid Composition in the Intramuscular Fat and Subcutaneous Fat
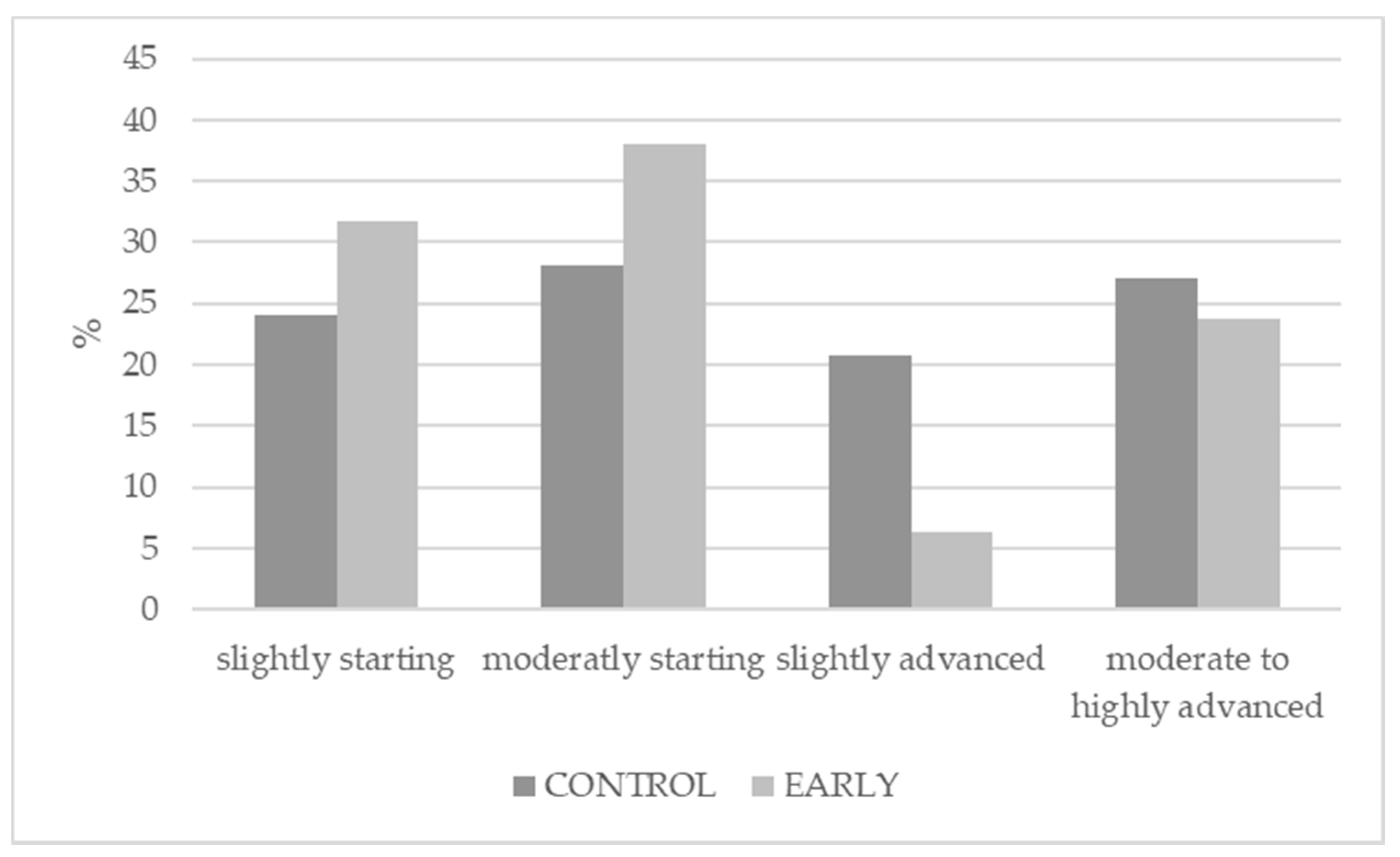
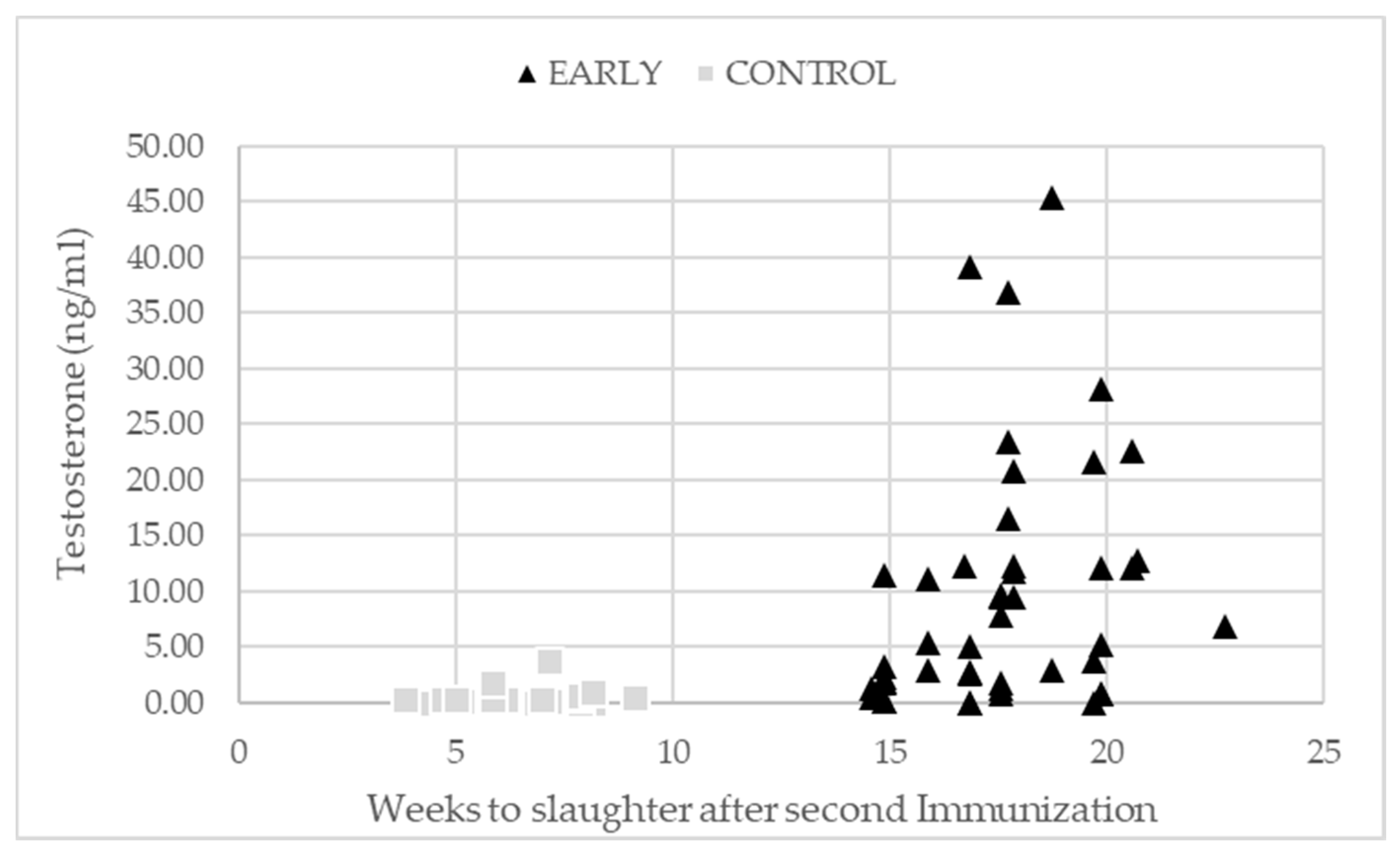
2.5. Testicular Development and Immunization Status
2.6. Animal Behaviour and Welfare
2.7. Statistical Analysis
- (1)
- Live weight: yijk = µ + Immi + Trial runj + Litterk + εijk
- (2)
- Daily gain: yijkl = µ + Immi + Trial runj + Litterk + Start weightl + εijkl
- (3)
- Feed efficiency: yi = µ + Immi + εi
- (4)
- Slaughtering performance: yijk = µ + Immi + Trial runj + Litterk + Slaughter weightm + εijkm
- (5)
- Meat and fat quality: yijk = µ + Immi + Trial runj + Litterk + εijk
- (6)
- Testes weight: yijk = µ + Immi + Trial runj + Litterk + Age at slaughtern + Slaughter weightm + εijknm
- (7)
- Immunization status: yijk = µ + Immi + Trial runj + Litterk + Age at slaughtern + εijk
- (8)
- Histology characteristics: yijk = µ + Immi + Trial runj + εij
- (9)
- Behavior: yijk = µ + Immi + Trial runj + Day sectiono + εijo
- (10)
- Appearance of scratches: yi = µ + Immi + εi
3. Results
3.1. Fattening and Slaughtering Performance
3.2. Boar Taint and Fatty Acid Composition
3.3. Testicular Development and Immunization Status
3.4. Animal Behaviour and Welfare
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Trial Run | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Control | 19 | 16 | 19 |
| Early | 20 | 15 | 20 |
| 39 | 31 | 39 | |
| LS Means | Imm | Trial Run | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Early | 1 | 2 | 3 | Imm | Trial Run | Live /Slaughter Weight | |
| n = 54 | n = 55 | n = 39 | n = 31 | n = 39 | ||||
| Feed conversion [kg feed −1 kg gain] during… | ||||||||
| Pre-fattening | 1.99 | 2.00 | 2.09 b | 1.62 a | 2.29 b | 0.909 | <0.001 | - |
| Standard error | 0.05–0.06 | |||||||
| Fattening | 2.29 | 2.29 | 2.42 b | 2.22 a | 2.23 a | 0.901 | 0.015 | - |
| Standard error | 0.03–0.04 | |||||||
| Daily weight gain [g] during… | ||||||||
| Pre-fattening | 783 | 824 | 751 a | 940 b | 718 a | 0.047 | <0.001 | <0.001 |
| Standard error | 14.35–19.67 | |||||||
| Fattening | 970 | 969 | 964 | 980 | 964 | 0.970 | 0.863 | <0.001 |
| Standard error | 16.34–24.21 | |||||||
| Slaughtering performance | ||||||||
| Dressing rate [%] | 77.80 | 77.77 | 77.16 a | 77.89 ab | 78.37 b | 0.918 | 0.041 | - |
| Standard error | 0.27–0.37 | |||||||
| Lean meat content [%] | 56.51 | 56.69 | 55.32 a | 56.46 a | 58.02 b | 0.657 | <0.001 | 0.149 |
| Standard error | 0.33–0.47 | |||||||
| Electrical conductivity [ms cm−1] | 1.74 | 2.00 | 1.77 | 1.85 | 1.91 | 0.035 | 0.721 | - |
| Standard error | 0.09–0.14 | |||||||
| Fatty acid composition | ||||||||
| SFA IMF (%) | 36.49 | 36.21 | 35.71 a | 37.36 b | 35.98 | 0.389 | <0.001 | - |
| Standard error | 0.23–0.32 | |||||||
| MUFA IMF (%) | 50.12 | 48.85 | 50.39 b | 51.36 b | 46.70 a | <0.001 | <0.001 | - |
| Standard error | 0.34–0.46 | |||||||
| PUFA IMF (%) | 13.37 | 14.98 | 13.88 b | 11.29 a | 17.35 c | 0.015 | <0.001 | - |
| Standard error | 0.46–0.61 | |||||||
| SFA SF (%) | 34.23 | 34.02 | 34.66 b | 34.72 b | 33.00 a | 0.453 | <0.001 | - |
| Standard error | 0.24–0.36 | |||||||
| MUFA SF (%) | 49.04 | 48.52 | 49.75 b | 48.53 a | 48.05 a | 0.034 | <0.001 | - |
| Standard error | 0.17–0.24 | |||||||
| PUFA SF (%) | 16.73 | 17.48 | 15.66 a | 16.73 a | 18.92 b | 0.021 | <0.001 | - |
| Standard error | 0.26–0.34 | |||||||
| Interaction of Immunization (Control, Early) and Trial Run (1–3) | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control × 1 | Control × 2 | Control × 3 | Early × 1 | Early × 2 | Early × 3 | Imm | Trial Run | Imm* Trial Run | |
| LS Means | n = 19 | n = 16 | n = 19 | n = 20 | n = 15 | n = 20 | |||
| Androstenone (ng/g fat) | 72.84 a | 50.00 a | 50.00 a | 569.5 b | 363.03 b | 97.65 ac | <0.001 | 0.016 | 0.032 |
| Standard error | 82.73–95.52 | ||||||||
| Skatole (ng/g fat) | 14.24 a | 20.56 a | 32.93 a | 77.52 b | 41.25 a | 25.79 a | 0.001 | 0.233 | 0.001 |
| Standard error | 9.49–11.03 | ||||||||
| Imm | Trial Run | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Early | 1 | 2 | 3 | Imm | Trial Run | Imm* Trial Run | Age at Slaughter | ||
| LS Means | n = 45 | n = 46 | n = 21 | n = 31 | n = 39 | |||||
| GnRH binding (%) | 46.08 | 29.55 | 35.16 | 39.63 | 38.66 | <0.001 | 0.424 | 0.115 | 0.007 | |
| Standard error | 1.76–2.66 | |||||||||
| Imm* Trial run | ||||||||||
| Control × 1 | Control × 2 | Control × 3 | Early × 1 | Early × 2 | Early × 3 | |||||
| n = 10 | n = 16 | n = 19 | n = 11 | n = 15 | n = 20 | |||||
| Testosterone (ng/mL) | 0.29 a | 0.50 a | 0.21 a | 17.80 b | 11.60 b | 4.58 a | <0.001 | 0.007 | 0.004 | 0.364 |
| Standard error | 1.67–2.24 | |||||||||
References
- Bonneau, M.; Weiler, U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Brewster, V.; Nevel, A. Immunocastration with Improvac (TM) reduces aggressive and sexual behaviours in male pigs. Appl. Anim. Behav. Sci. 2013, 145, 32–36. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance1. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork Production with Immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef]
- Brunius, C.; Zamaratskaia, G.; Andersson, K.; Chen, G.; Norrby, M.; Madej, A.; Lundström, K. Early immunocastration of male pigs with Improvac®—Effect on boar taint, hormones and reproductive organs. Vaccine 2011, 29, 9514–9520. [Google Scholar] [CrossRef]
- Einarsson, S.; Brunius, C.; Wallgren, M.; Lundström, K.; Andersson, K.; Zamaratskaia, G.; Rodriguez-Martinez, H. Effects of early vaccination with Improvac® on the development and function of reproductive organs of male pigs. Anim. Reprod. Sci. 2011, 127, 50–55. [Google Scholar] [CrossRef][Green Version]
- Richtlinie für die Stationsprüfung auf Mastleistung, Schlachtkörperwert und Fleischbeschaffenheit beim Schwein. Bundesverband Rind und Schwein e.V. 2019. Available online: https://www.rind-schwein.de/services/files/brs/richtlinien/20190624%20Richtlinie%20NKP%20Deckblatt%20ObenSans.pdf (accessed on 2 November 2020).
- Fischer, J.; Elsinghorst, P.W.; Bücking, M.; Tholen, E.; Petersen, B.; Wüst, M. Development of a Candidate Reference Method for the Simultaneous Quantitation of the Boar Taint Compounds Androstenone, 3α-Androstenol, 3β-Androstenol, Skatole, and Indole in Pig Fat by Means of Stable Isotope Dilution Analysis–Headspace Solid-Phase Microextraction–Gas Chromatography/Mass Spectrometry. Anal. Chem. 2011, 83, 6785–6791. [Google Scholar] [CrossRef]
- Trautmann, J.; Meier-Dinkel, L.; Gertheiss, J.; Mörlein, D. Boar taint detection: A comparison of three sensory protocols. Meat Sci. 2016, 111, 92–100. [Google Scholar] [CrossRef]
- Liu, X.; Trautmann Mörlein, J.; Wigger, R.; Zhou, G.H.; Mörlein, D. Fatty acid composition and its association with chemical and sensory analysis of boar taint. Food Chem. 2017, 231. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.; Sell, J. Effects of dietary conjugated linoleic acid and linoleic: Linolenic acid ratio on polyunsaturated fatty acid status in laying hens. Poult. Sci. 2000, 79, 1749–1756. [Google Scholar] [CrossRef]
- Sauer, F.; Schmoll, F.; Sattler, T. Einfluss des Alters bei der zweiten Improvac®-Vakzination auf Hodengewicht, Hodenhistologie und Ebergeruchsstoffe von männlichen Mastschweinen im Vergleich zu intakten Mastebern und Kastraten. Wien. Tierärztliche Mon. 2014, 101, 103–109. [Google Scholar]
- Kress, K.; Weiler, U.; Schmucker, S.; Čandek-Potokar, M.; Vrecl, M.; Fazarinc, G.; Škrlep, M.; Batorek-Lukač, N.; Stefanski, V. Influence of Housing Conditions on Reliability of Immunocastration and Consequences for Growth Performance of Male Pigs. Animals 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Dalmau, A.; Velarde, A.; Scott, K.; Edwards, S.; Butterworth, A.; Veissier, I.; Keeling, L.; Overbeke, G.; Bedaux, V. Welfare Quality® Assessment for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Grela, E.R.; Kowalczuk-Vasilev, E.; Klebaniuk, R. Performance, pork quality and fatty acid composition of entire males, surgically castrated or immunocastrated males, and female pigs reared under organic system. Pol. J. Vet. Sci. 2013, 16, 7. [Google Scholar] [CrossRef]
- Zoels, S.; Reiter, S.; Ritzmann, M.; Weiß, C.; Numberger, J.; Schütz, A.; Lindner, P.; Stefanski, V.; Weiler, U. Influences of Immunocastration on Endocrine Parameters, Growth Performance and Carcass Quality, as Well as on Boar Taint and Penile Injuries. Animals 2020, 10, 346. [Google Scholar] [CrossRef]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.; Brunius, C.; Zamaratskaia, G.; Lundström, K. Early vaccination with Improvac®: Effects on performance and behaviour of male pigs. Animal 2012, 6, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Turkstra, J.; Zeng, X.; Van Diepen, J.T.M.; Jongbloed, A.; Oonk, H.; Van de Wiel, D.; Meloen, R. Performance of male pigs immunized against GnRH is related to the time of onset of biological response. J. Anim. Sci. 2002, 80, 2953–2959. [Google Scholar] [CrossRef]
- Weissmann, F.; Honikel, K. Zur Frage einer Referenzmethode zum Ausschluss von PSE-Fleisch in der Wareneingangskontrolle. Mitt. Bundesanst. Fur Fleischforsch. Kulmb. 1998, 1, 300–303. [Google Scholar]
- Mörlein, D.; Link, G.; Werner, C.; Wicke, M. Suitability of three commercially produced pig breeds in Germany for a meat quality program with emphasis on drip loss and eating quality. Meat Sci. 2007, 77, 504–511. [Google Scholar] [CrossRef]
- Sladek, Z.; Prudikova, M.; Knoll, A.; Kulich, P.; Steinhauserova, I.; Borilova, G. Effect of early immunocastration on testicular histology in pigs. Veterinární Med. 2018, 63, 18–27. [Google Scholar] [CrossRef]
- Claus, R.; Rottner, S.; Rueckert, C. Individual return to Leydig cell function after GnRH-immunization of boars. Vaccine 2008, 26, 4571–4578. [Google Scholar] [CrossRef] [PubMed]
- Rottner, S.; Claus, R. Return of testicular function after vaccination of boars against GnRH: Consequences on testes histology. Anim. Int. J. Anim. Biosci. 2009, 3, 1279. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Rydhmer, L.; Andersson, H.K.; Chen, G.; Lowagie, S.; Andersson, K.; Lundström, K. Long-term effect of vaccination against gonadotropin-releasing hormone, using Improvac™, on hormonal profile and behaviour of male pigs. Anim. Reprod. Sci. 2008, 108, 37–48. [Google Scholar] [CrossRef]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Bonneau, M.; Conseil, G.; Giovanni, F.; Mounier, A.-M.; Peignier, Y. Effects of age and live weight on fat 5α-androstenone levels in young boars fed two planes of nutrition. Reprod. Nutr. Dév. 1987, 27, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Edwards, S.A.; Jensen, B.B.; Rousing, T.; Sørensen, J.T. Weight and season affects androstenone and skatole occurrence in entire male pigs in organic pig production. Animal 2015, 9, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Babol, J.; Madej, A.; Squires, E.; Lundström, K. Age-related Variation of Plasma Concentrations of Skatole, Androstenone, Testosterone, Oestradiol-17β, Oestrone Sulphate, Dehydroepiandrosterone Sulphate, Triiodothyronine and IGF-1 in Six Entire Male Pigs. Reprod. Domest. Anim. 2004, 39, 168–172. [Google Scholar] [CrossRef]
- Mörlein, J.; Meier-Dinkel, L.; Gertheiss, J.; Schnäckel, W.; Mörlein, D. Sustainable use of tainted boar meat: Blending is a strategy for processed products. Meat Sci. 2019, 152, 65–72. [Google Scholar] [CrossRef]
- Meier-Dinkel, L.; Gertheiss, J.; Schnäckel, W.; Mörlein, D. Consumers’ perception and acceptance of boiled and fermented sausages from strongly boar tainted meat. Meat Sci. 2016, 118, 34–42. [Google Scholar] [CrossRef]
- Pauly, C.; Spring, P.; O’Doherty, J.V.; Ampuero Kragten, S.; Bee, G. Growth performance, carcass characteristics and meat quality of group-penned surgically castrated, immunocastrated (Improvac®) and entire male pigs and individually penned entire male pigs. Animal 2009, 3, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Lindner, J.; Judas, M.; Höreth, R. Schlachtkörperzusammensetzung und Gewebebeschaffenheit von schweren Schweinen–II. Mitteilung: Merkmale der Fleisch-und Fettqualität. Arch. Anim. Breed. 2006, 49, 279–292. [Google Scholar] [CrossRef]
- Bee, G.; Für Nutztiere, E.F. Das Fettzahl-konforme Schwein: Die MUFA-PUFA-Norm. SchR Inst. Nutztierwiss 2004, 25, 37–51. [Google Scholar]
- Johansson, L.; Lundström, K.; Jonsäll, A. Effects of RN genotype and silage feed on fat content and fatty acid composition of fresh and cooked pork loin. Meat Sci. 2002, 60, 17–24. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, S.; Algers, B.; Svedberg, J. Description and Evaluation of a Scoring System of Clinical Health in Laying Hens. Laying Hens in Loose Housing Systems. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2000. Acta Universitatis Agriculturae Sueciae Veterinaria 73. [Google Scholar]
- Rydhmer, L.; Lundström, K.; Andersson, K. Immunocastration reduces aggressive and sexual behaviour in male pigs. Animal 2010, 4, 965–972. [Google Scholar] [CrossRef]
- Althouse, G.C. 15 Applied Andrology in Swine. Anim. Androl. Theor. Appl. 2014, 404. [Google Scholar] [CrossRef]


| Immunization | p-Values | |||||
|---|---|---|---|---|---|---|
| Control | Early | Imm | Trial Run | Age at Slaughter | Slaughter Weight | |
| LS Means | n = 54 | n = 55 | ||||
| Testes weight (g) | 225 | 345 | <0.001 | 0.384 | <0.001 | 0.013 |
| Standard error | 23.61–34.55 | |||||
| Relative frequencies (%) | n = 106 | n = 108 | ||||
| Testes | ||||||
| Hypoplasia | 9.4 | 41.7 | <0.001 | 0.144 | - | - |
| Atrophied | 90.6 | 58.3 | <0.001 | 0.144 | - | - |
| Epididymis | ||||||
| Azoospermia | 20.8 | 51.9 | <0.001 | 0.120 | - | - |
| Oligospermia | 75.5 | 41.7 | <0.001 | 0.649 | - | - |
| Teratozoospermia | 78.3 | 46.3 | <0.001 | 0.171 | - | - |
| Imm | Trial Run | p-Values | |||||
|---|---|---|---|---|---|---|---|
| Relative Frequencies (%) of Injuries | Control | Early | 1 | 2 | 3 | Imm | Trial Run |
| n = 266 | n = 259 | n = 200 | n = 164 | n = 161 | |||
| Total | 41.7 | 45.6 | 58.0 | 40.9 | 28.6 | 0.625 | <0.001 |
| Ears | 15.8 | 17.4 | 18.5 | 20.1 | 19.5 | 0.722 | 0.051 |
| Head to shoulder | 24.1 | 23.2 | 32.5 | 20.1 | 16.2 | 0.602 | <0.001 |
| Shoulder to flank | 14.7 | 19.3 | 24.0 | 14.6 | 10.6 | 0.238 | 0.004 |
| Ham | 6.0 | 5.8 | 11.0 | 4.3 | 1.2 | 0.685 | 0.002 |
| Legs | 1.1 | 2.3 | 3.5 | 1.2 | 0.0 | 0.393 | 0.431 |
| Foreskin | 0.4 | 1.5 | 0.0 | 0.6 | 2.5 | 0.157 | 0.418 |
| Testes | 0.7 | 0.0 | 0.0 | 1.2 | 0.0 | 0.960 | 0.998 |
| Tail | 2.6 | 3.9 | 4.5 | 0.6 | 4.4 | 0.439 | 0.150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, D.; Baldinger, L.; Bussemas, R.; Büttner, S.; Weißmann, F.; Ciulu, M.; Mörlein, J.; Mörlein, D. Early Immunocastration of Pigs: From Farming to Meat Quality. Animals 2021, 11, 298. https://doi.org/10.3390/ani11020298
Werner D, Baldinger L, Bussemas R, Büttner S, Weißmann F, Ciulu M, Mörlein J, Mörlein D. Early Immunocastration of Pigs: From Farming to Meat Quality. Animals. 2021; 11(2):298. https://doi.org/10.3390/ani11020298
Chicago/Turabian StyleWerner, Daniela, Lisa Baldinger, Ralf Bussemas, Sinje Büttner, Friedrich Weißmann, Marco Ciulu, Johanna Mörlein, and Daniel Mörlein. 2021. "Early Immunocastration of Pigs: From Farming to Meat Quality" Animals 11, no. 2: 298. https://doi.org/10.3390/ani11020298
APA StyleWerner, D., Baldinger, L., Bussemas, R., Büttner, S., Weißmann, F., Ciulu, M., Mörlein, J., & Mörlein, D. (2021). Early Immunocastration of Pigs: From Farming to Meat Quality. Animals, 11(2), 298. https://doi.org/10.3390/ani11020298






