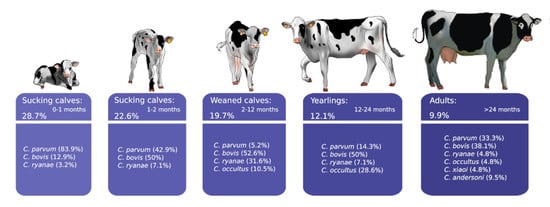
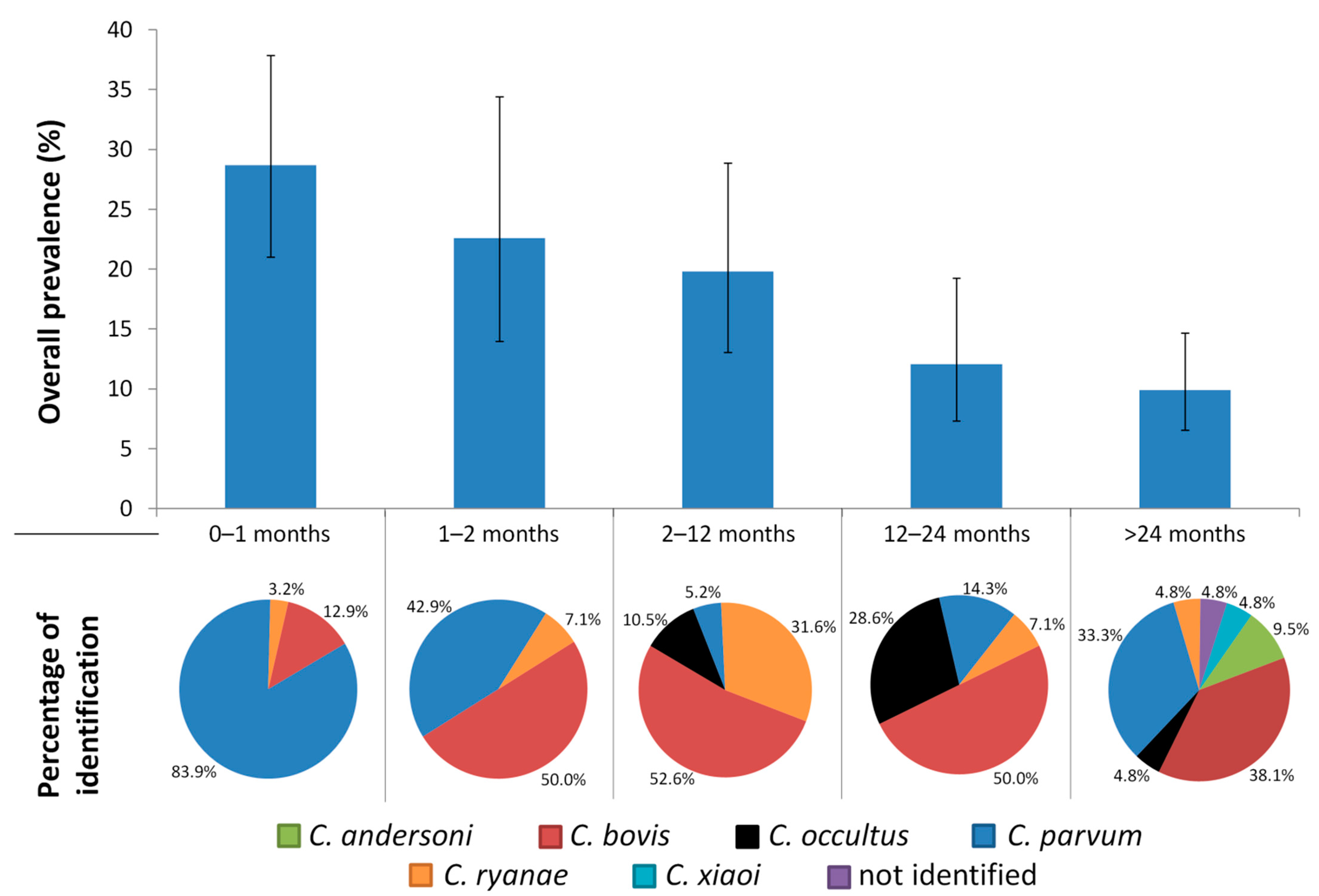
The Age-Related Cryptosporidium Species Distribution in Asymptomatic Cattle from North-Western Spain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Study Area and Characteristics of Farms
2.3. Sampling of Animals
2.4. Molecular Methods
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Katzer, F. Looking for Cryptosporidium: The application of advances in detection and diagnosis. Trends Parasitol. 2013, 29, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Šlapeta, J. Cryptosporidiosis and Cryptosporidium species in animals and humans: A thirty colour rainbow? Int. J. Parasitol. 2013, 43, 957–970. [Google Scholar] [CrossRef]
- Xiao, L.; Fayer, R. Molecular characterization of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 2008, 38, 1239–1255. [Google Scholar] [CrossRef]
- Santín, M. Cryptosporidium and Giardia in ruminants. Vet. Clin. N. Am.–Food A 2020, 36, 223–238. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Ash, A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016, 40, 315–323. [Google Scholar] [CrossRef]
- Azami, M.; Moghaddam, D.D.; Salehi, R.; Salehi, M. The identification of Cryptosporidium species (protozoa) in Ifsahan, Iran by PCR-RFLP analysis of the 18S rRNA gene. Mol. Biol. (Mosk) 2007, 41, 934–939. [Google Scholar] [CrossRef]
- Chen, F.; Huang, K. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle from farms in China. J. Vet. Sci. 2012, 13, 15–22. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Zhao, X.; Xu, H.; Wu, W.; Wang, Y.; Guo, Y.; Wang, L.; Feng, Y.; Xiao, L. Occurrence and molecular characterization of Cryptosporidium spp. and Enterocytozoon bieneusi in dairy cattle, beef cattle and water buffaloes in China. Vet. Parasitol. 2015, 207, 220–227. [Google Scholar] [CrossRef]
- Mirhashemi, M.E.; Zintl, A.; Grant, T.; Lucy, F.; Mulcahy, G.; De Waal, T. Molecular epidemiology of Cryptosporidium species in livestock in Ireland. Vet. Parasitol. 2016, 216, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Trout, J.M.; Fayer, R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet. Parasitol. 2008, 155, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Yang, F.; Zhang, L.; Cao, J.; Zhang, X.; Ling, H.; Liu, A.; Shen, Y. Distribution and genetic characterizations of Cryptosporidium spp. in pre-weaned dairy calves in Northeastern China’s Heilongjiang Province. PLoS ONE 2013, 8, e54857. [Google Scholar] [CrossRef]
- Shaw, H.J.; Innes, E.A.; Morrison, L.J.; Katzer, F.; Wells, B. Long-term production effects of clinical cryptosporidiosis in neonatal calves. Int. J. Parasitol. 2020, 50, 371–376. [Google Scholar] [CrossRef]
- Díaz, P.; Quílez, J.; Chalmers, R.M.; Panadero, R.; López, C.; Sánchez-Acedo, C.; Morrondo, P.; Díez-Baños, P. Genotype and subtype analysis of Cryptosporidium isolates from calves and lambs in Galicia (NW Spain). Parasitology 2010, 137, 1187–1193. [Google Scholar] [CrossRef]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Robinson, G.; del Cacho, E.; Sánchez-Acedo, C. Cryptosporidium species and subtype analysis from dairy calves in Spain. Parasitology 2008, 135, 1613–1620. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of Cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef]
- Fayer, R.; Santín, M.; Trout, J.M.; Greiner, E. Prevalence of species and genotypes of Cryptosporidium found in 1-2-year-old dairy cattle in the eastern United States. Vet. Parasitol. 2006, 135, 105–112. [Google Scholar] [CrossRef]
- Fayer, R.; Santín, M.; Trout, J.M. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet. Parasitol. 2007, 145, 260–266. [Google Scholar] [CrossRef]
- Santín, M.; Trout, J.M.; Xiao, L.; Zhou, L.; Greiner, E.; Fayer, R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004, 122, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Feltus, D.C.; Giddings, C.W.; Khaitsa, M.L.; McEvoy, J.M. High prevalence of Cryptosporidium bovis and the deer-like genotype in calves compared to mature cows in beef cow-calf operations. Vet. Parasitol. 2008, 151, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Clifton-Hadley, F.A.; Cheney, T.; Giles, M. Prevalence and molecular typing of Cryptosporidium in dairy cattle in England and Wales and examination of potential on-farm transmission routes. Vet. Parasitol. 2014, 204, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Chang, Y.; Yu, F.; Zhang, S.; Wang, R.; Zhang, L. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Gansu, northwest China. Parasite 2020, 27, 62. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ortega, Y.; He, G.; Das, P.; Xu, M.; Zhang, X.; Fayer, R.; Gatei, W.; Cama, V.; Xiao, L. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 2007, 144, 1–9. [Google Scholar] [CrossRef]
- Qi, M.; Wang, H.; Jing, B.; Wang, D.; Wang, R.; Zhang, L. Occurrence and molecular identification of Cryptosporidium spp. in dairy calves in Xinjiang, northwestern China. Vet. Parasitol. 2015, 212, 404–407. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; Almeida, A.; González-Warleta, M.; Correia Da Costa, J.M.; Mezo, M. Prevalence and preliminary genetic characterization of Cryptosporidium spp. isolated from asymptomatic heifers in Galicia (NW Spain). J. Eukaryot. Microbiol. 2006, 53 (Suppl. 1), 22–23. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; Almeida, A.; González-Warleta, M.; Correia Da Costa, J.M.; Rumbo-Lorenzo, C.; Mezo, M. Occurrence of Cryptosporidium parvum and Giardia duodenalis in healthy adult domestic ruminants. Parasitol. Res. 2007, 101, 1443–1448. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; García-Presedo, I.; Almeida, A.; González-Warleta, M.; Correia Da Costa, J.M.; Mezo, M. Cryptosporidium spp. and Giardia duodenalis in two areas of Galicia (NW Spain). Sci. Total Environ. 2011, 409, 2451–2459. [Google Scholar] [CrossRef]
- MAPA. Análisis Provincial del Número de Animales Según Tipos, 2018 (Noviembre). Anuario de Estadística 2018. 2020. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2018/default.aspx?parte=3&capitulo=08&grupo=1&seccion=2 (accessed on 31 August 2020).
- Consellería do Medio Rural. Estrutura por Tamaño de Explotación. In: Anuario de Estatística Agraria 2018. 2020. Available online: https://mediorural.xunta.gal/es/recursos/estadisticas/estadistica-agraria/2018 (accessed on 31 August 2020).
- Ireland-Perry, R.L.; Stallings, C.C. Fecal Consistency as Related to Dietary Composition in Lactating Holstein Cows. J. Dairy Sci. 1993, 76, 1074–1082. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; Carro-Corral, C.; González-Warleta, M.; Mezo, M. Prevalence and intensity of infection of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Galicia (NW Spain). J. Vet. Med. B 2006, 53, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Navarro, E.; Prieto, A.; Pérez-Creo, A.; Viña, M.; Díaz-Cao, J.M.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; et al. Cryptosporidium species in post-weaned and adult sheep and goats from N.W. Spain: Public and animal health significance. Vet. Parasitol. 2018, 254, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Alderisio, K.A.; Xiao, L. Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl. Environ. Microbiol. 2005, 71, 4446–4454. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Pavlasek, I.; Ryan, U. Identification of novel Cryptosporidium genotypes from avian hosts. Appl. Environ. Microbiol. 2006, 72, 7548–7553. [Google Scholar] [CrossRef]
- Alves, M.; Xiao, L.; Sulaiman, I.; Lal, A.A.; Matos, O.; Antunes, F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 2003, 41, 2744–2747. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 2005, 43, 2805–2809. [CrossRef]
- Silva, F.M.; Lopes, R.S.; Araújo-Junior, J.P. Identification of Cryptosporidium species and genotypes in dairy cattle in Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 22–28. [Google Scholar] [CrossRef]
- Langkjaer, R.B.; Vigre, H.; Enemark, H.L.; Maddox-Hyttel, C. Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology 2007, 134, 339–350. [Google Scholar] [CrossRef]
- Amer, S.; Zidan, S.; Adamu, H.; Ye, J.; Roellig, D.; Xiao, L.; Feng, Y. Prevalence and characterization of Cryptosporidium spp. in dairy cattle in Nile River delta provinces, Egypt. Exp. Parasitol. 2013, 135, 518–523. [Google Scholar] [CrossRef]
- Khan, S.M.; Debnath, C.; Pramanik, A.K.; Xiao, L.; Nozaki, T.; Ganguly, S. Molecular characterization and assessment of zoonotic transmission of Cryptosporidium from dairy cattle in West Bengal, India. Vet. Parasitol. 2010, 171, 41–47. [Google Scholar] [CrossRef]
- Manyazewal, A.; Francesca, S.; Pal, M.; Gezahegn, M.; Tesfaye, M.; Lucy, M.; Teklu, W.; Getachew, T. Prevalence, risk factors and molecular characterization of Cryptosporidium infection in cattle in Addis Ababa and its environs, Ethiopia. Vet. Parasitol. Reg. Stud. 2018, 13, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Silverlås, C.; de Verdier, K.; Emanuelson, U.; Mattsson, J.G.; Björkman, C. Cryptosporidium infection in herds with and without calf diarrhoeal problems. Parasitol. Res. 2010, 107, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Kváč, M.; Kouba, M.; Vítovec, J. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet. Parasitol. 2006, 137, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.; Almeida, A.; Castro, A.; de Lurdes Delgado, M.; Soares, S.; da Costa, J.M.; Canada, N. Molecular characterization of Cryptosporidium and Giardia isolates from cattle from Portugal. Vet. Parasitol. 2007, 147, 47–50. [Google Scholar] [CrossRef]
- Coklin, T.; Farber, J.; Parrington, L.; Dixon, B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet. Parasitol. 2007, 150, 297–305. [Google Scholar] [CrossRef]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef]
- Gong, C.; Cao, X.F.; Deng, L.; Li, W.; Huang, X.M.; Lan, J.C.; Xiao, Q.C.; Zhong, Z.J.; Feng, F.; Zhang, Y.; et al. Epidemiology of Cryptosporidium infection in cattle in China: A review. Parasite 2017, 24, 1. [Google Scholar] [CrossRef]
- Ares-Mazás, M.E.; Fernández-da Ponte, B.; Vergara-Castiblanco, C.A.; Freire-Santos, F.; Quilez-Cinca, J.; Causape-Valenzuela, A.C.; Sánchez-Acedo, C. Oocysts, IgG levels and immunoblot patterns determined for Cryptosporidium parvum in bovine examined during a visit to a farm (northeastern Spain). Vet. Parasitol. 1999, 81, 185–193. [Google Scholar] [CrossRef]
- Murakoshi, F.; Xiao, L.; Matsubara, R.; Sato, R.; Kato, Y.; Sasaki, T.; Fukuda, Y.; Tada, C.; Nakai, Y. Molecular characterization of Cryptosporidium spp. in grazing beef cattle in Japan. Vet. Parasitol. 2012, 187, 123–128. [Google Scholar] [CrossRef]
- Liu, A.; Wang, R.; Li, Y.; Zhang, L.; Shu, J.; Zhang, W.; Feng, Y.; Xiao, L.; Ling, H. Prevalence and distribution of Cryptosporidium spp. in dairy cattle in Heilongjiang Province, China. Parasitol. Res. 2009, 105, 797–802. [Google Scholar] [CrossRef]
- Ondrácková, Z.; Kvác, M.; Sak, B.; Kvetonová, D.; Rost, M. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle in South Bohemia, the Czech Republic. Vet. Parasitol. 2009, 165, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, G.; Zhao, J.; Lu, Q.; Wang, H.; Zhang, L.; Jian, F.; Ning, C.; Xiao, L. Cryptosporidium andersoni is the predominant species in post-weaned and adult dairy cattle in China. Parasitol. Int. 2011, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kváč, M.; Vlnatá, G.; Ježková, J.; Horčičková, M.; Konečný, R.; Hlásková, L.; McEvoy, J.; Sak, B. Cryptosporidium occultus sp. n. (Apicomplexa: Cryptosporidiidae) in rats. Eur. J. Protistol. 2018, 63, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, H.; Jex, A.R.; Koehler, A.V.; Rajapakse, R.P.; Udayawarna, K.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. First molecular characterization of Cryptosporidium and Giardia from bovines (Bos taurus and Bubalus bubalis) in Sri Lanka: Unexpected absence of C. parvum from pre-weaned calves. Parasites Vectors 2014, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Fukuda, Y.; Tada, C.; Sato, R.; Duong, B.; Nguyen, D.T.; Nakai, Y. Molecular characterization of Cryptosporidium in native beef calves in central Vietnam. Parasitol. Res. 2012, 111, 1817–1820. [Google Scholar] [CrossRef]
- Liang, N.; Wu, Y.; Sun, M.; Chang, Y.; Lin, X.; Yu, L.; Hu, S.; Zhang, X.; Zheng, S.; Cui, Z.; et al. Molecular epidemiology of Cryptosporidium spp. in dairy cattle in Guangdong Province, South China. Parasitology 2019, 146, 28–32. [Google Scholar] [CrossRef]
- Sevá, A.D.P.; Funada, M.R.; Souza, S.D.O.; Nava, A.; Richtzenhain, L.J.; Soares, R.M. Occurrence and molecular characterization of Cryptosporidium spp. isolated from domestic animals in a rural area surrounding Atlantic dry forest fragments in Teodoro Sampaio municipality, State of São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2010, 19, 249–253. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; González-Losada, Y.A.; Mezo-Menéndez, M.; Ares-Mazas, E. A study of cryptosporidiosis in a cohort of neonatal calves. Vet. Parasitol. 2002, 106, 11–17. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Santín, M. Clinical and subclinical infections with Cryptosporidium in animals. N. Z. Vet. J. 2013, 61, 1–10. [Google Scholar] [CrossRef]
- Meireles, M.V.; de Oliveira, F.P.; Teixeira, W.F.; Coelho, W.M.; Mendes, L.C. Molecular characterization of Cryptosporidium spp. in dairy calves from the state of São Paulo, Brazil. Parasitol. Res. 2011, 109, 949–951. [Google Scholar] [CrossRef]
- Yildirim, A.; Adanir, R.; Inci, A.; Yukari, B.A.; Duzlu, O.; Onder, Z.; Ciloglu, A.; Simsek, E. Prevalence and genotyping of bovine Cryptosporidium species in the Mediterranean and Central Anatolia Region of Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101425. [Google Scholar] [CrossRef] [PubMed]
- Silverlås, C.; Blanco-Penedo, I. Cryptosporidium spp. in calves and cows from organic and conventional dairy herds. Epidemiol. Infect. 2013, 141, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, T.; Koehler, A.V.; Hu, M.; Gasser, R.B. Molecular investigation of Cryptosporidium and Giardia in pre- and post-weaned calves in Hubei Province, China. Parasites Vectors 2017, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Åberg, M.; Emanuelson, U.; Troell, K.; Björkman, C. Infection dynamics of Cryptosporidium bovis and Cryptosporidium ryanae in a Swedish dairy herd. Vet. Parasitol. X 2019, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Rieux, A.; Paraud, C.; Pors, I.; Chartier, C. Molecular characterization of Cryptosporidium isolates from beef calves under one month of age over three successive years in one herd in western France. Vet. Parasitol. 2014, 202, 171–179. [Google Scholar] [CrossRef]
- Åberg, M.; Emanuelson, U.; Troell, K.; Björkman, C. A single-cohort study of Cryptosporidium bovis and Cryptosporidium ryanae in dairy cattle from birth to calving. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100400. [Google Scholar] [CrossRef]
- Wegayehu, T.; Karim, R.; Anberber, M.; Adamu, H.; Erko, B.; Zhang, L.; Tilahun, G. Prevalence and genetic characterization of Cryptosporidium species in dairy calves in Central Ethiopia. PLoS ONE 2016, 11, e0154647. [Google Scholar] [CrossRef]
- Maikai, B.V.; Umoh, J.U.; Kwaga, J.K.P.; Lawal, I.A.; Maikai, V.A.; Cama, V.; Xiao, L. Molecular characterization of Cryptosporidium spp. in native breeds of cattle in Kaduna State, Nigeria. Vet. Parasitol. 2011, 178, 241–245. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, H.; Huang, Y.; Xu, L.; Rao, L.; Wang, S.; Wang, W.; Yi, Y.; Zhou, X.; Wu, Y.; et al. Cryptosporidium spp. in wild rats (Rattus spp.) from the Hainan Province, China: Molecular detection, species/genotype identification and implications for public health. Int. J. Parasitol. Parasites Wildl. 2019, 9, 317–321. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Hu, S.; Zhao, W.; Zhao, J.; Kváč, M.; Guo, Y.; Li, N.; Feng, Y.; Xiao, L. Common occurrence of divergent Cryptosporidium species and Cryptosporidium parvum subtypes in farmed bamboo rats (Rhizomys sinensis). Parasites Vectors 2020, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liu, H.; Jiang, Y.; Yin, J.; Yuan, Z.; Shen, Y.; Cao, J. First report of Cryptosporidium viatorum and Cryptosporidium occultus in humans in China, and of the unique novel C. viatorum subtype XVaA3h. BMC Infect. Dis. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Hijjawi, N.; Mukbel, R.; Yan, R.; Ryan, U. Genetic characterization of Cryptosporidium in animal and human isolates from Jordan. Vet. Parasitol. 2016, 228, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Santín, M. Cryptosporidium xiaoi n. sp. (Apicomplexa: Cryptosporidiidae) in sheep (Ovis aries). Vet. Parasitol. 2009, 164, 192–200. [Google Scholar] [CrossRef]
- Díaz, P.; Quílez, J.; Prieto, A.; Navarro, E.; Pérez-Creo, A.; Fernández, G.; Panadero, R.; López, C.; Díez-Baños, P.; Morrondo, P. Cryptosporidium species and subtype analysis in diarrhoeic pre-weaned lambs and goat kids from north-western Spain. Parasitol. Res. 2015, 114, 4099–4105. [Google Scholar] [CrossRef]
- Díaz, P.; Quílez, J.; Robinson, G.; Chalmers, R.M.; Díez-Baños, P.; Morrondo, P. Identification of Cryptosporidium xiaoi in diarrhoeic goat kids (Capra hircus) in Spain. Vet. Parasitol. 2010, 172, 132–134. [Google Scholar] [CrossRef]
- Abal-Fabeiro, J.L.; Maside, X.; Llovo, J.; Bartolomé, C. Aetiology and epidemiology of human cryptosporidiosis cases in Galicia (NW Spain), 2000–2008. Epidemiol. Infect. 2015, 143, 3022–3035. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; García-Presedo, I.; Almeida, A.; González-Warleta, M.; Da Costa, J.M.; Mezo, M. Detection of Cryptosporidium spp. and Giardia duodenalis in surface water: A health risk for humans and animals. Water Res. 2009, 43, 4133–4142. [Google Scholar] [CrossRef]
- Gómez-Couso, H.; Méndez-Hermida, F.; Castro-Hermida, J.A.; Ares-Mazás, E. Cryptosporidium contamination in harvesting areas of bivalve molluscs. J. Food Prot. 2006, 69, 185–190. [Google Scholar] [CrossRef]
| Age Class (Months) | Number of C. parvum Positives | IIaA15G2R1 n (%) | IIaA16G3R1 n (%) | Not Identified n (%) |
|---|---|---|---|---|
| G-1 (0–1) | 26 | 18 (69.2) | 2 (7.7) | 6 (23.1) |
| G-2 (1–2) | 6 | 4 (66.7) | 1 (16.7) | 1 (16.7) |
| G-3 (2–12) | 1 | 0 (0) | 1 (100) | 0 (0) |
| G-4 (12–24) | 2 | 1 (50) | 0 (0) | 1 (50) |
| G-5 (>24) | 7 | 3 (42.9) | 0 (0) | 4 (57.1) |
| Total | 42 | 26 (61.9) | 4 (9.5) | 12 (28.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, P.; Navarro, E.; Remesar, S.; García-Dios, D.; Martínez-Calabuig, N.; Prieto, A.; López-Lorenzo, G.; López, C.M.; Panadero, R.; Fernández, G.; et al. The Age-Related Cryptosporidium Species Distribution in Asymptomatic Cattle from North-Western Spain. Animals 2021, 11, 256. https://doi.org/10.3390/ani11020256
Díaz P, Navarro E, Remesar S, García-Dios D, Martínez-Calabuig N, Prieto A, López-Lorenzo G, López CM, Panadero R, Fernández G, et al. The Age-Related Cryptosporidium Species Distribution in Asymptomatic Cattle from North-Western Spain. Animals. 2021; 11(2):256. https://doi.org/10.3390/ani11020256
Chicago/Turabian StyleDíaz, Pablo, Esther Navarro, Susana Remesar, David García-Dios, Néstor Martínez-Calabuig, Alberto Prieto, Gonzalo López-Lorenzo, Ceferino Manuel López, Rosario Panadero, Gonzalo Fernández, and et al. 2021. "The Age-Related Cryptosporidium Species Distribution in Asymptomatic Cattle from North-Western Spain" Animals 11, no. 2: 256. https://doi.org/10.3390/ani11020256
APA StyleDíaz, P., Navarro, E., Remesar, S., García-Dios, D., Martínez-Calabuig, N., Prieto, A., López-Lorenzo, G., López, C. M., Panadero, R., Fernández, G., Díez-Baños, P., & Morrondo, P. (2021). The Age-Related Cryptosporidium Species Distribution in Asymptomatic Cattle from North-Western Spain. Animals, 11(2), 256. https://doi.org/10.3390/ani11020256