Invasion Risk and Potential Impact of Alien Freshwater Fishes on Native Counterparts in Klang Valley, Malaysia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
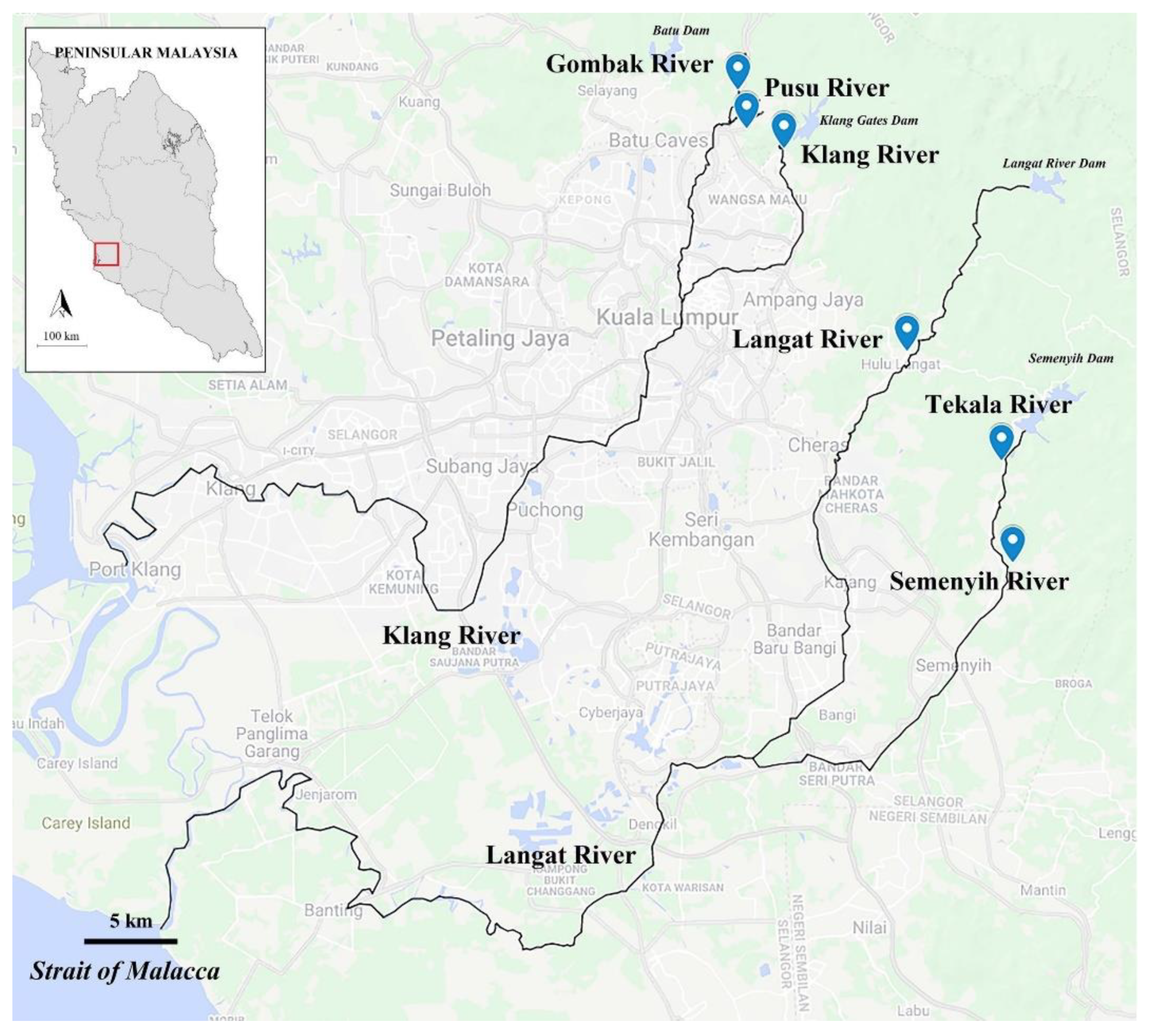
2.1. Study Area
2.2. Fish Sampling
2.3. Water Quality Parameters and the Surrounding Anthropogenic Characteristics
2.4. Invasiveness Screening
2.5. Fish Community Structures
2.6. Stomach Contents Analysis
2.7. Feeding Intensity, Stomach Fullness Index, Frequency of Occurrence, and Volumetric Measurement
2.8. Index of Preponderance, Diet Overlap, and Trophic Level
2.9. Stable Isotopes Analysis
2.10. Statistical Analyses
3. Results
3.1. Fish Checklist and Community Structures
3.2. Water Quality Parameters and Anthropogenic Factors across Sites
3.3. Invasiveness Screening
3.4. Relationships between Fish Occurrences vs. Water Quality Parameters and Anthropogenic Factors
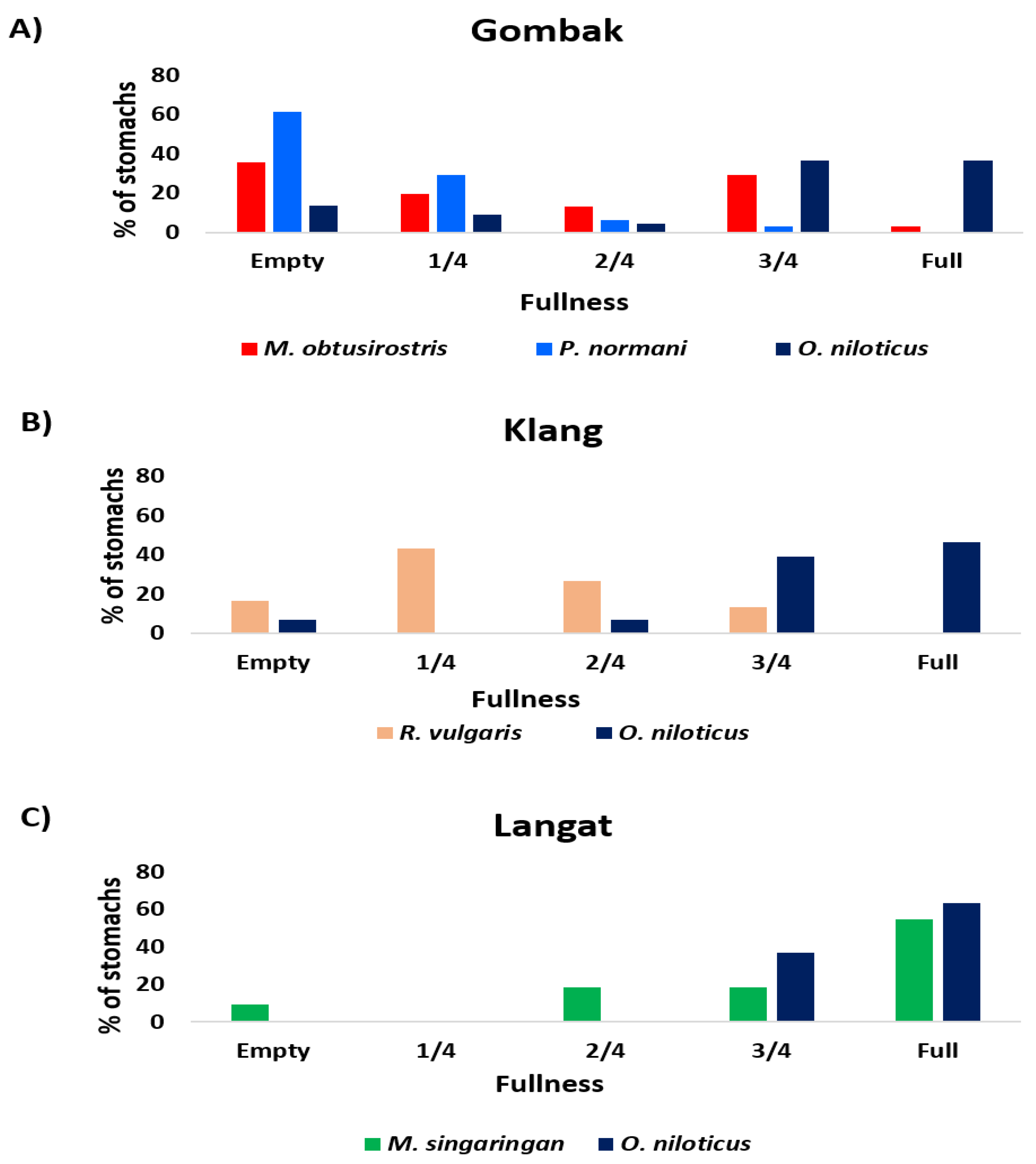
3.5. Feeding Intensity and Stomach Fullness Index
3.6. Importance of the Food Items
3.7. Diet Overlap and Trophic Level
3.8. Stable Isotope Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilgers, L.; Herder, F.; Hadiaty, R.; Pfaender, J. Alien attack: Trophic interactions of flowerhorn cichlids with endemics of ancient Lake Matano (Sulawesi, Indonesia). Evol. Ecol. Res. 2018, 19, 561–574. [Google Scholar]
- Nagelkerke, L.A.; van Onselen, E.; van Kessel, N.; Leuven, R.S. Functional feeding traits as predictors of invasive success of alien freshwater fish species using a food-fish model. PLoS ONE 2018, 13, e0197636. [Google Scholar] [CrossRef]
- Khairul-Adha, R.; Yuzine, E.; Aziz, A. The influence of alien fish species on native fish community structure in Malaysian waters. Kuroshio Sci. 2013, 7, 81–93. [Google Scholar]
- Saba, A.O.; Ismail, A.; Zulkifli, S.Z.; Halim, M.R.A.; Wahid, N.A.A.; Amal, M.N.A. Species composition and invasion risks of alien ornamental freshwater fishes from pet stores in Klang Valley, Malaysia. Sci. Rep. 2020, 10, 17205. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Piria, M.; Simonović, P.; Kalogianni, E.; Vardakas, L.; Koutsikos, N.; Zanella, D.; Ristovska, M.; Apostolou, A.; Adrović, A.; Mrdak, D.; et al. Alien freshwater fish species in the Balkans—Vectors and pathways of introduction. Fish Fish. 2018, 19, 138–169. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, H.R.; Teimori, A.; Owfi, F.; Abbasi, K.; Coad, B.W. Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran. J. Ichthyol. 2014, 1, 62–72. [Google Scholar]
- Saba, A.O.; Ismail, A.; Zulkifli, S.Z.; Shohaimi, S.; Jamil, N.R.; Nawi, N.M.; Ghani, I.F.A.; Halim, M.R.A.; Amal, M.N.A. Checklists, production trends, and potential ecological and socioeconomic impacts of non-native freshwater fishes in Malaysia: A review. Aquat. Invasions 2020, 15, 646–670. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Ertürk Gürkan, S.; Yalçin Özdilek, Ş. The effects of trout culture on diet and food availability of native freshwater fish populations. Aquacult Res. 2019, 50, 1212–1219. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A.; Bacher, S. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 2018, 19, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Irons, K.S.; Sass, G.G.; McClelland, M.A.; Stafford, J.D. Reduced condition factor of two native fish species coincident with invasion of non-native Asian carps in the Illinois River, USA Is this evidence for competition and reduced fitness? J. Fish Biol. 2007, 71, 258–273. [Google Scholar] [CrossRef]
- Minder, M.; Arsenault, E.R.; Erdenee, B.; Pyron, M. Dietary specificity and overlap in endorheic river fishes: How do native and non-native species compare? J. Fish Biol. 2020, 97, 453–464. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, D.R.; de Castro, D.M.P.; Callisto, M.; de Moura Chaves, A.J.; Moreira, M.Z.; Pompeu, P.S. Stable isotopes and stomach content analyses indicate omnivorous habits and opportunistic feeding behavior of an invasive fish. Aquat. Ecol. 2019, 53, 365–381. [Google Scholar] [CrossRef]
- Bradbeer, S.J.; Ngatunga, B.P.; Turner, G.F.; Genner, M.J. Relative growth of invasive and indigenous tilapiine cichlid fish in Tanzania. Afr. J. Aquat. Sci. 2020, 45, 378–381. [Google Scholar] [CrossRef]
- Ribeiro, F.; Leunda, P.M. Non-native fish impacts on Mediterranean freshwater ecosystems: Current knowledge and research needs. Fish. Manag. Ecol. 2012, 19, 142–156. [Google Scholar] [CrossRef]
- Elfidasari, D.; Wijayanti, F.; Sholihah, A. Trophic level and position of Pterygoplichthys pardalis in Ciliwung River (Jakarta, Indonesia) ecosystem based on the gut content analysis. Biodiversitas 2020, 21, 2862–2870. [Google Scholar] [CrossRef]
- Shalloof, K.A.S.; Alaa, M.; Aly, W. Feeding habits and trophic levels of cichlid species in tropical reservoir, Lake Nasser, Egypt. Egypt. J. Aquat. Res. 2020, 46, 159–165. [Google Scholar] [CrossRef]
- Manko, P. Stomach Content Analysis in Freshwater Fish Feeding Ecology; University of Prešov: Prešov, Slovakia, 2016; 116p. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Pasquaud, S.; Elie, P.; Jeantet, C.; Billy, I.; Martinez, P.; Girardin, M. A preliminary investigation of the fish food web in the Gironde estuary, France, using dietary and stable isotope analyses. Estuar. Coast. Shelf Sci. 2008, 78, 267–279. [Google Scholar] [CrossRef]
- Malek, A.J.; Collie, J.S.; Taylor, D.L. Trophic structure of a coastal fish community determined with diet and stable isotope analyses. J. Fish Biol. 2016, 89, 1513–1536. [Google Scholar] [CrossRef]
- McClain-Counts, J.P.; Demopoulos, A.W.; Ross, S.W. Trophic structure of mesopelagic fishes in the Gulf of Mexico revealed by gut content and stable isotope analyses. Mar. Ecol. 2017, 38, e12449. [Google Scholar] [CrossRef]
- Pacioglu, O.; Zubrod, J.P.; Schulz, R.; Jones, J.I.; Pârvulescu, L. Two is better than one: Combining gut content and stable isotope analyses to infer trophic interactions between native and invasive species. Hydrobiologia 2019, 839, 25–35. [Google Scholar] [CrossRef]
- Sukeri, N.F.M.; Rashid, Z.A.; Saba, A.O.; Halim, M.R.A.; Amal, M.N.A. The influences of water quality on fish occurrences in Kuala Mai, Pahang River and Ulu Tembeling, Tembeling River, Pahang, Malaysia. Pertanika J. Trop. Agric. Sci. 2020, 43, 163–182. [Google Scholar]
- Saba, A.O.; Rasli, N.F.; Ismail, A.; Zulkifli, S.Z.; Ghani, I.F.A.; Muhammad-Rasul, A.H.; Amal, M.N.A. A report on introduced Amazon sailfin catfish, Pterygoplichthys pardalis in Gombak Basin, Selangor, with notes on two body patterns of the species. Pertanika J. Trop. Agric. Sci. 2020, 43, 693–703. [Google Scholar]
- Chong, V.C.; Lee, P.K.Y.; Lau, C.M. Diversity, extinction risk and conservation of Malaysian fishes. J. Fish Biol. 2010, 76, 2009–2066. [Google Scholar] [CrossRef]
- Samat, A.; Shukor, M.N.; Mazlan, A.G.; Arshad, A.; Fatimah, M.Y. Length-weight relationship and condition factor of Pterygoplichthys pardalis (Pisces: Loricariidae) in Malaysia Peninsular. RJFH 2008, 3, 48–53. [Google Scholar]
- Khairul-Adha, R.A.; Daud, S.K.; Siraj, S.S.; Arshad, A.; Esa, Y.; Ibrahim, E.R. Freshwater fish diversity and composition in Batang Kerang floodplain, Balai Ringin, Sarawak. Pertanika J. Trop. Agric. Sci. 2009, 32, 7–16. [Google Scholar]
- Naji, A.; Ismail, A.; Kamrani, E.; Sohrabi, T. Correlation of MT levels in livers and gills with heavy metals in wild tilapia (Oreochromis mossambicus) from the Klang River, Malaysia. Bull. Environ. Contam. Toxicol. 2014, 92, 674–679. [Google Scholar] [CrossRef]
- Jalal, K.C.A.; Alifah, F.K.; Faizul, H.N.N.; Mamun, A.A.; Kader, M.A.; Ashraf, M.A. Diversity and community composition of fishes in the Pusu River (Gombak, Malaysia). J. Coast. Res. 2018, 82, 150–155. [Google Scholar] [CrossRef]
- Suhaila, J.; Deni, S.M.; Zin, W.W.; Jemain, A.A. Trends in peninsular Malaysia rainfall data during the southwest monsoon and northeast monsoon seasons: 1975–2004. Sains Malays. 2010, 39, 533–542. [Google Scholar]
- Wong, C.L.; Liew, J.; Yusop, Z.; Ismail, T.; Venneker, R.; Uhlenbrook, S. Rainfall characteristics and regionalization in Peninsular Malaysia based on a high-resolution gridded data set. Water 2016, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.F.A.; Ishak, A.G. The importance of internal migration: In the context of urban planning decision making. In Proceedings of the International Conference on Built Environment in Developing Countries, Penang, Malaysia, 2–3 December 2009. [Google Scholar]
- Kottelat, M. The fishes of the inland waters of Southeast Asia: A catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull. Zool. 2013, 27, 1–663. [Google Scholar]
- Zakaria-Ismail, M.; Fatimah, A.; Khaironizam, M.Z. Fishes of the Freshwater Ecosystems of Peninsular Malaysia; Lambert Academic Publishing: Saarbrücken, Germany, 2019; 356p. [Google Scholar]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Trakan, A.S.; Miossec, L.; Goulletquer, P. Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Copp, G.H.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; Macleod, A.; Cowx, I.G.; Nunn, A.D.; Occhipinti-Ambrogi, A.; Savini, D.; et al. European non-native species in aquaculture risk analysis scheme—A summary of assessment protocols and decision support tools for use of alien species in aquaculture. Fish. Manag. Ecol. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Lawson, L.L., Jr.; Hill, J.E.; Vilizzi, L.; Hardin, S.; Copp, G.H. Revisions of the Fish Invasiveness Screening Kit (FISK) for its application in warmer climatic zones, with reference to Peninsular Florida. Risk Anal. 2013, 33, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Vilizzi, L.; Copp, G.H.; Adamovich, B.; Almeida, D.; Chan, J.; Davison, P.I.; Dembski, S.; Ekmekçi, F.G.; Ferincz, Á.; Forneck, S.C.; et al. A global review and meta-analysis of applications of the freshwater Fish Invasiveness Screening Kit. Rev. Fish Biol. 2019, 29, 529–569. [Google Scholar] [CrossRef] [Green Version]
- Lawson, L.L., Jr.; Hill, J.E.; Hardin, S.; Vilizzi, L.; Copp, G.H. Evaluation of the Fish Invasiveness Screening Kit (FISK v2) for Peninsular Florida. Manag. Biol. Invasions 2015, 6, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Killi, N.; Tarkan, A.S.; Kozic, S.; Copp, G.H.; Davison, P.I.; Vilizzi, L. Risk screening of the potential invasiveness of non-native jellyfishes in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 150, 110728. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1962. [Google Scholar]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley Interscience: Hoboken, NJ, USA, 1969. [Google Scholar]
- Whittaker, R.H. Evolution of species diversity in land communities. Evol. Biol. 1977, 10, 1–67. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Wilson, M.V.; Shmida, A. Measuring beta diversity with presence-absence data. J. Ecol. 1984, 72, 1055–1064. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software. 2001. Available online: https://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 20 January 2021).
- Widarmanto, N.; Haeruddin, H.; Purnomo, P. Kebiasaan Makanan, Luas Relung dan Tingkat Trofik Komunitas ikan di Estuari Kaliwlingi Kabupaten Brebes. BAWAL 2019, 11, 69–78. [Google Scholar] [CrossRef]
- Huynh, M.; Serediak, N. Algae Identification Field Guide Agriculture and Agric-Food Canada; Agri-Environment Services Branch, Majesty the Queen in Right of Canada: Ottawa, ON, Canada, 2006; 40p. [Google Scholar]
- Van Vuuren, S.; Taylor, J.; Gerber, A.; Van Ginkel, C. Easy Identification of the Most Common Freshwater Algae; North-West University and Department of Water Affairs and Forestry: Pretoria, South Africa, 2006; 200p. [Google Scholar]
- Phan, D.D.; Nguyen, V.K.; Le, T.N.; Dang, N.T.; Ho, T.H. Identification Handbook of Freshwater Zooplankton of the Mekong River and Its Tributaries; Mekong River Commission: Vientiane, Laos, 2015; 207p. [Google Scholar]
- Zacharia, P.; Abdurahiman, K.; Mohamed, K. Methods of stomach content analysis of fishes. In Winter School On towards Ecosystem-Based Management of Marine Fisheries—Building Mass Balance Trophic and Simulation Models; RC of Central Marine Fisheries Research Institute: Mangalore, India, 2004. [Google Scholar]
- Willis, C.M.; Richardson, J.; Smart, T.; Cowan, J.; Biondo, P. Diet composition, feeding strategy, and diet overlap of 3 sciaenids along the southeastern United States. Fish. Bull. 2015, 113, 290–302. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Santi, E.P.; Rahardjo, M.F. Diet of scalloped perchlet, Ambassis nalua (Hamilton, 1822) in Pabean Bay, West Java. J. Iktiologi Indones. 2017, 17, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Escalona, V.H.; Abitia-Cardenas, L.A.; Campos-Davila, L.; Galvan-Magaña, F. Trophic interrelations of the three most abundant fish species from Laguna San Ignacio, Baja California Sur, Mexico. Bull. Mar. Sci. 2000, 66, 361–373. [Google Scholar]
- Pauly, D.; Froese, R.; Sala, P.S.; Palomares, M.L.; Christensen, V.; Rius, J. TrophLab Manual; ICLARM: Manila, Philippines, 2000. [Google Scholar]
- Pauly, D.; Palomares, M.L. Approaches for dealing with three sources of bias when studying the fishing down marine food web phenomenon. Fishing Down the Mediterranean Food Webs? In Proceedings of the CIESM Workshop Series 12, Kerkyra, Greece, 26–30 July 2000. [Google Scholar]
- Nakamura, Y.; Horinouchi, M.; Shibuno, T.; Tanaka, Y.; Miyajima, T.; Koike, I.; Kurokura, H.; Sano, M. Evidence of ontogenetic migration from mangroves to coral reefs by black-tail snapper Lutjanus fulvus: Stable isotope approach. Mar. Ecol. Prog. Ser. 2008, 355, 257–266. [Google Scholar] [CrossRef]
- Zulkifli, Z.; Mohamat-Yusuff, F.; Ismail, A.; Miyazaki, N. Food preference of the giant mudskipper Periophthalmodon schlosseri (Teleostei: Gobiidae). Knowl. Manag. Aquat. Ecosyst. 2012, 405, 1–10. [Google Scholar]
- Shuai, F.; Li, X.; Liu, Q.; Zhu, S.; Wu, Z.; Zhang, Y. Nile tilapia (Oreochromis niloticus) invasions disrupt the functional patterns of fish community in a large subtropical river in China. Fish. Manag. Ecol. 2019, 26, 578–589. [Google Scholar] [CrossRef]
- Almeida, D.; Ribeiro, F.; Leunda, P.M.; Vilizzi, L.; Copp, G.H. Effectiveness of FISK, an invasiveness screening tool for non-native freshwater fishes, to perform risk identification assessments in the Iberian Peninsula. Risk Anal. 2013, 33, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Zengeya, T.A.; Robertson, M.P.; Booth, A.J.; Chimimba, C.T. A qualitative ecological risk assessment of the invasive Nile tilapia, Oreochromis niloticus in a sub-tropical African river system (Limpopo River, South Africa). Aquat. Conserv. 2013, 23, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Adhikari, S.; Tiwari, P.K. Importance of Soil and Water Quality Management in Freshwater Aquaculture with Special Reference to Catfish Farming. In Proceedings of the Mass Breeding and Culture Technique of Catfishes, Odisha, India, 16–21 July 2018; p. 25. [Google Scholar]
- García-Berthou, E. The characteristics of invasive fishes: What has been learned so far? J. Fish Biol. 2007, 71, 33–55. [Google Scholar] [CrossRef]
- Nordin, N.F.; Ibrahim, N.H.; Chowdhury, A.J. Physicochemical parameters and bacterial composition in Sungai Pusu, Gombak. Sci. Herit. J. 2018, 2, 10–20. [Google Scholar] [CrossRef]
- Saba, A.O.; Ismail, A.; Zulkifli, S.Z.; Shohaimi, S.; Amal, M.N.A. Public knowledge and perceptions of the impacts and importance of alien fish species in Malaysia: Implications for freshwater biodiversity and conservation. Manag. Biol. Invasions 2021, 12, 441–456. [Google Scholar] [CrossRef]
- Vicente, I.S.; Fonseca-Alves, C.E. Impact of introduced Nile tilapia (Oreochromis niloticus) on non-native aquatic ecosystems. Pak. J. Biol. Sci. 2013, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- De Iongh, H.H.; Van Zon, J.C. Assessment of impact of the introduction of exotic fish species in north-east Thailand. Aquac. Res. 1993, 24, 279–289. [Google Scholar] [CrossRef]
- Ansah, Y.B.; Frimpong, E.A.; Hallerman, E.M. Genetically improved tilapia strains in Africa: Potential benefits and negative impacts. Sustainability 2014, 6, 3697–3721. [Google Scholar] [CrossRef] [Green Version]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ellender, B.R.; Marr, S.M.; Weyl, O.L.; Zengeya, T.; Wasserman, R.J.; Alexander, M.E.; Ivey, P.; Woodford, D.J. Evaluating invasion risk for freshwater fishes in South Africa. Bothalia 2017, 47, 1–10. [Google Scholar]
- Perdikaris, C.; Koutsikos, N.; Vardakas, L.; Kommatas, D.; Simonovic, P.; Paschos, I.; Detsis, V.; Vilizzi, L.; Copp, G.H. Risk screening of non-native translocated and traded aquarium freshwater fishes in Greece using Fish Invasiveness Screening Kit. Fish. Manag. Ecol. 2016, 23, 32–43. [Google Scholar] [CrossRef]
- Mendoza, R.; Luna, S.; Aguilera, C. Risk assessment of the ornamental fish trade in Mexico: Analysis of freshwater species and effectiveness of the FISK (Fish Invasiveness Screening Kit). Biol. Invasions 2015, 17, 3491–3502. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J.D. Ecological impacts of non-native freshwater fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Herder, F.; Freyhof, J. Resource partitioning in a tropical stream fish assemblage. J. Fish Biol. 2006, 69, 571–589. [Google Scholar] [CrossRef]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Peterson, A. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. 2006, 63, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Zengeya, T.A.; Booth, A.J.; Bastos, A.D.; Chimimba, C.T. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ. Biol. Fishes 2011, 92, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Zengeya, T.A.; Booth, A.J.; Chimimba, C.T. Broad niche overlap between invasive Nile tilapia Oreochromis niloticus and indigenous congenerics in Southern Africa: Should we be concerned? Entropy 2015, 17, 4959–4973. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.A.; Bart, A.N.; Yi, Y.; Rakocy, J.E.; Diana, J.S. The effect of the introduction of Nile tilapia (Oreochromis niloticus L.) on small indigenous fish species (mola, Amblypharyngodon mola, Hamilton; chela, Chela cachius, Hamilton; punti, Puntius sophore, Hamilton). Aquac. Res. 2010, 41, 904–912. [Google Scholar] [CrossRef]
- Gochuru, N.N.; Manyala, J.O.; Raburu, P.O. Some aspects of reproduction and feeding habits of Nile tilapia (Oreochromis niloticus) in three dams in Uasin Gishu County, Kenya. Lakes Reserv. Res. Manag. 2019, 24, 181–189. [Google Scholar] [CrossRef]
- De Moraes, C.R.; de Attayde, J.L.; Henry-Silva, G.G. Stable isotopes of C and N as dietary indicators of Nile tilapia (Oreochromis niloticus) cultivated in net cages in a tropical reservoir. Aquac. Rep. 2020, 18, 100458. [Google Scholar] [CrossRef]
- Lerner, J.E.; Ono, K.; Hernandez, K.M.; Runstadler, J.A.; Puryear, W.B.; Polito, M.J. Evaluating the use of stable isotope analysis to infer the feeding ecology of a growing US gray seal (Halichoerus grypus) population. PLoS ONE 2018, 13, e0192241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, F.; Lek, S.; Li, X.; Zhao, T. Biological invasions undermine the functional diversity of fish community in a large subtropical river. Biol. Invasions 2018, 20, 2981–2996. [Google Scholar] [CrossRef]
- Birck, T.; Message, H.J.; Baumgartner, G.; Yahaut, N. Foraging behavior interactions between the invasive Nile tilapia (Cichliformes: Cichlidae) and three large native predators. Neotrop. Ichthyol. 2019, 17, e180116. [Google Scholar] [CrossRef]



| Order | Family | Species | Abbr | Number of Individuals Per Site | % of Occurrences | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | G | K | T | S | L | Total | |||||
| Cyprinidontiformes | Poecilidae | Poecilia reticulata Peters, 1859 * | P.reti | 55 | 75 | 2 | 0 | 0 | 8 | 140 | 15.4 |
| Cyprinidontiformes | Aplocheilidae | Aplocheilus armatus (van Hasselt 1823) | A.arma | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0.2 |
| Cypriniformes | Cyprinidae | Mystacoleucus obtusirostris (Valenciennes, 1842) | M.obtu | 6 | 64 | 0 | 18 | 4 | 23 | 115 | 12.7 |
| Cypriniformes | Cyprinidae | Poropuntius normani Smith, 1931 | P.norm | 3 | 49 | 0 | 4 | 12 | 0 | 68 | 7.5 |
| Cypriniformes | Cyprinidae | Hampala macrolepidota Kuhl and Van Hasselt, 1823 | H.macro | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0.2 |
| Cypriniformes | Cyprinidae | Osteochilus vittatus (Valenciennes, 1842) | O.vitt | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0.4 |
| Cypriniformes | Cyprinidae | Barbodes banksi (Herre, 1940) | B.bank | 0 | 2 | 0 | 4 | 4 | 0 | 10 | 1.1 |
| Cypriniformes | Cyprinidae | Neolissochilus soroides (Duncker, 1904) | N.soro | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.1 |
| Cypriniformes | Cyprinidae | Barbonymus gonionotus (Bleeker, 1849) * | B.goni | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0.2 |
| Cypriniformes | Danionidae | Esomus metallicus Ahl, 1923 | E.meta | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 0.4 |
| Cypriniformes | Danionidae | Rasbora vulgaris Duncker, 1904 | R.vulga | 0 | 0 | 48 | 5 | 8 | 0 | 61 | 6.7 |
| Perciformes | Cichlidae | Oreochromis niloticus (Linnaeus, 1758) * | O.nilo | 272 | 27 | 46 | 0 | 2 | 69 | 416 | 45.9 |
| Perciformes | Cichlidae | Oreochromis sp. * | C.spp | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0.2 |
| Perciformes | Oshpronemidae | Trichopsis vittata (Cuvier, 1831) | T.vita | 0 | 0 | 6 | 0 | 1 | 0 | 7 | 0.8 |
| Perciformes | Eleotridae | Oxyeleotris marmorata (Bleeker, 1852) | O.mamo | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0.2 |
| Siluriformes | Loricariidae | Pterygoplichthys pardalis (Castelnau, 1855) * | P.pard | 28 | 0 | 0 | 0 | 0 | 1 | 29 | 3.2 |
| Siluriformes | Loricariidae | Pterygoplichthys disjunctivus (Weber, 1991) * | P.disj | 8 | 0 | 0 | 0 | 0 | 19 | 27 | 3.0 |
| Siluriformes | Bagridae | Mystus singaringan (Bleeker, 1846) | M.singa | 0 | 0 | 0 | 0 | 0 | 11 | 11 | 1.2 |
| Siluriformes | Bagridae | Hemibagrus sp. | H.spp | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.1 |
| Siluriformes | Clariidae | Clarias sp. | Cl.spp | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0.3 |
| Total Fish Individuals | 376 | 217 | 112 | 32 | 32 | 138 | 907 | 100 | |||
| River | Dmn | H’ | J | C |
|---|---|---|---|---|
| Pusu | 0.361 | 0.944 | 0.485 | 0.551 |
| Gombak | 0.339 | 1.366 | 0.849 | 0.273 |
| Klang | 0.850 | 1.304 | 0.594 | 0.358 |
| Tekala | 0.884 | 1.242 | 0.772 | 0.373 |
| Semenyih | 1.315 | 1.801 | 0.866 | 0.198 |
| Langat | 0.851 | 1.537 | 0.667 | 0.307 |
| River | Pusu | Gombak | Klang | Tekala | Semenyih | Langat |
|---|---|---|---|---|---|---|
| Pusu | 0.333 | 0.750 | 0.667 | 0.467 | 0.412 | |
| Gombak | 0.714 | 0.400 | 0.231 | 0.600 | ||
| Klang | 0.714 | 0.529 | 0.684 | |||
| Tekala | 0.385 | 0.867 | ||||
| Semenyih | 0.667 | |||||
| Langat |
| River | Species | Mean ± SD | n | Min | Max | %FS |
|---|---|---|---|---|---|---|
| Gombak | Mystcoleucus obtusirotris | 9.69 ± 1.46 | 31 | 7.30 | 13.00 | 64.50 |
| Poropuntius normani | 7.88 ± 2.20 | 31 | 6.60 | 13.40 | 41.90 | |
| Oreochromis niloticus * | 6.78 ± 0.91 | 22 | 5.30 | 8.90 | 86.40 | |
| Klang | Rasbora vulgaris | 11.42 ± 4.85 | 30 | 5.00 | 21.50 | 92.90 |
| Oreochromis niloticus * | 8.74 ± 0.58 | 28 | 7.70 | 9.80 | 83.30 | |
| Langat | Mystus singaringan | 14.15 ± 1.38 | 11 | 10.90 | 16.50 | 90.90 |
| Oreochromis niloticus * | 10.51 ±1.92 | 30 | 6.50 | 14.30 | 100.00 |
| River | Species | Mean ± SD |
|---|---|---|
| Gombak | Mystacoleucus obtusirostris | 0.38 ± 0.26 a |
| Poropuntius normani | 0.23 ± 0.15 a | |
| Oreochromis niloticus * | 1.05 ± 0.72 b | |
| Klang | Rasbora vulgaris | 0.29 ± 0.25 a |
| Oreochromis niloticus * | 1.26 ± 0.95 b | |
| Langat | Mystus singaringan | 0.35 ± 0.30 a |
| Oreochromis niloticus * | 1.17 ± 0.55 b |
| Mystacoleucus obtusirostris n = 31 | Poropuntius normani n = 31 | Oreochromis niloticus * n = 22 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Category | FO (%) | V (%) | IP (%) | FO (%) | V (%) | IP (%) | FO (%) | V (%) | IP (%) |
| Cyanobacteria | 70.00 | 2.68 | 2.27 | 53.85 | 12.22 | 8.18 | 94.74 | 6.46 | 6.56 |
| Multicellular green algae | 90.00 | 21.56 | 23.48 | 100.00 | 11.71 | 14.56 | 52.63 | 3.65 | 1.06 |
| Cladophora | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 21.05 | 1.57 | 0.35 |
| Other multicellular algae | 90.00 | 21.56 | 23.48 | 100.00 | 11.71 | 14.56 | 31.58 | 2.08 | 0.70 |
| Unicellular green algae | 0.00 | 0.00 | 0.00 | 23.08 | 0.61 | 0.09 | 84.62 | 7.42 | 4.80 |
| Chlamydomonas | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 42.11 | 1.52 | 0.68 |
| Chroococcus | 0.00 | 0.00 | 0.00 | 7.69 | 0.31 | 0.03 | 78.95 | 3.60 | 3.04 |
| Synedra | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 57.89 | 1.52 | 0.94 |
| Other unicellular algae | 0.00 | 0.00 | 0.00 | 15.38 | 0.31 | 0.06 | 15.79 | 0.79 | 0.13 |
| Euglena | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 36.84 | 0.73 | 0.29 |
| Red algae | 10.00 | 2.82 | 0.17 | 23.08 | 1.12 | 0.32 | 0.00 | 0.00 | 0.00 |
| Compsopogon | 5.00 | 2.26 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other red algae | 5.00 | 0.56 | 0.03 | 23.08 | 1.12 | 0.32 | 0.00 | 0.00 | 0.00 |
| Detritus | 100.00 | 48.27 | 58.39 | 100.00 | 52.85 | 65.71 | 100.00 | 45.42 | 48.63 |
| Insect part | 50.00 | 13.53 | 8.18 | 30.77 | 8.35 | 3.19 | 0.00 | 0.00 | 0.00 |
| Diatoms | 70.00 | 4.09 | 3.46 | 84.62 | 4.07 | 4.29 | 100.00 | 27.04 | 28.95 |
| Worms | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.53 | 0.11 | 0.01 |
| Unidentified | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.26 | 0.11 | 0.01 |
| Plant parts | 10.00 | 0.35 | 0.04 | 15.38 | 5.70 | 1.09 | 0.00 | 0.00 | 0.00 |
| Mud | 50.00 | 6.62 | 4.01 | 61.54 | 3.36 | 2.57 | 100.00 | 9.05 | 9.69 |
| Zooplankton | 5.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rotifer | 5.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rasbora vulgaris n = 30 | Oreochromis niloticus * n = 28 | |||||
|---|---|---|---|---|---|---|
| Food Category | FO (%) | V (%) | IP (%) | FO (%) | V (%) | IP (%) |
| Cyanobacteria | 16.00 | 0.45 | 0.08 | 100.00 | 3.57 | 3.88 |
| Multicellular green algae | 76.00 | 4.11 | 3.42 | 100.00 | 11.89 | 11.13 |
| Cladophora | 4.00 | 0.15 | 0.01 | 14.81 | 1.92 | 0.31 |
| Other multicellular algae | 76.00 | 3.96 | 3.41 | 100.00 | 9.97 | 10.83 |
| Unicellular green algae | 0.00 | 0.00 | 0.00 | 100.00 | 5.99 | 5.83 |
| Chlamydomonas | 0.00 | 0.00 | 0.00 | 11.11 | 0.10 | 0.01 |
| Chroococcus | 0.00 | 0.00 | 0.00 | 100.00 | 4.61 | 5.01 |
| Closterium | 0.00 | 0.00 | 0.00 | 59.26 | 1.25 | 0.80 |
| Other unicellular green algae | 0.00 | 0.00 | 0.00 | 3.70 | 0.03 | 0.00 |
| Diatoms | 8.00 | 0.22 | 0.02 | 100.00 | 17.47 | 18.98 |
| Red algae | 4.00 | 0.60 | 0.03 | 3.70 | 0.03 | 0.00 |
| Compsopogon | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other red algae | 4.00 | 0.60 | 0.03 | 3.70 | 0.03 | 0.00 |
| Detritus | 100.00 | 38.82 | 43.96 | 100.00 | 40.40 | 43.89 |
| Unidentified | 8.00 | 3.29 | 0.30 | 14.81 | 1.04 | 0.17 |
| Plant parts | 28.00 | 6.66 | 2.11 | 70.37 | 6.50 | 4.97 |
| Mud | 0.00 | 0.00 | 0.00 | 100.00 | 8.99 | 9.76 |
| Zooplankton | 0.00 | 0.00 | 0.00 | 33.3 | 0.67 | 0.10 |
| Unidentified zooplankton | 0.00 | 0.00 | 0.00 | 14.81 | 0.20 | 0.03 |
| Rotifer | 0.00 | 0.00 | 0.00 | 11.11 | 0.30 | 0.04 |
| Daphnia | 0.00 | 0.00 | 0.00 | 14.81 | 0.17 | 0.03 |
| Fish egg | 8.00 | 0.82 | 0.07 | 11.11 | 0.24 | 0.03 |
| Fish scale | 4.00 | 0.60 | 0.03 | 11.11 | 0.07 | 0.01 |
| Worms | 4.00 | 0.30 | 0.01 | 66.67 | 1.21 | 0.88 |
| Insect part | 100.00 | 44.13 | 49.97 | 18.52 | 1.92 | 0.39 |
| Mystus singaringan n = 11 | Oreochromis niloticus * n = 30 | |||||
|---|---|---|---|---|---|---|
| Food Category | FO (%) | V (%) | IP (%) | FO (%) | V (%) | IP (%) |
| Cyanobacteria | 60.00 | 1.22 | 0.95 | 60.00 | 1.31 | 0.95 |
| Multicellular green algae | 100.00 | 8.24 | 10.70 | 96.67 | 18.12 | 17.38 |
| Cladophora | 0.00 | 0.00 | 0.00 | 73.33 | 11.84 | 10.52 |
| Other algae | 100.00 | 8.24 | 10.70 | 90.00 | 6.28 | 6.85 |
| Unicellular green algae | 0.00 | 0.00 | 0.00 | 80.00 | 2.64 | 2.18 |
| Colestrium | 0.00 | 0.00 | 0.00 | 6.67 | 0.06 | 0.00 |
| Chroococcus | 0.00 | 0.00 | 0.00 | 3.33 | 0.03 | 0.00 |
| Other unicellular green algae | 0.00 | 0.00 | 0.00 | 70.00 | 2.56 | 2.17 |
| Red algae | 70.00 | 11.70 | 5.95 | 60.00 | 7.78 | 3.40 |
| Compsopogon | 10.00 | 4.88 | 0.63 | 36.67 | 6.28 | 2.79 |
| Other red algae | 60.00 | 6.82 | 5.31 | 33.33 | 1.50 | 0.61 |
| Detritus | 100.00 | 27.47 | 35.68 | 96.67 | 24.76 | 29.01 |
| Insect part | 70.00 | 13.94 | 12.67 | 30.00 | 1.89 | 0.69 |
| Diatoms | 20.00 | 0.41 | 0.11 | 53.33 | 0.94 | 0.61 |
| Worms | 70.00 | 15.67 | 14.25 | 83.33 | 18.17 | 18.36 |
| Unidentified | 10.00 | 1.83 | 0.24 | 16.67 | 1.06 | 0.21 |
| Plant parts | 100.00 | 11.39 | 14.80 | 96.67 | 13.50 | 15.83 |
| Mud | 70.00 | 3.36 | 3.05 | 100.00 | 9.36 | 11.35 |
| Zooplankton | 40.00 | 2.85 | 0.95 | 6.67 | 0.44 | 0.04 |
| Unidentified zooplankton | 30.00 | 1.63 | 0.63 | 6.67 | 0.44 | 0.04 |
| Rotifer | 20.00 | 1.22 | 0.32 | 0.00 | 0.00 | 0.00 |
| Fish scales | 30.00 | 1.53 | 0.59 | 3.33 | 0.03 | 0.00 |
| Leech | 10.00 | 0.41 | 0.05 | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba, A.O.; Ismail, A.; Zulkifli, S.Z.; Ghani, I.F.A.; Halim, M.R.A.; Ibrahim, M.A.; Mukhtar, A.; Aziz, A.A.; Wahid, N.A.A.; Amal, M.N.A. Invasion Risk and Potential Impact of Alien Freshwater Fishes on Native Counterparts in Klang Valley, Malaysia. Animals 2021, 11, 3152. https://doi.org/10.3390/ani11113152
Saba AO, Ismail A, Zulkifli SZ, Ghani IFA, Halim MRA, Ibrahim MA, Mukhtar A, Aziz AA, Wahid NAA, Amal MNA. Invasion Risk and Potential Impact of Alien Freshwater Fishes on Native Counterparts in Klang Valley, Malaysia. Animals. 2021; 11(11):3152. https://doi.org/10.3390/ani11113152
Chicago/Turabian StyleSaba, Abdulwakil Olawale, Ahmad Ismail, Syaizwan Zahmir Zulkifli, Intan Faraha A. Ghani, Muhammad Rasul Abdullah Halim, Musa Adamu Ibrahim, Aqilah Mukhtar, Azharuddin Abd Aziz, Noor Azrizal Abdul Wahid, and Mohammad Noor Azmai Amal. 2021. "Invasion Risk and Potential Impact of Alien Freshwater Fishes on Native Counterparts in Klang Valley, Malaysia" Animals 11, no. 11: 3152. https://doi.org/10.3390/ani11113152
APA StyleSaba, A. O., Ismail, A., Zulkifli, S. Z., Ghani, I. F. A., Halim, M. R. A., Ibrahim, M. A., Mukhtar, A., Aziz, A. A., Wahid, N. A. A., & Amal, M. N. A. (2021). Invasion Risk and Potential Impact of Alien Freshwater Fishes on Native Counterparts in Klang Valley, Malaysia. Animals, 11(11), 3152. https://doi.org/10.3390/ani11113152







