First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Polymerase Chain Reaction (PCR) Detection and Prevalence of EHP
2.3. Histopathology and Transmission Electron Microscopy (TEM)
3. Results
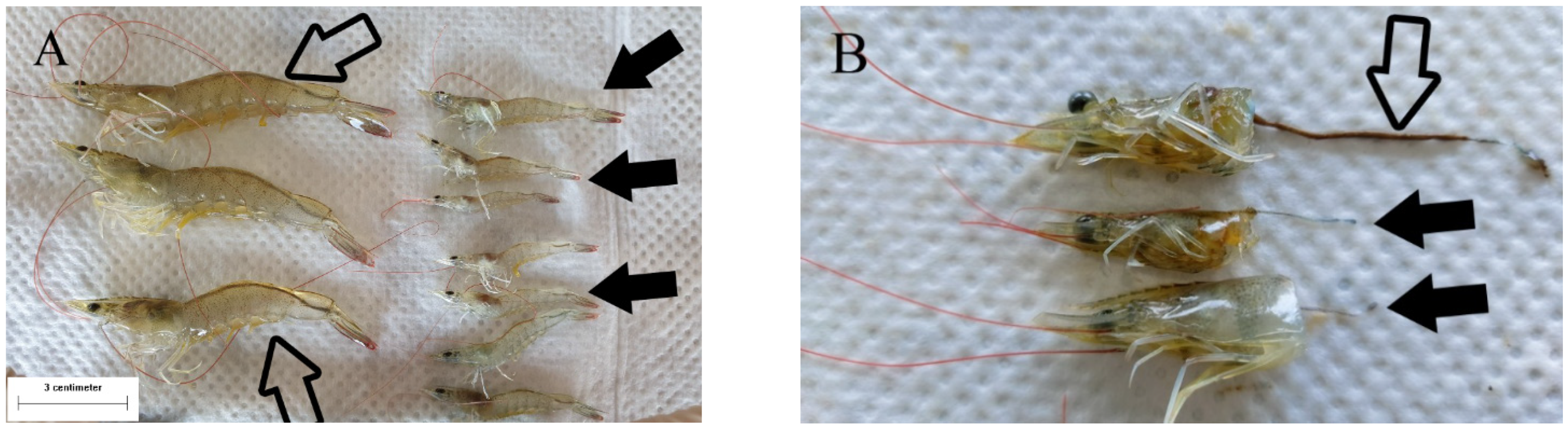
3.1. Macroscopic Observation of Shrimp with Delayed Growth
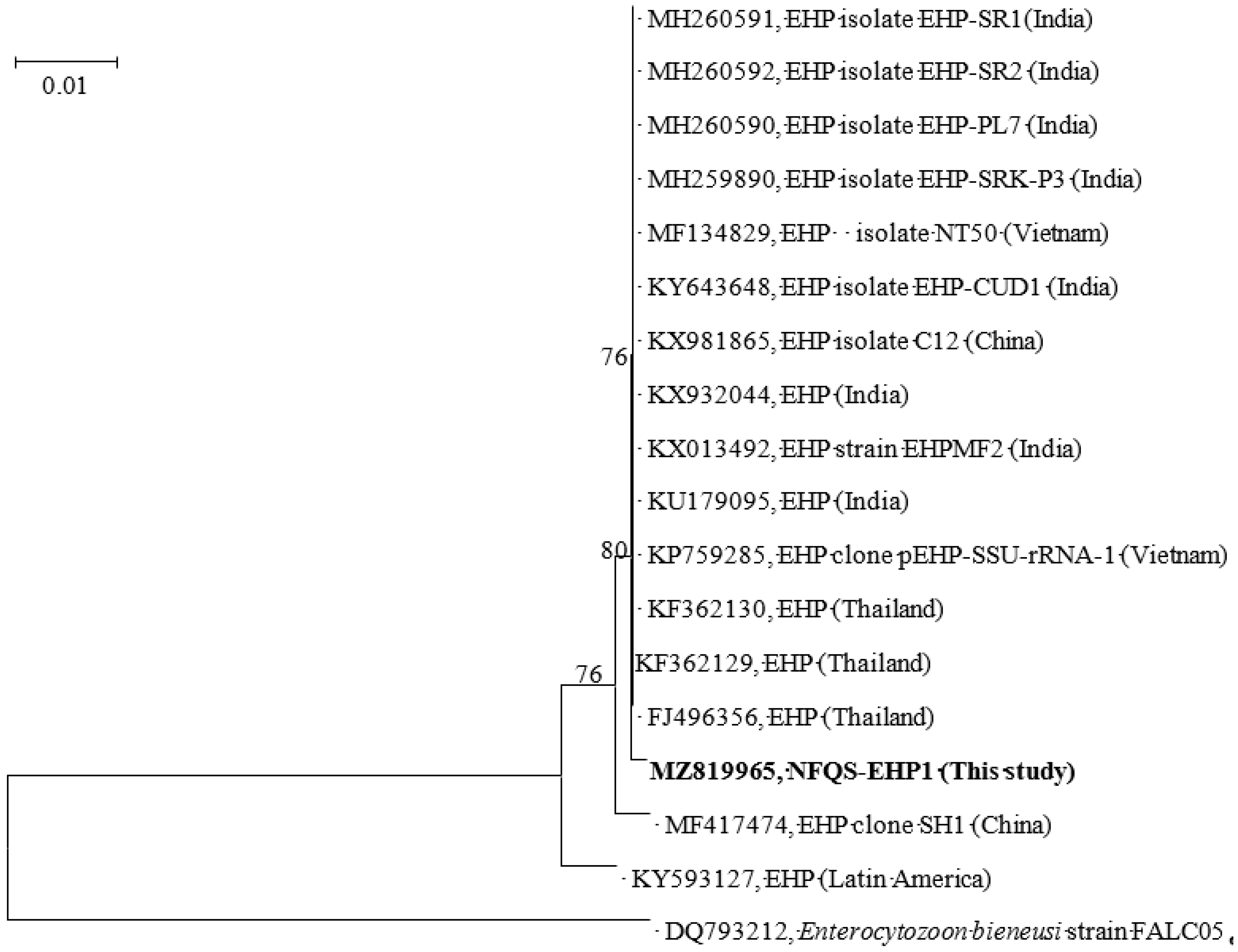
3.2. Nested PCR and Prevalence of EHP Infection
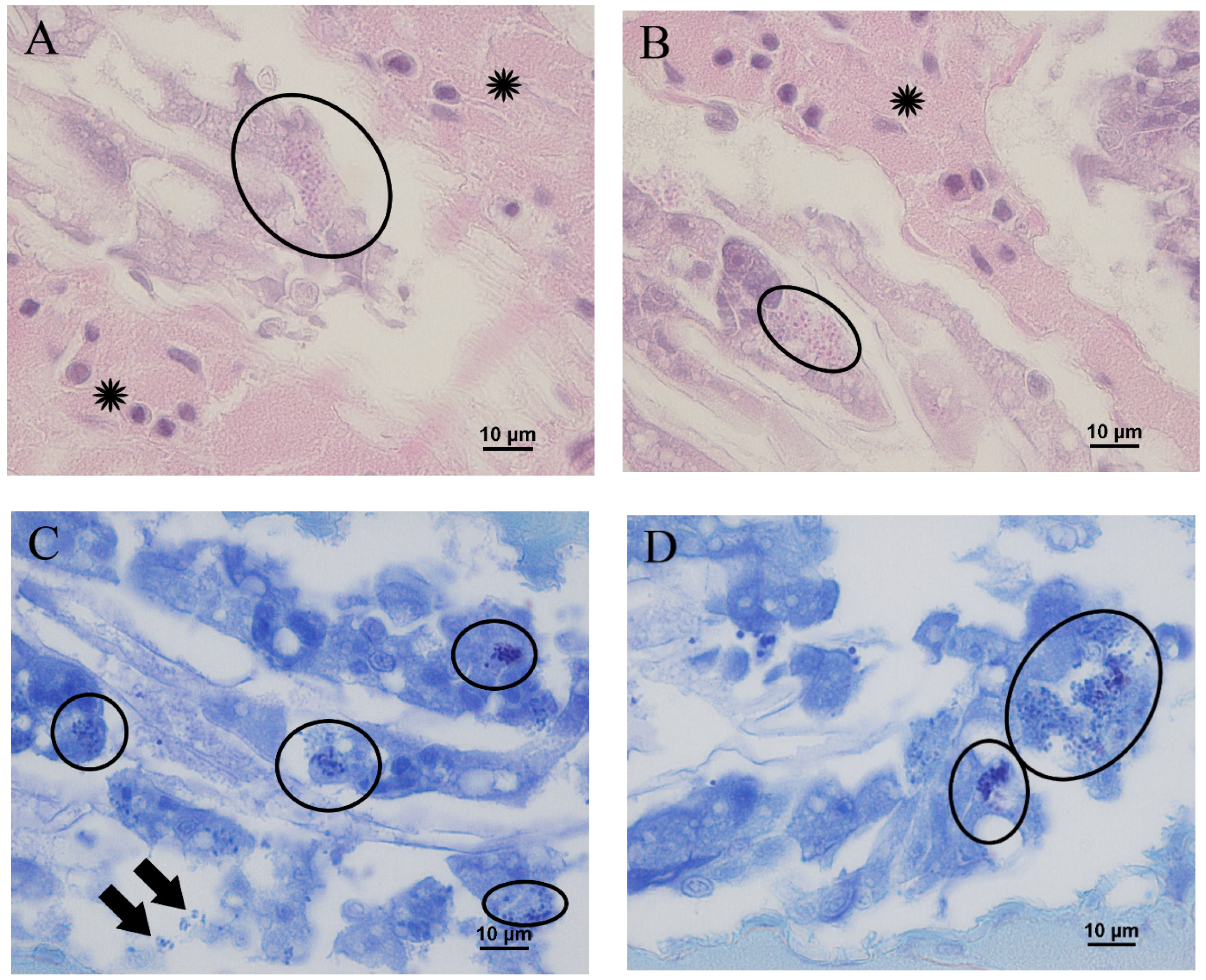
3.3. Microscopic Observation of Shrimp with Delayed Growth
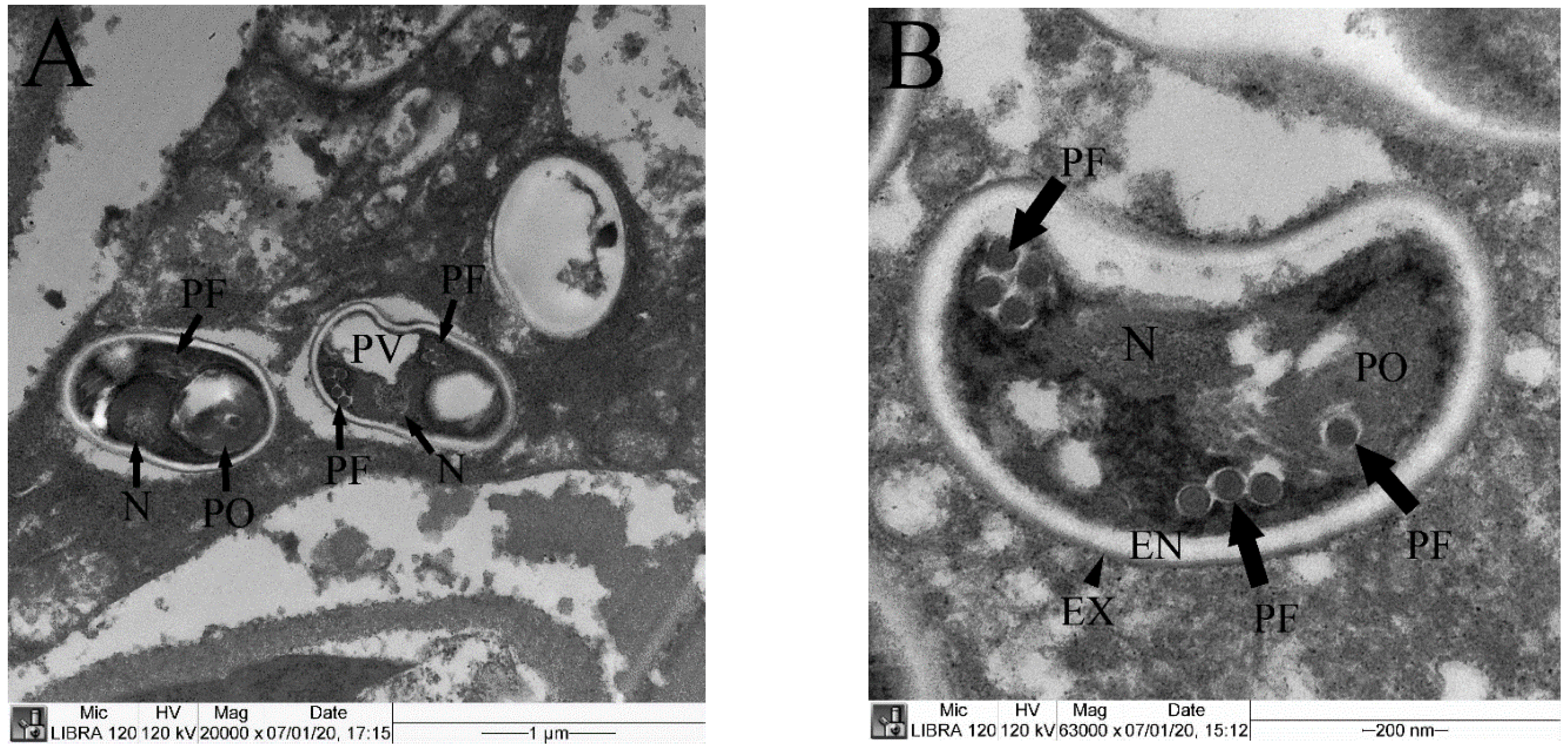
3.4. TEM Observation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaffari, N.; Sanchez-Flores, A.; Doan, R.; Garcia-Orozco, K.D.; Chen, P.L.; Ochoa-Leyva, A.; Lopez-Zavala, A.A.; Carrasco, J.S.; Hong, C.; Brieba, L.G. Novel transcriptome assembly and improved annotation of the whiteleg shrimp (Litopenaeus vannamei), a dominant crustacean in global seafood mariculture. Sci. Rep. 2014, 4, 7081. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agriculture Organization of the United Nations). Fisheries and Aquaculture Information and Statistics Branch. 2021. Available online: http://www.fao.org/figis/servlet/SQServlet?file=/usr/local/tomcat/8.5.16/figis/webapps/figis/temp/hqp_8773744000615448316.xml&outtype=html (accessed on 18 August 2021).
- KOSIS. Fishery Production Survey. 2021. Available online: https://kosis.kr/statisticsList/statisticsListIndex.do?vwcd=MT_ZTITLE&menuId=M_01_01#content-group (accessed on 18 August 2021).
- Kim, S.-R.; CWR, G.; SHMP, W.; Shin, G.-W. Detection and genetic characteristic of Yellow-head virus genotype 8 (YHV-8) Cultured Litopanaeus vanamei, in Korea. J. Fish Pathol. 2020, 33, 77–81. [Google Scholar]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Chayaburakul, K.; Nash, G.; Pratanpipat, P.; Sriurairatana, S.; Withyachumnarnkul, B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis. Aquat. Org. 2004, 60, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourtip, S.; Wongtripop, S.; Stentiford, G.D.; Bateman, K.S.; Sriurairatana, S.; Chavadej, J.; Sritunyalucksana, K.; Withyachumnarnkul, B. Enterocytozoon hepatopenaei sp. nov.(Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships. J. Invertebr. Pathol. 2009, 102, 21–29. [Google Scholar] [CrossRef]
- Tang, K.F.; Pantoja, C.R.; Redman, R.M.; Han, J.E.; Tran, L.H.; Lightner, D.V. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. J. Invertebr. Pathol. 2015, 130, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Shivam, S.; Praveena, P.E.; Rajan, J.J.S.; Kumar, T.S.; Avunje, S.; Jagadeesan, V.; Babu, S.P.; Pande, A.; Krishnan, A.N. Emergence of Enterocytozoon hepatopenaei (EHP) in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture 2016, 454, 272–280. [Google Scholar] [CrossRef]
- Tang, K.F.; Aranguren, L.F.; Piamsomboon, P.; Han, J.E.; Maskaykina, I.Y.; Schmidt, M.M. Detection of the microsporidian Enterocytozoon hepatopenaei (EHP) and Taura syndrome virus in Penaeus vannamei cultured in Venezuela. Aquaculture 2017, 480, 17–21. [Google Scholar] [CrossRef]
- Tang, K.F.; Han, J.E.; Aranguren, L.F.; White-Noble, B.; Schmidt, M.M.; Piamsomboon, P.; Risdiana, E.; Hanggono, B. Dense populations of the microsporidian Enterocytozoon hepatopenaei (EHP) in feces of Penaeus vannamei exhibiting white feces syndrome and pathways of their transmission to healthy shrimp. J. Invertebr. Pathol. 2016, 140, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aldama-Cano, D.J.; Sanguanrut, P.; Munkongwongsiri, N.; Ibarra-Gámez, J.C.; Itsathitphaisarn, O.; Vanichviriyakit, R.; Flegel, T.W.; Sritunyalucksana, K.; Thitamadee, S. Bioassay for spore polar tube extrusion of shrimp Enterocytozoon hepatopenaei (EHP). Aquaculture 2018, 490, 156–161. [Google Scholar] [CrossRef]
- Chaijarasphong, T.; Munkongwongsiri, N.; Stentiford, G.D.; Aldama-Cano, D.J.; Thansa, K.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. The shrimp microsporidian Enterocytozoon hepatopenaei (EHP): Biology, pathology, diagnostics and control. J. Invertebr. Pathol. 2020, 107458. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Lee, S.C.; Park, S.C.; Jeon, H.J.; Kim, K.Y.; Lee, Y.S.; Park, S.; Han, S.-H.; Kim, J.H.; Choi, S.-K. Molecular detection of Enterocytozoon hepatopenaei and Vibrio parahaemolyticus-associated acute hepatopancreatic necrosis disease in Southeast Asian Penaeus vannamei shrimp imported into Korea. Aquaculture 2020, 517, 734812. [Google Scholar] [CrossRef]
- Tangprasittipap, A.; Srisala, J.; Chouwdee, S.; Somboon, M.; Chuchird, N.; Limsuwan, C.; Srisuvan, T.; Flegel, T.W.; Sritunyalucksana, K. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei. BMC Vet. Res. 2013, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. PLoS ONE 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Peeler, E.; Oidtmann, B. Demonstrating freedom from Gyrodactylus salaris (Monogenea: Gyrodactylidae) in farmed rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2008, 79, 47–56. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Frelier, P.; Sis, R.; Bell, T.; Lewis, D. Microscopic and ultrastructural studies of necrotizing hepatopancreatitis in Pacific white shrimp (Penaeus vannamei) cultured in Texas. Vet. Pathol. 1992, 29, 269–277. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano, R. ‘Bright-red’syndrome in Pacific white shrimp Litopenaeus vannamei is caused by Vibrio harveyi. Dis. Aquat. Org. 2010, 92, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, V.; Gopalakrishnan, A. A novel report of phytopathogenic fungi Gilbertella persicaria infection on Penaeus monodon. Aquaculture 2014, 430, 224–229. [Google Scholar] [CrossRef]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Sivakumar, S.; Vimal, S.; Abdul Majeed, S.; Taju, G.; Haribabu, P.; Uma, A.; Sahul Hameed, A. Biochemical changes and tissue distribution of Enterocytozoon hepatopenaei (EHP) in naturally and experimentally EHP-infected whiteleg shrimp, Litopenaeus vannamei (Boone, 1931), in India. J. Fish Dis. 2017, 40, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Wei, P.; Shen, H.; Wan, X.; Jin, M.; Li, X.; Shi, H.; Qiao, Y.; Jiang, G.; Gu, W. Proteomic and metabolomic responses in hepatopancreas of whiteleg shrimp Litopenaeus vannamei infected by microsporidian Enterocytozoon hepatopenaei. Fish Shellfish Immunol. 2019, 87, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Adult growth hormone deficiency. Indian J. Endocrinol. Metab. 2011, 15, S197. [Google Scholar] [CrossRef]
- Saito, H.; Inoue, T.; Fukatsu, K.; Ming-Tsan, L.; Inaba, T.; Fukushima, R.; Muto, T. Growth hormone and the immune response to bacterial infection. Horm. Res. Paediatr. 1996, 45, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Biju, N.; Sathiyaraj, G.; Raj, M.; Shanmugam, V.; Baskaran, B.; Govindan, U.; Kumaresan, G.; Kasthuriraju, K.K.; Chellamma, T.S.R.Y. High prevalence of Enterocytozoon hepatopenaei in shrimps Penaeus monodon and Litopenaeus vannamei sampled from slow growth ponds in India. Dis. Aquat. Org. 2016, 120, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Aranguren, L.F.; Han, J.E.; Tang, K.F. Enterocytozoon hepatopenaei (EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei. Aquaculture 2017, 471, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Gomes, R.S., Jr.; de Lima, J.P.V.; Cavalli, R.O.; Correia, E.d.S. Acute toxicity of ammonia and nitrite to painted river prawn, Macrobrachium carcinus, larvae. J. World Aquac. Soc. 2016, 47, 239–247. [Google Scholar] [CrossRef]
- Nkuba, A.C.; Mahasri, G.; Lastuti, N.D.R.; Mwendolwa, A.A. Correlation of Nitrite and Ammonia with Prevalence of Enterocytozoon hepatopenaei (EHP) in Shrimp (Litopenaeus vannamei) on Several Super-Intensive Ponds in East Java, Indonesia. J. Ilm. Perikan. Dan Kelaut. 2021, 13, 58–67. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Sudhakaran, R. Exploring the potentiality of Artemia salina to act as a reservoir for microsporidian Enterocytozoon hepatopenaei of penaeid shrimp. Biocatal. Agric. Biotechnol. 2020, 25, 101607. [Google Scholar] [CrossRef]
- Desrina, D.; Prayitno, S.B.; Haditomo, A.H.C.; Latritiani, R.; Sarjito, S. Detection of Enterocytozoon hepatopenaei (EHP) DNA in the polychaetes from shrimp ponds suffering white feces syndrome outbreaks. Biodivers. J. Biol. Divers. 2020, 21, 369–374. [Google Scholar] [CrossRef]
- Salachan, P.V.; Jaroenlak, P.; Thitamadee, S.; Itsathitphaisarn, O.; Sritunyalucksana, K. Laboratory cohabitation challenge model for shrimp hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei (EHP). BMC Vet. Res. 2016, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Flores, R.; Mai, H.N.; Noble, B.L.; Schofield, P.J.; Dhar, A.K. Detection of Enterocytozoon hepatopenaei using an invasive but non-lethal sampling method in shrimp (Penaeus vannamei). J. Microbiol. Methods 2019, 162, 38–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-S.; Jang, G.-I.; Kim, S.-M.; Kim, Y.-S.; Jeon, Y.-G.; Oh, Y.-K.; Hwang, J.-Y.; Kwon, M.-G. First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea. Animals 2021, 11, 3150. https://doi.org/10.3390/ani11113150
Kim B-S, Jang G-I, Kim S-M, Kim Y-S, Jeon Y-G, Oh Y-K, Hwang J-Y, Kwon M-G. First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea. Animals. 2021; 11(11):3150. https://doi.org/10.3390/ani11113150
Chicago/Turabian StyleKim, Bo-Seong, Gwang-Il Jang, Su-Mi Kim, Young-Sook Kim, Yu-Gyeong Jeon, Yun-Kyeong Oh, Jee-Youn Hwang, and Mun-Gyeong Kwon. 2021. "First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea" Animals 11, no. 11: 3150. https://doi.org/10.3390/ani11113150
APA StyleKim, B.-S., Jang, G.-I., Kim, S.-M., Kim, Y.-S., Jeon, Y.-G., Oh, Y.-K., Hwang, J.-Y., & Kwon, M.-G. (2021). First Report of Enterocytozoon hepatopenaei Infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) Cultured in Korea. Animals, 11(11), 3150. https://doi.org/10.3390/ani11113150





