Optimal Standardized Ileal Digestible Total Sulfur Amino Acids to Lysine REQUIREMENTS Are Increased in Nursery Pigs Raised under Antibiotic-Free Feeding Regime
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.2. Experiment 2
2.3. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Experiment 2
3.2.1. SID TSAA to Lys Ratios on Growth Performance of Nursery Pigs Raised without Antibiotics
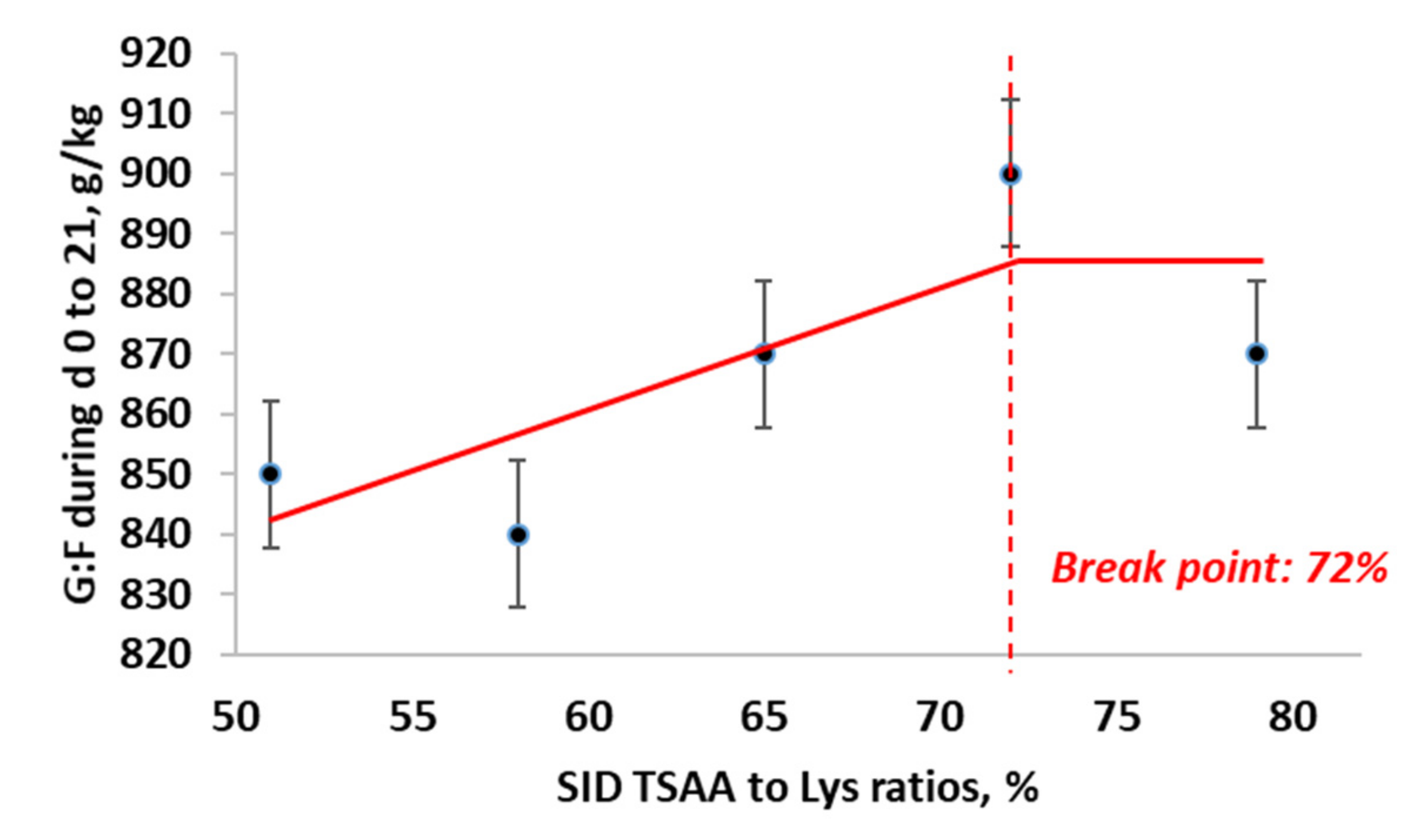
3.2.2. Estimation of Optimal Ratio of SID TSAA to Lys in Nursery Pigs Raised without Antibiotics
4. Discussion
4.1. Antibiotics Growth Promoter on Growth Performance and Amino Acid Requirement
4.2. Function of Methionine and Increased Methionine Requirement without AGP
4.3. Optimal SID TSAA:Lys in Nursery Pigs without AGP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, G.F.; Gaines, A.M.; Ratliff, B.W.; Srichana, P.; Allee, G.L.; Perryman, K.R.; Knight, C.D. Estimation of the true ileal digestible lysine and sulfur amino acid requirement and comparison of the bioefficacy of 2-hydroxy-4-(methylthio) butanoic acid and DL-methionine in eleven- to twenty-six-kilogram nursery pigs. J. Anim. Sci. 2006, 84, 1709–1721. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Swain, B.K.; Johri, T.S. Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2000, 41, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Carew, L.B.; McMurtry, J.P.; Alster, F.A. Effects of methionine deficiencies on plasma levels of thyroid hormones, insulin-like growth factors-I and -II, liver and body weights, and feed intake in growing chickens. Poult. Sci. 2003, 82, 1932–1938. [Google Scholar] [CrossRef]
- Bauchart-Thevret, C.; Stoll, B.; Chacko, S.; Burrin, D.G. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Moretó, M.; Miró, L.; Amat, C.; Polo, J.; Manichanh, C.; Pérez-Bosque, A. Dietary supplementation with spray-dried porcine plasma has prebiotic effects on gut microbiota in mice. Sci. Rep. 2020, 10, 2926. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.E.; Notzold, R.A.; Jensen, A.H. The tryptophan requirement of the young pig. J. Anim. Sci. 1955, 14, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Bikker, P.; Dirkzwager, A. Withdrawal of antimicrobial growth promoters from pig diets increases the amino acid requirements for growth performance. In Progress in Research on Energy and Protein Metabolism; Souffrant, W.B., Metges, C., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2003; pp. 593–596. [Google Scholar]
- Capozzalo, M.M.; Resink, J.W.; Htoo, J.K.; Kim, J.C.; de Lange, F.M.; Mullan, B.P.; Hansen, C.F.; Pluske, J.R. Determination of the optimum standardised ileal digestible sulphur amino acids to lysine ratio in weaned pigs challenged with enterotoxigenic Escherichia coli. Anim. Feed Sci. Technol. 2017, 227, 118–130. [Google Scholar] [CrossRef] [Green Version]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 9780309489034. [Google Scholar]
- PIC. Nutrient Specifications Manual; PIC North America: Hendersonville, TN, USA, 2016. [Google Scholar]
- Gonçalves, M.A.D.; Gourley, K.M.; Dritz, S.S.; Tokach, M.D.; Bello, N.M.; DeRouchey, J.M.; Woodworth, J.C.; Goodband, R.D. Effects of amino acids and energy intake during late gestation of high-performing gilts and sows on litter and reproductive performance under commercial conditions. J. Anim. Sci. 2016, 94, 1993–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milliken, G.A.; Johnson, D.E. Analysis of Messy Data: Designed Experiments, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 1, ISBN 1584883340. [Google Scholar]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Maria Cardinal, K.; Kipper, M.; Andretta, I.; Machado Leal Ribeiro, A. Withdrawal of antibiotic growth promoters from broiler diets: Performance indexes and economic impact. Poult. Sci. 2019, 98, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Smiricky-Tjardes, M.R.; Albin, D.M.; Wubben, J.E.; Gabert, V.M.; Deplancke, B.; Bane, D.; Anderson, D.B.; Gaskins, H.R. Molecular ecological analysis of porcine ileal microbiota responses to antimicrobial growth promoters. J. Anim. Sci. 2003, 81, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.B.; McCracken, V.J.; Aminov, R.I.; Simpson, J.M.; Mackie, R.I.; Verstegen, M.W.A.; Gaskins, H.R. Gut microbiology and growth-promoting antibiotics in swine. Nutr. Abstr. Rev. Ser. B Livest. Feed. Feed. 2000, 70, 101–108. [Google Scholar]
- Mu, C.; Yang, Y.; Yu, K.; Yu, M.; Zhang, C.; Su, Y.; Zhu, W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids 2017, 49, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Mu, C.; Yang, Y.; Zhang, C.; Su, Y.; Huang, Z.; Yu, K.; Zhu, W. Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets. Amino Acids 2017, 49, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.T.; Curry, S.; Trachsel, J.M.; Schroyen, M.; Gabler, N.K. Evaluating nursery pig responses to in-feed sub-therapeutic antibiotics. PLoS ONE 2019, 14, e0216070. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zhang, C.; Yang, Y.; Mu, C.; Su, Y.; Yu, K.; Zhu, W. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J. Anim. Sci. Biotechnol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Redox control of the transsulfuration and glutathione biosynthesis pathways. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 85–92. [Google Scholar] [CrossRef]
- Jones, D.P. Extracellular redox state: Refining the definition of oxidative stress in aging. Rejuvenation Res. 2006, 9, 169–181. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [Green Version]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aw, T.Y. Cellular redox: A modulator of intestinal epithelial cell proliferation. News Physiol. Sci. 2003, 18, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, T.; Iwakiri, R.; Fujimoto, K.; Aw, T.Y. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001, 15, 2131–2139. [Google Scholar] [CrossRef] [Green Version]
- Pias, E.K.; Yee Aw, T. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. FASEB J. 2002, 16, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedijk, M.A.; Stoll, B.; Chacko, S.; Schierbeek, H.; Sunehag, A.L.; Van Goudoever, J.B.; Burrin, D.G. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2007, 104, 3408–3413. [Google Scholar] [CrossRef] [Green Version]
- Shoveller, A.K.; Brunton, J.A.; Pencharz, P.B.; Ball, R.O. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J. Nutr. 2003, 133, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Liu, L.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed Sci. Technol. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Shen, Y.B.; Weaver, A.C.; Kim, S.W. Effect of feed grade L-methionine on growth performance and gut health in nursery pigs compared with conventional DL-methionine. J. Anim. Sci. 2014, 92, 5530–5539. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.D.; Dibner, J.J. Comparative absorption of 2-hydroxy-4-(methylthio)-butanoic acid and L-methionine in the broiler chick. J. Nutr. 1984, 114, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.D.; Atwell, C.A.; Vázquez-Añón, M.; Dibner, J.J. Comparative in vitro and in vivo absorption of 2-hydroxy-4(methylthio) butanoic acid and methionine in the broiler chicken. Poult. Sci. 2005, 84, 1397–1405. [Google Scholar] [CrossRef]
- Knight, C.D.; Atwell, C.A.; Wuelling, C.W.; Ivey, F.J.; Dibner, J.J. The Relative Effectiveness of 2-Hydroxy-4-(Methylthio) Butanoic Acid and DL-Methionine in Young Swine. J. Anim. Sci. 1998, 76, 781–787. [Google Scholar] [CrossRef]
- Zimmermann, B.; Mosenthin, R.; Rademacher, M.; Lynch, P.B.; Esteve-Garcia, E. Comparative studies on the relative efficacy of DL-methionine and liquid methionine hydroxy analogue in growing pigs. Asian-Australas. J. Anim. Sci. 2005, 18, 1003–1010. [Google Scholar] [CrossRef]
- Kim, B.G.; Lindemann, M.D.; Rademacher, M.; Brennan, J.J.; Cromwell, G.L. Efficacy of DL-methionine hydroxy analog free acid and DL-methionine as methionine sources for pigs. J. Anim. Sci. 2006, 84, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Moeser, A.J.; Vander Klok, C.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A.T. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning Is Associated with an Upregulation of Expression of Inflamatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, E.A.L.; Grimble, R.F. Cysteine and methionine supplementation modulate the effect of tumor necrosis factor α on protein synthesis, glutathione and zinc concentration of liver and lung in rats fed a low protein diet. J. Nutr. 1994, 124, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. The effects of sulfur amino acid intake on immune function in humans. J. Nutr. 2006, 136, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Kahindi, R.; Regassa, A.; Htoo, J.; Nyachoti, M. Optimal sulfur amino acid to lysine ratio for post weaning piglets reared under clean or unclean sanitary conditions. Anim. Nutr. 2017, 3, 380–385. [Google Scholar] [CrossRef] [PubMed]




| P1 | P2 | P3 | ||
|---|---|---|---|---|
| Ingredients | no AGP-51% 1 | AGP-51% 1 | ||
| Corn, yellow dent | 38.71 | 56.70 | 64.48 | 62.77 |
| Soybean meal, 47.5% CP | 15.00 | 25.00 | 31.00 | 31.00 |
| Whey, dried | 30.00 | 8.20 | 0.00 | 0.00 |
| Plasma spray-dried | 7.00 | 0.00 | 0.00 | 0.00 |
| Fish meal, menhaden | 5.15 | 7.00 | 0.00 | 0.00 |
| L-lysine HCl | 0.19 | 0.26 | 0.21 | 0.21 |
| L-threonine | 0.04 | 0.08 | 0.11 | 0.12 |
| MHA 2 | 0.19 | 0.16 | 0.00 | 0.00 |
| L-tryptophan | 0.02 | 0.04 | 0.00 | 0.03 |
| Choice white grease | 1.83 | 0.32 | 1.00 | 1.70 |
| Monocalcium phosphate 21% | 0.17 | 0.25 | 0.98 | 0.97 |
| Limestone | 0.55 | 0.72 | 1.11 | 1.10 |
| Salt | 0.25 | 0.49 | 0.61 | 0.60 |
| Mecadox 2.5 (0.55%) | 0.00 | 0.00 | 0.00 | 1.00 |
| Vitamin and mineral premix 3 | 0.50 | 0.50 | 0.50 | 0.50 |
| Zinc oxide, 72% Zn | 0.40 | 0.28 | 0.00 | 0.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient composition | ||||
| ME 4, kcal/kg | 3395 | 3307 | 3402 | 3402 |
| CP 4, % | 23.90 | 21.80 | 20.30 | 20.30 |
| SID 5, % | ||||
| Lys | 1.47 | 1.31 | 1.10 | 1.10 |
| Thr | 0.88 | 0.79 | 0.74 | 0.74 |
| Met | 0.47 | 0.48 | 0.28 | 0.28 |
| Met+Cys | 0.85 | 0.76 | 0.56 | 0.56 |
| Trp | 0.29 | 0.26 | 0.22 | 0.22 |
| Ca, % | 0.85 | 0.85 | 0.75 | 0.75 |
| STTD 6 P, % | 0.53 | 0.40 | 0.33 | 0.33 |
| Ingredients | P1-51% 1 | P2-51% 2 | P3-51% 3 |
|---|---|---|---|
| Corn, yellow dent | 20.74 | 36.39 | 63.15 |
| Oat groats | 20.00 | 15.00 | 0.00 |
| Soybean meal, 47.5% CP | 15.00 | 20.00 | 31.00 |
| HP300 4 | 2.50 | 5.00 | 0.00 |
| Whey powder | 30.00 | 15.00 | 0.00 |
| Plasma spray-dried | 4.00 | 2.00 | 0.00 |
| Fish meal | 3.00 | 1.50 | 0.00 |
| Soy oil | 2.00 | 2.00 | 0.00 |
| Choice white grease | 0.00 | 0.00 | 2.50 |
| Dicalcium phosphate 18.5% | 1.05 | 1.05 | 1.65 |
| Limestone | 0.54 | 0.76 | 0.50 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin premix 5 | 0.11 | 0.11 | 0.11 |
| Mineral premix 5 | 0.29 | 0.29 | 0.29 |
| ZnO, 72% Zn | 0.28 | 0.28 | 0.00 |
| L-lysine HCl | 0.15 | 0.23 | 0.34 |
| MHA 6 | 0.04 | 0.05 | 0.06 |
| L-threonine | 0.00 | 0.04 | 0.09 |
| L-tryptophan | 0.00 | 0.00 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient composition | |||
| ME 7, kcal/kg | 3478 | 3454 | 3404 |
| CP 7, % | 21.7 | 21.91 | 20.46 |
| Fermentable fiber, % | 8.51 | 10.49 | 12.13 |
| SID 8, % | |||
| Lys | 1.31 | 1.28 | 1.20 |
| Thr | 0.79 | 0.77 | 0.72 |
| Met | 0.32 | 0.33 | 0.33 |
| Met+Cys | 0.67 | 0.65 | 0.61 |
| Trp | 0.27 | 0.25 | 0.23 |
| Ca, % | 0.85 | 0.79 | 0.70 |
| Total P, % | 0.81 | 0.71 | 0.70 |
| STTD 9 P, % | 0.57 | 0.44 | 0.39 |
| AGP | SID TSAA:Lys, % | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | No | Yes | 51 | 58.5 | 66 | SEM | AGP | TSAA | AGP × TSAA |
| Initial BW, kg | 7.9 | 7.9 | 7.9 | 8.0 | 7.9 | 0.1 | 0.85 | 0.91 | 0.50 |
| Final BW, kg | 16.3 | 16.7 | 16.1 | 16.5 | 16.8 | 0.2 | 0.03 | 0.03 | 0.39 |
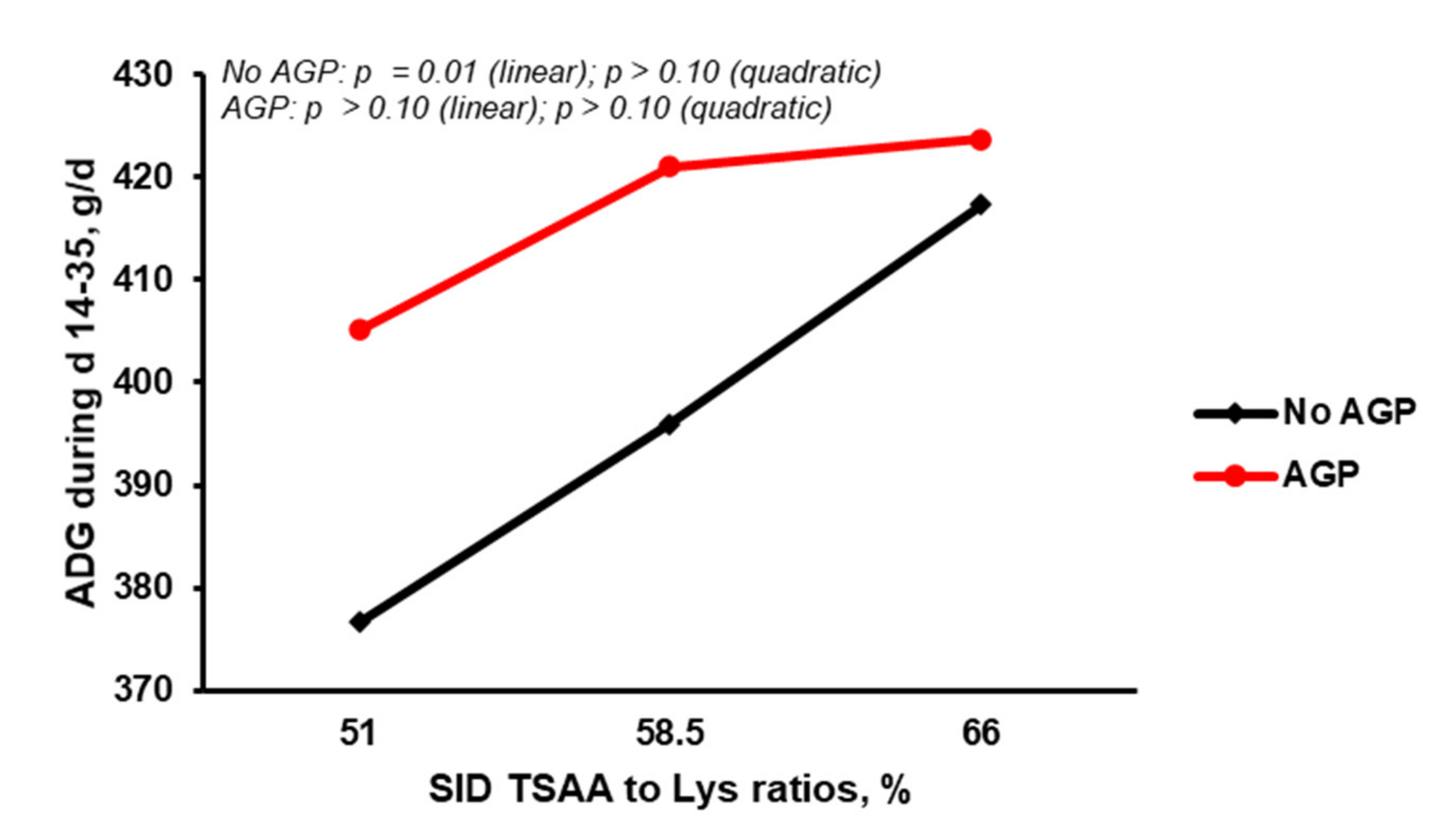
| ADG, g/d | 397 | 417 | 391 | 409 | 421 | 9 | 0.01 | 0.01 | 0.39 |
| ADFI, g/d | 616 | 647 | 616 | 633 | 645 | 12 | <0.01 | 0.06 | 0.96 |
| G:F, g/g | 0.643 | 0.645 | 0.634 | 0.645 | 0.653 | 0.009 | 0.83 | 0.06 | 0.08 |
| SID TSAA:Lys, % | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | 51 | 58 | 65 | 72 | 79 | SEM | Diet | Linear | Quadratic | Cubic |
| BW, kg | ||||||||||
| d 0 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | <.01 | 0.91 | 0.85 | 0.43 | 0.71 |
| d 7 | 6.6 | 6.6 | 6.6 | 6.5 | 6.6 | 0.1 | 0.94 | 0.77 | 0.60 | 0.53 |
| d 21 | 10.5 | 10.6 | 10.8 | 10.8 | 10.6 | 0.1 | 0.30 | 0.19 | 0.09 | 0.76 |
| d 42 | 21.2 | 21.7 | 21.9 | 21.6 | 21.9 | 0.2 | 0.02 | 0.09 | 0.19 | 0.30 |
| ADG, g/d | ||||||||||
| d 0 to 7 | 211 | 214 | 209 | 208 | 218 | 8 | 0.93 | 0.77 | 0.58 | 0.50 |
| d 7 to 21 | 278 | 289 | 302 | 302 | 287 | 7 | 0.07 | 0.15 | 0.01 | 0.42 |
| d 21 to 42 | 511 | 526 | 532 | 516 | 535 | 8 | 0.23 | 0.33 | 0.71 | 0.26 |
| d 0 to 21 | 256 | 264 | 271 | 271 | 264 | 6 | 0.31 | 0.19 | 0.09 | 0.77 |
| d 0 to 42 | 384 | 395 | 401 | 394 | 401 | 4 | 0.02 | 0.01 | 0.15 | 0.10 |
| ADFI, g/d | ||||||||||
| d 0 to 7 | 237 | 240 | 232 | 236 | 235 | 6 | 0.94 | 0.68 | 0.87 | 0.80 |
| d 7 to 21 | 325 | 351 | 351 | 340 | 335 | 11 | 0.45 | 0.84 | 0.09 | 0.39 |
| d 21 to 42 | 728 | 718 | 748 | 737 | 721 | 9 | 0.14 | 0.86 | 0.37 | 0.15 |
| d 0 to 21 | 296 | 314 | 311 | 305 | 301 | 8 | 0.54 | 0.95 | 0.14 | 0.39 |
| d 0 to 42 | 511 | 514 | 524 | 520 | 511 | 6 | 0.40 | 0.71 | 0.09 | 0.50 |
| G:F, g/g | ||||||||||
| d 0 to 7 | 0.889 | 0.894 | 0.900 | 0.880 | 0.924 | 0.023 | 0.73 | 0.45 | 0.56 | 0.39 |
| d 7 to 21 | 0.837 | 0.830 | 0.863 | 0.900 | 0.868 | 0.021 | 0.16 | 0.29 | 0.95 | 0.08 |
| d 21 to 42 | 0.707 | 0.736 | 0.721 | 0.702 | 0.746 | 0.014 | 0.18 | 0.46 | 0.73 | 0.08 |
| d 0 to 21 | 0.846 | 0.843 | 0.873 | 0.896 | 0.865 | 0.012 | 0.03 | 0.12 | 0.90 | 0.09 |
| d 0 to 42 | 0.754 | 0.770 | 0.766 | 0.758 | 0.767 | 0.011 | 0.85 | 0.30 | 0.77 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, P.; Almeida, F.; Orlando, U.; Gonçalves, M.; Hancock, D.; Vazquez-Añón, M. Optimal Standardized Ileal Digestible Total Sulfur Amino Acids to Lysine REQUIREMENTS Are Increased in Nursery Pigs Raised under Antibiotic-Free Feeding Regime. Animals 2021, 11, 3143. https://doi.org/10.3390/ani11113143
Ren P, Almeida F, Orlando U, Gonçalves M, Hancock D, Vazquez-Añón M. Optimal Standardized Ileal Digestible Total Sulfur Amino Acids to Lysine REQUIREMENTS Are Increased in Nursery Pigs Raised under Antibiotic-Free Feeding Regime. Animals. 2021; 11(11):3143. https://doi.org/10.3390/ani11113143
Chicago/Turabian StyleRen, Ping, Ferdinando Almeida, Uislei Orlando, Marcio Gonçalves, Deana Hancock, and Mercedes Vazquez-Añón. 2021. "Optimal Standardized Ileal Digestible Total Sulfur Amino Acids to Lysine REQUIREMENTS Are Increased in Nursery Pigs Raised under Antibiotic-Free Feeding Regime" Animals 11, no. 11: 3143. https://doi.org/10.3390/ani11113143
APA StyleRen, P., Almeida, F., Orlando, U., Gonçalves, M., Hancock, D., & Vazquez-Añón, M. (2021). Optimal Standardized Ileal Digestible Total Sulfur Amino Acids to Lysine REQUIREMENTS Are Increased in Nursery Pigs Raised under Antibiotic-Free Feeding Regime. Animals, 11(11), 3143. https://doi.org/10.3390/ani11113143





