1. Introduction
In the last few years, mesenchymal stem cells (MSCs) have shown promise as a therapy in the treatment of musculoskeletal diseases (MSDs), including different pathological conditions such as disorders of muscle, joint, bone, nerves, and tendons [
1]. The disease burden of MSDs in veterinary medicine is considerable and represents a significant threat to animal welfare worldwide, particularly in canine and equine species [
2,
3]. MSDs are related to various factors, such as intensive and repetitive works, sport-related trauma, ageing, and genetic background [
1]. However, the MSCs ability to undergo multi-lineage differentiation has long been considered a critical feature to justify their clinical application. Currently, it is proved that the therapeutic effect of MSCs is mainly related to the secretion of a wide variety of trophic factors, encompassed under the name of secretome [
4,
5], playing an important paracrine activity.
MSCs secretome is composed of a combination of soluble factors (mainly cytokines and growth factors) and insoluble extracellular vesicles, including exosomes and microvesicles. The numerous different features associated with the secretome suggest that it could be used as an MSCs substitute [
5] to mediate their biological activity, including anti-inflammatory and tissue regenerative properties [
6]. The use of MSC-secretome as an alternative to their parental cells brings several advantages in regenerative medicine: the use of a cell-free product would reduce the unwanted immune reaction, and the tumorigenic risk related to the MSCs proliferation and differentiation capability; it is also easier to handle, store and ensure a sterile product by filtration [
7,
8,
9]. We recently proposed a GMP-compliant human secretome production process that allowed us to obtain a stable and effective product named Lyosecretome (freeze-dried secretome) from human adipose tissue-derived MSCs [
10,
11,
12,
13,
14]; this process included an ultrafiltration step for the concentration and the purification of MSCs-derived secretome, and the following lyophilization, obtaining a powder dosage form ensuring an improved long-term stability of the secretome.
In the current work, we applied the same procedure to obtain canine Lyosecretome from adipose tissue-derived MSCs, as a candidate for in vivo testing on naturally occurring diseases, thanks to the promising regenerative potential of MSCs secretome. Canine Lyosecretome was first characterized through quality controls regarding physicochemical properties, vesicles morphology and size distribution, protein and lipid content; furthermore, the requirements for the preparation of injectable pharmaceutical dosage forms were evaluated. Next, in vitro potency tests were performed to assess proliferative effects on different cell lines. Finally, the proof of principle of the safety of the canine allogenic Lyosecretome was performed by intraarticular administration to osteoarthritic dogs. As far as we know, this study is the first canine study based on MSCs secretome in naturally occurring musculoskeletal diseases. The application of Lyosecretome to animals affected by spontaneous osteoarthritis, avoiding the use of experimentally induced disease models, could provide interesting information about the safety and feasibility of an allogenic cell-free regenerative medicine approach in companion animals that share with the owner’s environment and lifestyle [
15], thus providing valuable clues for osteoarthritis therapy in the human counterpart.
3. Results and Discussion
Injectable Lyosecretome (freeze-dried secretome) formulations have been prepared by standardized processes under ISO9001 clinical grade. Overall, three batches were produced starting from three different cell lines, as summarized in
Table 7.
After ultrafiltration to concentrate and purify the cell culture supernatant, the product was added to the cryoprotectant and freeze-dried. A filtering module with a 5 kDa molecular weight cut-off was used to retain in the ultrafiltrate both extracellular vesicles (EVs) and proteins with weight above the cut-off. The use of this membrane size was justified by preliminary investigations, demonstrating a higher immunomodulatory potency in 5 kDa ultrafiltrate (containing both EVs and soluble factors) instead of the 300 kDa ultrafiltrate (containing only EVs) [
10]. Furthermore, Bari et al. [
11] demonstrated that the concentration of IL-6 was higher in samples prepared by ultrafiltration rather than ultracentrifugation. Since IL-6 is a key player in the immunomodulatory features of the MSCs secretome, the large amounts of IL-6 discarded in the ultracentrifugation procedure suggest ultrafiltration is an effective procedure to prepare EV fraction. Similarly, higher anti-elastase activity was demonstrated for the whole secretome and attributed to the large amount of Alpha-1-Antitrypsin, the main inhibitor of neutrophil elastase, in the soluble fraction of the secretome [
14]. Accordingly, Mitchell and colleagues found that EVs and soluble molecules act synergistically to promote tissue regeneration [
23], supporting low cut-off ultrafiltration as a valuable process to prepare MSCs secretome.
The pharmaceutical quality of Lyosecretome was defined by setting up a complete characterization of total protein and lipid content, physical-chemical properties, and particle size. The amount of proteins and lipids for each batch produced is reported in
Table 8. It is worth noting that no systematic error influenced the calibration for both assays as the intercept of the curve equation is not statically significant, and the plot of the residuals had an ordinary distribution of the error (data not shown). Overall, the yield of each batch is different, meaning that the cell line and, more in general, the variability within the same species can strongly influence the secretome composition in terms of protein and lipid quantity (
p < 0.05). Maximum protein production was obtained in batch n. 1; by contrast, lipid amount is higher in batch n. 2. This aspect underlines that there is no correlation between the two assays, probably depending on cell density at the starvation time that can influence secretome production.
The physical-chemical characterization confirmed the simultaneous presence of both proteins and lipids in the Lyosecretome. In detail, FTIR spectra revealed low-intensity bands at around 1653 cm
−1 and 1547 cm
−1 (amide I C=O stretching vibrations and amide II N-H bending vibrations of the peptide groups, respectively), absorbance bands at about 1457 cm
−1 and 1377 cm
−1 (CH
2 and CH
3 groups) and 1260 and 880 cm
−1 (due to the stretching vibrations typical of phospholipids, triglycerides and cholesterol esters, and to the vibrations bands of mannitol) [
10,
11]. By DSC and TGA analysis, it was instead confirmed that the lyophilization process occurred successfully (data not shown).
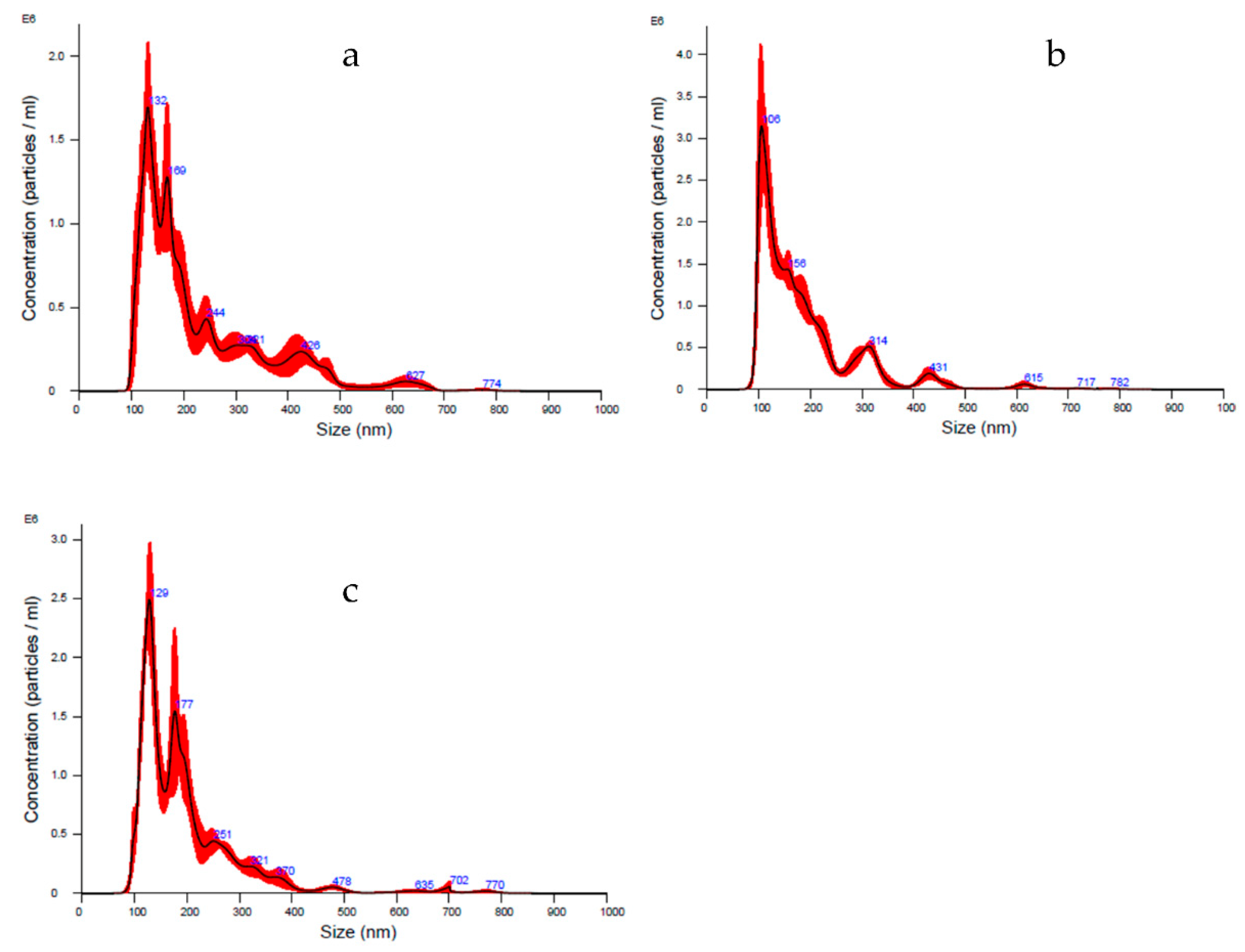
Lyosecretome particles’ size distribution and characterization were evaluated using NTA technology. In detail, the diameter ranges between 190–230 nm, matching the choice to consider both exosomes (40–120 nm) and microvesicles (250–1000 nm). Furthermore, a heterogeneous population is shown by means analyzing curve, whereas d
10 is set between 106–122 nm, d
50 is set between 157–180 nm, and d
90 is set between 316–400 nm. Thanks to this technique, the particle concentration per mL was estimated, ranging between 1.86 × 10
8 and 2.60 × 10
8 particle/mL (
Table 9 and
Figure 1).
Finally, since the Lyosecretome production was aimed at in vivo application, to guarantee the quality of the final product, microbiological tests were conducted before and after lyophilization, thus, on both secretome solution and lyophilized secretome (Lyosecretome) powder. Analyses were performed at a certified institute (IZSLER, Brescia, Italy), and the sterility conditions were ascertained in the same establishment. The results proved the absence of any mycoplasma and bacterial endotoxin level in each examined sample (data not shown).
Regarding the in vitro efficacy, Lyosecretome proliferation potency was assessed by MTT assay. Canine tenocytes, chondrocytes and adipose tissue-derived MSCs were chosen as target cells, the latter because the tissues resident MSCs can mediate tissue regeneration [
24]. Lyosecretome stimulated cell metabolic activity in a dose-dependent trend for all cell types; in detail, the treatment with higher Lyosecretome concentrations reached almost 85% of cell metabolic activity compared to 10% FBS supplemented cultures. As shown in
Figure 2, the dose-dependent effect was evaluated up to 200,000 cell equivalent/well. The effect of Lyosecretome treatment was statistically different for the three cell types (
p < 0.0001).
The anti-elastase activity of canine Lyosecretome demonstrated a dose-dependent trend for all the tested concentrations of 2, 5, 10 and 20 mg/mL (
Figure 3). The assay consists of an enzymatic reaction which was evaluated for up to 40 min. At the higher concentration of 20 mg/mL, the activity reached 85%, considering Epigallocatechin gallate as a positive control due to its inhibition of elastase activity.
Despite this study has not performed a qualitative analysis of protein, RNA, and lipid contents of canine Lyosecretome, proteomic analyses of equine [
2] and human [
10] Lyosecretome revealed proteins involved in controlling the inflammatory pathways activated in osteoarthritic joints and proteins related to cartilage biology. This suggests using Lyosecretome as a substitute for cells in treating musculoskeletal system diseases [
25]. Furthermore, microvesicles are considered safer both from the point of view of immuno-reactivity and for the lower potential risks compared to the administration of in vitro expanded cells [
26]. Still, before testing Lyosecretome efficacy, the in vivo safety must be evaluated. In this regard, this study enrolled five dogs affected by bilateral OA to test Lyosecretome for clinical use. One dog was excluded from the beginning for recent nonsteroidal anti-inflammatory drugs (NSAIDs) treatment (Animal 5,
Table 1), and another dog was excluded before the second administration of the treatment for a recurrent skin infection that could negatively affect the intra-articular treatment (Animal 4,
Table 1).
Lyosecretome and a placebo, consisting of mannitol (used in Lyosecretome formulation as a stabilizer), were resuspended in hyaluronic acid and injected in each animal’s right and left joint, respectively. The choice to treat both affected joints with hyaluronic acid, whose lubricating properties support its widespread use in treating OA in dogs [
27], was made to ensure effective therapy in the control of clinical symptoms for patients enrolled in the study. The data collected with the questionnaire (
Table 6) and reported in
Table S1 have been analyzed to evaluate the onset of undesired effects following therapy (see
Supplementary materials, Figures S4 and S5). No systemic adverse reactions were observed, even after the second administration. After each treatment, short-time side effects were observed for all the patients’ as reluctance to trot or gallop. However, these adverse effects resolved within two days without the need for additional treatment. Since the administration of Lyosecretome (in the right joint) and placebo (in the left joint) co-occurred, it was impossible to attribute the observed symptoms to one of the two treatments. Similar sides effects were also observed by Lee et al. [
28]; the authors reported that following hyaluronic acid intraarticular injection, a dog showed a mild systemic inflammatory reaction, and two dogs showed non-weight-bearing lameness.
Only patient 3 was affected by swelling at the level of both the right and left joints (See
Supplementary Figure S2) accompanied by complaining and groaning and not ease of movement for both lying down and moving after a long rest period. Thus, patient 3 likely showed an adverse reaction that was not directly attributable to the administration of Lyosecretome, as the same response was evidenced even when administering the placebo. Furthermore, the reported effects are commonly noticed immediately after intra-articular injections when animals’ lameness score is enhanced [
29,
30]. Finally, regarding the clinical data, the examination by the veterinary practitioners, performed at days 40 and 80 after the first treatment, were used to assess the possible onset of unexpected symptoms after therapy administration. No significant results were observed in terms of lameness and pain worsening.
In conclusion, given the limited number of enrolled patients, the present work does not provide any direct evidence of the efficacy or safety of the Lyosecretome in the treatment of canine OA. Nevertheless, it is the proof-of-concept for veterinary clinical-grade MSC-secretome production; moreover, the feasibility of two consecutive allogeneic Lyosecretome intraarticular administration in osteoarthritic dogs is proved, without evidence of adverse reactions. Therefore, this paper represents the premise for patient recruitment in a safety/efficacy clinical trial, with a proper number of cases.