Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting
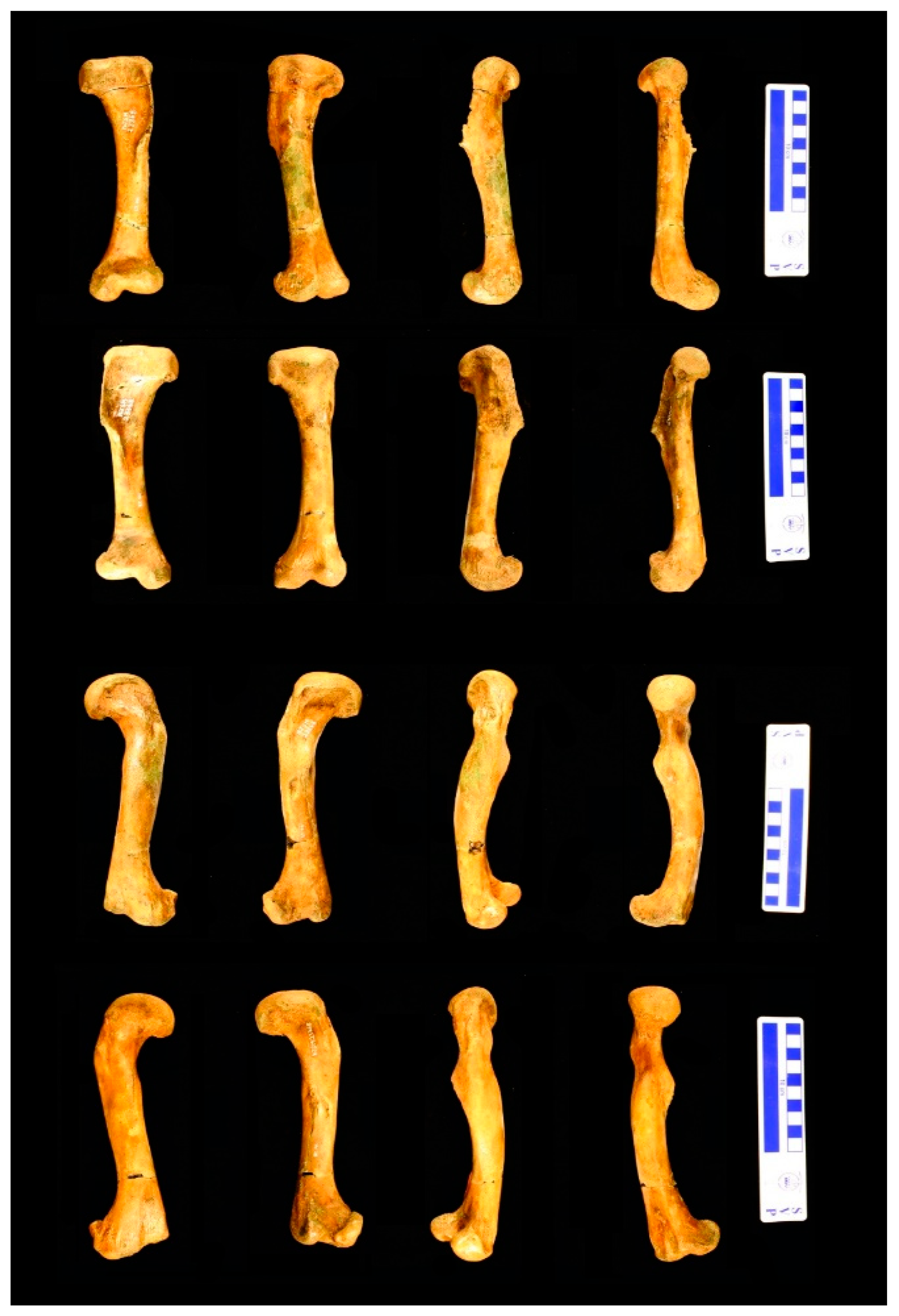
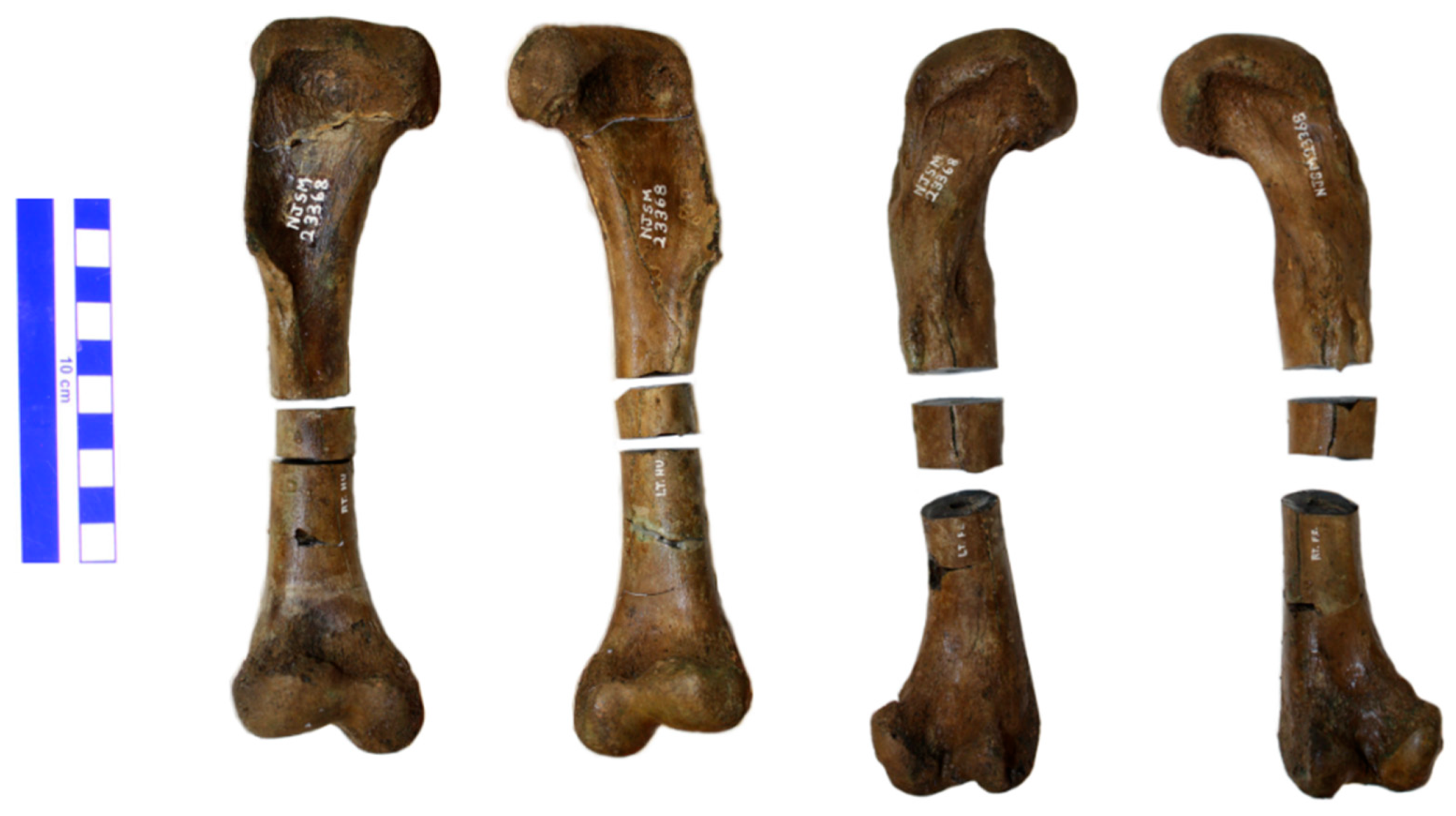
2.2. Specimen
2.3. Preparation
2.4. Imaging and Analysis
3. Results
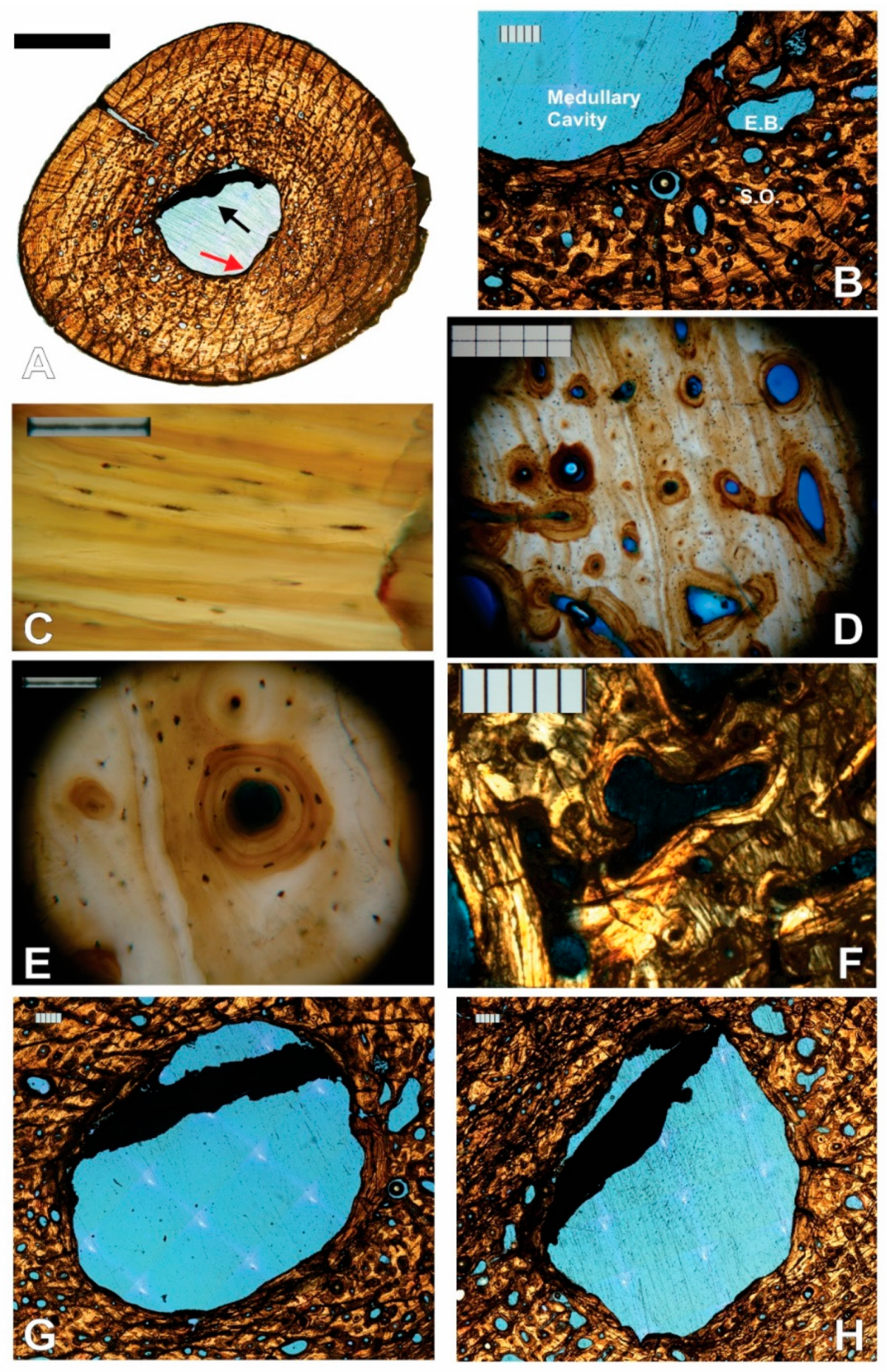
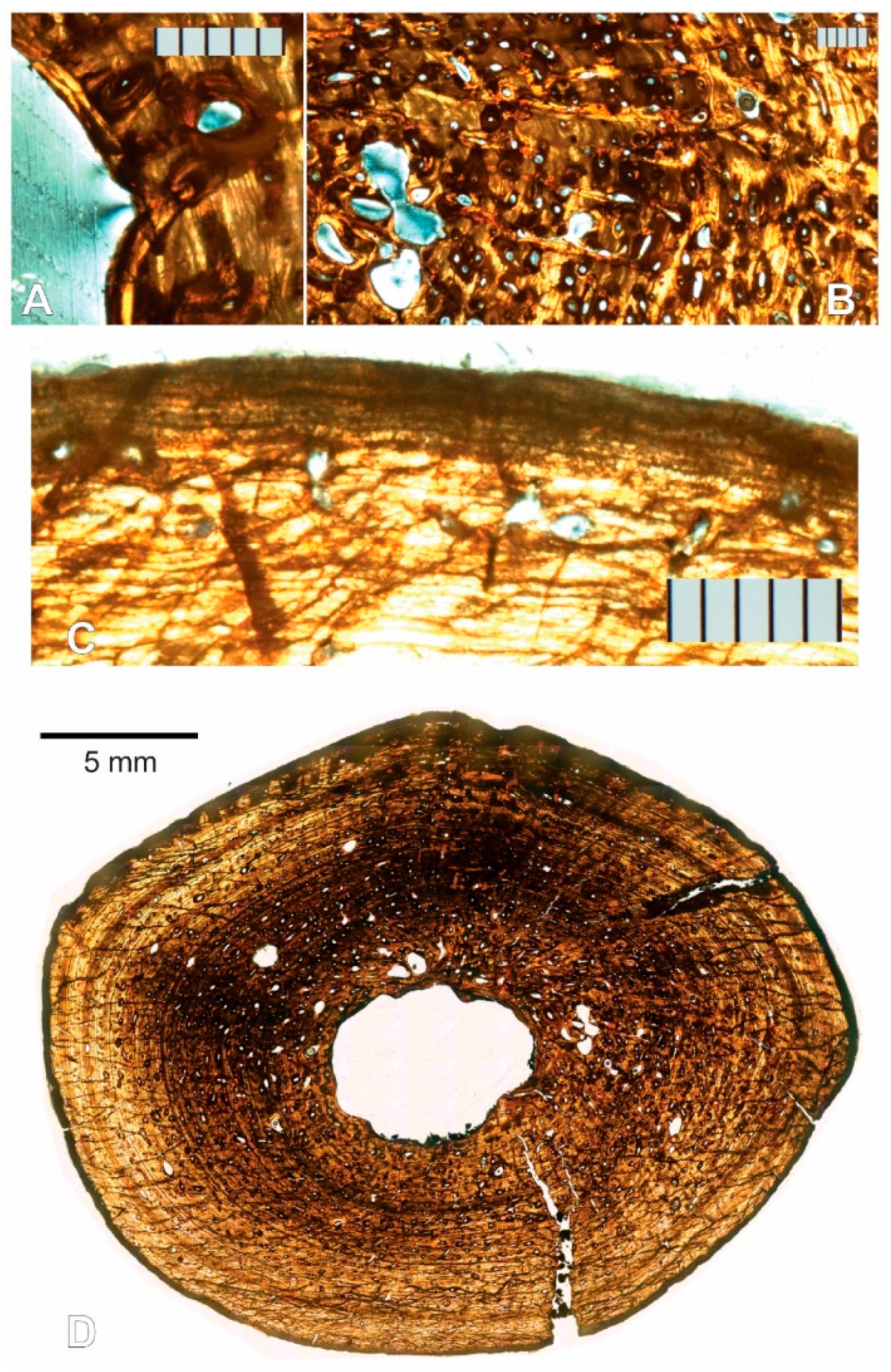
3.1. Paleohistological Description
3.1.1. Left Humerus
3.1.2. Right Humerus
3.1.3. Left Femur
3.1.4. Right Femur
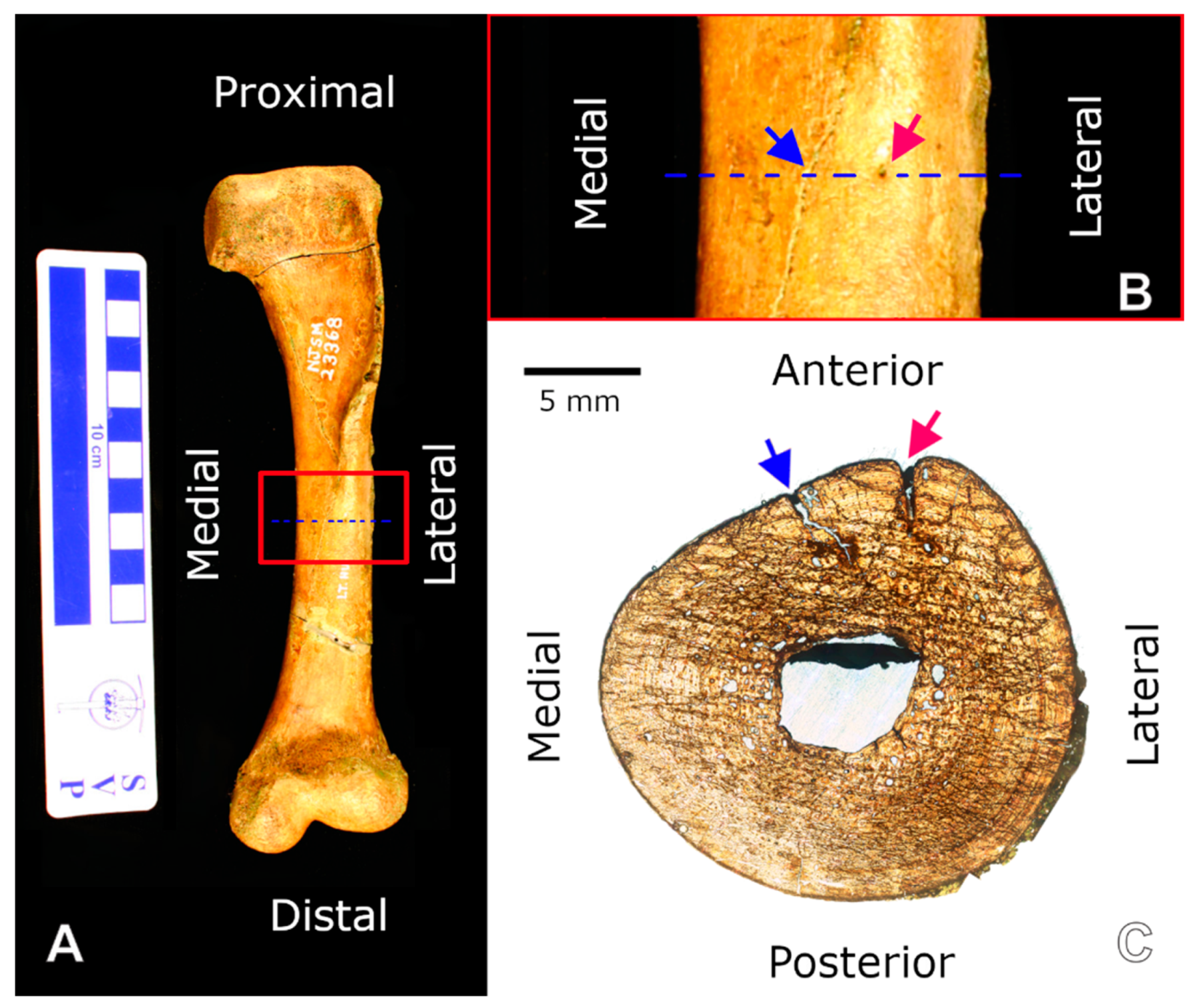
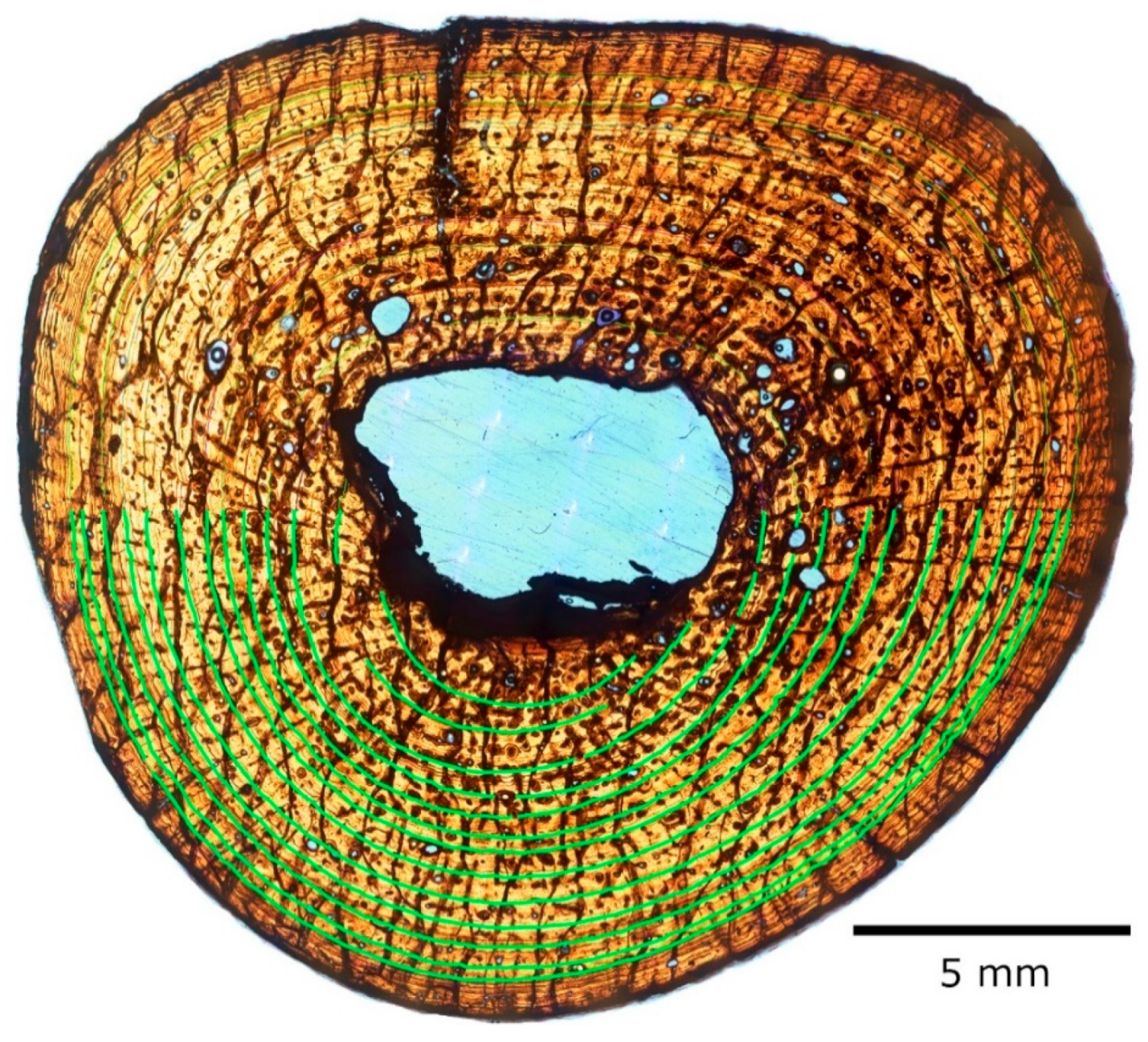
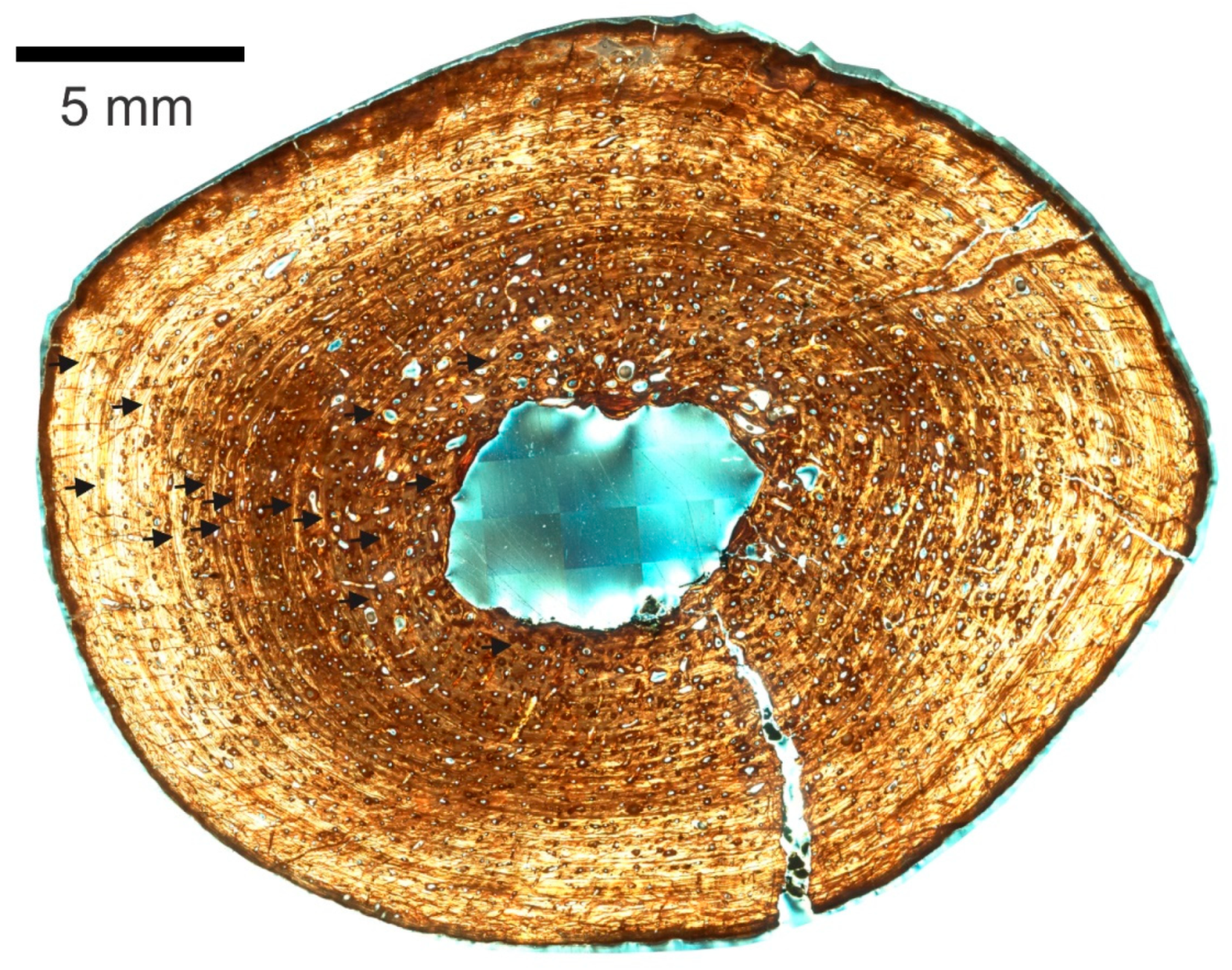
3.2. Skeletochronology
3.2.1. Right Humerus
3.2.2. Right Femur
4. Discussion
- (1)
- If using the thickest band for retrocalculation, the humerus indicates the specimen would have been 15 to 16 years old (and growing, as in each model) at the time of death. The femur results are congruent.
- (2)
- If using the penultimate band in the humerus, it would have been 18 to 22. The femur, in contrast, estimates 26.
- (3)
- If using a mean band thickness, the humerus suggests it would have been 17 to 18. The femur data suggest 19.
- (4)
- If using a mean percentage increase, the humerus results in an estimate of 14 to 15, while the femur estimates 18.
- (5)
- If using a parabolic model, the humerus indicates the specimen would have been 17 to 18, and the femur 18.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiller, T.A. A dyrosaurid crocodilian from the Cretaceous (Maastrichtian) Escondido Formation of Coahuila, Mexico. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2012. [Google Scholar]
- Ehret, D.; Hastings, A. An Eocene occurrence of a dyrosaurid (Crocodylomorpha, Mesoeucrocodylia) from Alabama, USA. In Proceedings of the Program and Abstracts of the 73rd Annual Meeting of the Society of Vertebrate Paleontology, Los Angeles, CA, USA, 30 October –2 November 2013; p. 121. [Google Scholar]
- Denton, R., Jr.; Dobie, J.; Parris, D. The marine crocodilian Hyposaurus in North America. In Ancient Marine Reptiles; Callaway, J., Nicholls, E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 375–397. [Google Scholar]
- Buffetaut, E. Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens. Mémoires Société Géologique Fr. 1982, 142, 1–88. [Google Scholar]
- Andrade, R.C.L.P.; Sayão, J.M. Paleohistology and lifestyle inferences of a dyrosaurid (Archosauria: Crocodylomorpha) from Paraíba Basin (Northeastern Brazil). PLoS ONE 2014, 9, e102189. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, W.B.; Parris, D.C.; Spamer, E.E. Paleontology, biostratigraphy, and depositional environments of the Cretaceous-Tertiary transition in the New Jersey Coastal Plain. Mosasaur 1986, 3, 1–35. [Google Scholar]
- Landman, N.H.; Johnson, R.O.; Edwards, L.E. Cephalopods from the Cretaceous/Tertiary boundary interval on the Atlantic Coastal Plain, with a description of the highest ammonite zones in North America. Part 2. Northeastern Monmouth County, New Jersey. Bull. Am. Mus. Nat. Hist. 2004, 287, 1–107. [Google Scholar] [CrossRef]
- Landman, N.H.; Johnson, R.O.; Garb, M.P.; Edwards, L.E.; Kyte, F.T. Cephalopods from the Cretaceous/Tertiary boundary interval on the Atlantic Coastal Plain, with a description of the highest ammonite zones in North America. Part 3. Manasquan River Basin, Monmouth County, New Jersey. Bull. Am. Mus. Nat. Hist. 2007, 303, 1–122. [Google Scholar] [CrossRef]
- Davidson, A.; Alderson, S.; Fox, M. Assembling an archival marking kit for paleontological specimens. In Proceedings of the 66th Annual Society of Vertebrate Paleontology Meeting, Ottawa, ON, Canada, 18–21 October 2006; Volume 54A. Available online: https://www.academia.edu/1385048/Assembling_an_Archival_Marking_Kit_for_Paleontological_Specimens (accessed on 31 August 2021).
- Ramanujan, S. Modular equations and approximations to Pi. Q. J. Pure Appl. Math. 1914, 45, 350–372. [Google Scholar]
- Francillon-Vieillot, H.; de Buffrénil, V.; Castanet, J.; Geraudie, J.; Meunier, F.; Sire, J.; Zylberberg, L.; de Ricqles, A. Microstructure and mineralization of vertebrate skeletal tissues. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 471–530. [Google Scholar]
- Ricqles, A.; Padian, K.; Horner, J.R. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Ann. Paléontologie 2003, 89, 67–101. [Google Scholar] [CrossRef]
- Klein, N.; Scheyer, T.; Tütken, T. Skeletochronology and isotopic analysis of a captive individual of Alligator mississippiensis Daudin, 1802. Foss. Rec. 2009, 12, 121–131. [Google Scholar] [CrossRef]
- Woodward, H.N.; Horner, J.R.; Farlow, J.O. Osteohistological evidence for determinate growth in the American Alligator. J. Herpetol. 2011, 45, 339–342. [Google Scholar] [CrossRef]
- Boles, Z. Vertebrate Taphonomy and Paleoecology of a Cretaceous-Paleogene Marine Bonebed. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2016. [Google Scholar]
- Horner, J.R.; Ricqles, A.; Padian, K. Variation in dinosaur skeletochronology indicators: Implications for age assessment and physiology. Paleobiology 1999, 25, 295–304. [Google Scholar] [CrossRef]
- Horner, J.R.; Padian, K. Age and growth dynamics of Tyrannosaurus rex. R. Soc. Lond. Proc. B 2004, 271, 1875–1880. [Google Scholar] [CrossRef] [Green Version]
- Woodward, H.; Padian, K.; Lee, A. Skeletochronology. In Bone Histology of Fossil Tetrapods; Padian, K., Lamm, E.-T., Eds.; University of California Press: Berkley, CA, USA; Los Angeles, CA, USA, 2013; pp. 195–215. [Google Scholar]
- Woodward, H.N.; Horner, J.R.; Farlow, J.O. Quantification of intraskeletal histovariablility in Alligator mississippiensis and implications for vertebrate osteohistology. PeerJ 2014, 2, e422. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, R.A. Skeletochronology of the limb elements of mosasaurs (Squamata; Mosasauridae). Trans. Kans. Acad. Sci. 2007, 110, 83–99. [Google Scholar] [CrossRef]
- Thorbjarnarson, J.B. Reproductive characteristics of the Order Crocodylia. Herpetologica 1996, 52, 8–24. [Google Scholar]
- Chinsamy, A. Bone histology and growth trajectory of the prosauropod dinosaur Massospondylus carinatus Owen. Mod. Geol. 1993, 18, 319–329. [Google Scholar]
- Peabody, F.E. Annual growth zones in living and fossil vertebrates. J. Morphol. 1961, 108, 11–62. [Google Scholar] [CrossRef]
- Castanet, J.; Smirina, E. Introduction to the skeletochronological method in amphibians and reptiles. Ann. Sci. Nat. Zool. Biol. Anim. 1990, 11, 191–196. [Google Scholar]
- Castanet, J.; Francillon-Viellot, H.; Meunier, F.J.; de Ricqles, A. Bone and individual aging. In Bone; Hall, B., Ed.; CRC Press: Boca Raton, FL, USA, 1993; Volume VII, pp. 245–283. [Google Scholar]
- Buffrénil, V. Mise en evidence de l’incidence des conditions de milieu sur la croissance de Crocodylus siamensis (Schneider, 1801) et valeur des marques de croissance squelettiques pour l’èvaluation de l’âge individuel. Arch. Zool. Exp. Gen. 1980, 121, 63–76. [Google Scholar]
- Hutton, J.M. Age determination of Nile crocodiles from the cortical stratification of bone. Copeia 1986, 2, 332–341. [Google Scholar] [CrossRef]
- Tucker, A.D. Validation of skeletochronology to determine age of freshwater crocodiles (Crocodylus johnstoni). Mar. Freshw. Res. 1997, 48, 343–351. [Google Scholar] [CrossRef]
- Hastings, A.K.; Bloch, J.I.; Jaramillo, C.A. A new blunt-snouted dyrosaurid, Anthracosuchus balrogus gen. et sp. nov. (Crocodylomorpha, Mesoeucrocodylia), from the Palaeocene of Colombia. Hist. Biol. 2015, 27, 998–1020. [Google Scholar] [CrossRef]
- Jouve, S.; Bouya, B.; Amaghzaz, M. A short-snouted dyrosaurid (Crocodyliformes, Mesoeucrocodylia) from the Paleocene of Morocco. Palaeontology 2005, 48, 359–369. [Google Scholar] [CrossRef]
- Callahan, W.R.; Pellegrini, R.A.; Schein, J.P.; McCauley, J.D.; Parris, D.C. A nearly complete specimen of Hyposaurus rogersii (Crocodylomorpha, Dyrosauridae) from the Late Cretaceous-Early Paleogene of New Jersey. In Proceedings of the Society of Vertebrate Paleontology 75th Annual Meeting, Dallas, TX, USA, 14–17 October 2015. [Google Scholar]
- Somma, L.A.; Fuller, P.; Foster, A. Crocodylus acutus Cuvier, 1807. U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL. Available online: https://nas.er.usgs.gov/queries/FactSheet.aspx?SpeciesID=223 (accessed on 28 July 2020).
- Richards, P.M. Evaluating the relative effects of life history stages in the conservation of the American crocodile (Crocodylus acutus) in Florida. Fla. Sci. 2003, 66, 273–286. [Google Scholar]
- Briggs-Gonzalez, V.; Bonenfant, C.; Basille, M.; Cherkiss, M.; Beauchamp, J.; Mazzotti, F. Life histories and conservation of long-lived reptiles, an illustration with the American crocodile (Crocodylus acutus). J. Anim. Ecol. 2017, 86, 1102–1113. [Google Scholar] [CrossRef] [Green Version]
- Mazzotti, F.J. Factors affecting the nesting success of the American crocodile, Crocodylus acutus, in Florida bay. Bull. Mar. Sci. 1989, 44, 220–228. [Google Scholar]
- Moler, P.E. American Crocodile Population Dynamics. Final Report to Study No. 7532, Florida Game and Fresh Water Fish Commission; Bureau of Wildlife Research: Tallahassee, FL, USA, 1991.
- Thorbjarnarson, J.B. American crocodile, Crocodylus acutus. In Crocodiles: Status, Survey and Conservation Action Plan; Manolis, S.C., Stevenson, C., Eds.; Crocodile Specialist Group: Darwin, Australia, 2010; pp. 46–53. [Google Scholar]
- Ferguson, M.W.J. Reproductive biology and embryology of the crocodilians. In Biology of the Reptilia; Gans, C., Billett, F., Maderson, P.F.A., Eds.; John Wiley and Sons: New York, NY, USA, 1985; Volume 14, pp. 329–491. [Google Scholar]
- Brochu, C.A. Closure of neurocentral sutures during crocodilian ontogeny: Implications for maturity assessment in fossil archosaurs. J. Vertebr. Paleontol. 1996, 16, 49–62. [Google Scholar] [CrossRef]
- Labarre, D.; Charruau, P.; Platt, S.G.; Rainwater, T.R.; Cedeño-Vázquez, J.R.; González-Cortés, H. Morphological diversity of the American crocodile (Crocodylus acutus) in the Yucatán peninsula. Zoomorphology 2017, 136, 387–401. [Google Scholar] [CrossRef]
- Cott, H.B. Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodilus niloticus) in Uganda and Northern Rhodesia. Trans. Zool. Soc. Lond. 1961, 29, 211–356. [Google Scholar] [CrossRef]
- Webb, G.J.W.; Messel, H.; Crawford, J.; Yerbury, M.J. Growth rates of Crocodylus porosus (Reptilia:Crocodilia) from Arnhem Land, Northern Australia. Aust. Wildl. Res. 1978, 5, 385–399. [Google Scholar] [CrossRef]
- Wink, C.S.; Elsey, R.M. Changes in femoral morphology during egg-laying in Alligator mississippiensis. J. Morphol. 1986, 189, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wink, C.S.; Elsey, R.M.; Hill, E.M. Changes in femoral robusticity and porosity during the reproductive cycle of the female alligator (Alligator mississippiensis). J. Morphol. 1987, 193, 317–321. [Google Scholar] [CrossRef]
- Ricqles, A.; Buffrénil, V. Bone histology, heterochronies, and the return of Tetrapods to life in water: Where are we? In Secondary Adaptation of Tetrapods to Life in Water; Mazin, J.-M., de Buffrénil, V., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2001; pp. 289–310. Available online: https://isbnsearch.org/isbn/9783931516888 (accessed on 26 October 2021).
- Troxell, E.L. Hyposaurus, a marine crocodilian. Am. J. Sci. 1925, 9, 489–514. [Google Scholar] [CrossRef]
- Parris, D.C. Biostratigraphy of the fossil crocodile Hyposaurus Owen from New Jersey. Investig. State Mus. 1986, 4, 1–16. [Google Scholar]
- Langston, W., Jr. Dyrosaurs (Crocodilia: Mesosuchia) from the Paleocene Umm Himar Formation, Kingdom of Saudi Arabia. Bull. Geol. Surv. 1995, 2093, A1–A19. [Google Scholar]
- Frey, E.; Salisbury, S. The kinematics of aquatic locomotion in Osteolaemus tetraspis Cope. In Crocodilian Biology and Evolution; Grigg, G., Seebacher, F., Franklin, C., Eds.; Surrey Beatty & Sons: Chipping Norton, Australia, 2001; pp. 165–179. [Google Scholar]
- Salisbury, S.W.; Frey, E. A biomechanical transformation model for the evolution of semi-spheroidal articulations between adjoining vertebral bodies in crocodilians. In Crocodilian Biology and Evolution; Grigg, G., Seebacher, F., Franklin, C., Eds.; Surrey Beatty & Sons: Chipping Norton, Australia, 2001; pp. 85–134. [Google Scholar]
- Schwarz-Wings, D.; Frey, E.; Martin, T. Reconstruction of the bracing system of the trunk and tail in hyposaurine dyrosaurids (Crocodylomorpha; Mesoeucrocodylia). J. Vertebr. Paleontol. 2009, 29, 453–472. [Google Scholar] [CrossRef]
- Swinton, W.E. On Congosaurus bequaerti Dollo. Ann. Musée Congo Belg. 1950, 13, 9–56. [Google Scholar]
- Taylor, M.A. Stomach stones for feeding or buoyancy? The occurrence and function of gastroliths in marine tetrapods. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1993, 341, 163–175. [Google Scholar]
- Henderson, D.M. Effects of stomach stones on the buoyancy and equilibrium of a floating crocodilian: A computational analysis. Can. J. Zool. 2003, 81, 1346–1357. [Google Scholar] [CrossRef]
- Uriona, T.J.; Farmer, C.G. Recruitment of the diaphragmaticus, ischiopubis and other respiratory muscles to control pitch and roll in the American alligator (Alligator mississippiensis). J. Exp. Biol. 2008, 211, 1141–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wings, O. A review of gastrolith function with implications for fossil vertebrates and a revised classification. Acta Palaeontol. Pol. 2007, 52, 1–16. [Google Scholar]
- Hua, S.; de Buffrénil, V. Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). J. Vertebr. Paleontol. 1996, 16, 703–717. [Google Scholar] [CrossRef]
- Buffetaut, E. Sokotosuchus ianwilsoni and the evolution of the dyrosaurid crocodilians. Niger. Field Monogr. 1979, 44 (Suppl. 1), 31–41. [Google Scholar]
- Buffetaut, E.; de Buffrénil, V.; de Ricqlès, A.; Spinar, Z.V. Remarques anatomiques et paléohistologiques sur Dyrosaurus phosphaticus, crocodilien mesosuchien des Phosphates yprésiens de Tunisie. Ann. Paléontologie 1982, 68, 327–341. [Google Scholar]



| LAG No. | Radius a− | Radius a+ | Percent Decrease from Previous Interval (a−) | Percent Decrease from Previous Interval (a+) | Radius b− | Radius b+ | Percent Decrease from Previous Interval (b−) | Percent Decrease from Previous INTERVAL (b+) | Calculated Circumference (Negative Radii) | Calculated Circumference (Positive Radii) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NP | NP | N/A | N/A | 3606 | NP | N/A | N/A | N/A | N/A |
| 2 | 3247 | NP | N/A | N/A | 4088 | NP | −54.7 | N/A | 23,120 | N/A |
| 3 | 4065 | 4621 | 39.37 | N/A | 4834 | 2903 | 39.2 | N/A | 28,009 | 23,948 |
| 4 | 4561 | 5278 | 5.97 | 25.1 | 5287 | 3572 | 11.4 | 8.5 | 30,981 | 28,062 |
| 5 | 5027 | 5770 | 16.85 | −64.1 | 5689 | 4183 | 7.1 | −22.1 | 33,698 | 31,469 |
| 6 | 5415 | 6578 | 26.58 | 43.7 | 6063 | 4930 | 30.7 | 39.7 | 36,086 | 36,340 |
| 7 | 5699 | 7032 | −102.71 | −90.4 | 6322 | 5381 | −85.6 | −59.7 | 37,790 | 39,172 |
| 8 | 6276 | 7898 | 9.60 | 16.1 | 6802 | 6101 | 3.5 | 12.0 | 41,103 | 44,161 |
| 9 | 6798 | 8624 | 15.80 | 21.6 | 7266 | 6735 | 0.3 | 27.2 | 44,195 | 48,436 |
| 10 | 7237 | 9194 | 10.26 | 6.6 | 7728 | 7196 | 24.0 | 20.7 | 47,027 | 51,681 |
| 11 | 7631 | 9726 | 1.63 | 30.8 | 8080 | 7562 | −7.7 | −7.0 | 49,368 | 54,523 |
| 12 | 8019 | 10,094 | 46.51 | 48.3 | 8458 | 7953 | 38.8 | 65.8 | 51,774 | 56,897 |
| 13 | 8226 | 10,285 | N/A | N/A | 8690 | 8087 | N/A | N/A | 53,155 | 57,924 |
| LAG No. | Radius a+ | Interval between CGMs (Radius a+) | Percent Decrease from Previous Interval (a+) | Radius b+ | Interval between CGMs (Radius b+) | Percent Decrease from Previous Interval (b+) | Calculated circumference (Positive Radii) |
|---|---|---|---|---|---|---|---|
| 1 | NP | N/A | N/A | 3395 | 407 | N/A | N/A |
| 2 | 4719 | 412 | N/A | 3802 | 283 | 30.4 | 26,848 |
| 3 | 5131 | 263 | 36.2 | 4086 | 258 | 9.1 | 29,049 |
| 4 | 5394 | 1252 | −376.5 | 4343 | 726 | −182.0 | 30,680 |
| 5 | 6646 | 773 | 38.3 | 5070 | 438 | 39.7 | 36,973 |
| 6 | 7419 | 1242 | −60.7 | 5507 | 572 | −30.6 | 40,832 |
| 7 | 8661 | 592 | 52.3 | 6079 | 510 | 10.8 | 46,662 |
| 8 | 9253 | 922 | −55.7 | 6589 | 670 | −31.3 | 50,123 |
| 9 | 10,175 | 829 | 10.1 | 7259 | 598 | 10.8 | 55,155 |
| 10 | 11,005 | 556 | 32.9 | 7857 | 618 | −3.4 | 59,668 |
| 11 | 11,561 | 603 | −8.3 | 8475 | 649 | −5.0 | 63,319 |
| 12 | 12,164 | 304 | 49.6 | 9124 | 428 | 34.1 | 67,220 |
| 13 | 12,468 | 278 | 8.5 | 9552 | 422 | 1.2 | 69,480 |
| 14 | 12,746 | N/A | N/A | 9974 | N/A | N/A | 71,644 |
| ID | Specimen No. | FL | CW |
|---|---|---|---|
| Hyposaurus rogersii | NJSM 23368 | 202.0 | 20.0 |
| Hyposaurus rogersii | NJSM 12293 | 250.0 | 27.15 |
| Hyposaurus rogersii | NJSM 11069 | Missing epiphyses | 35.45 |
| Hyposaurus natator | YPM 985 | 250.0 | 23.0 |
| Hyposaurus natator | YPM 753 | 285.0 | 28.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrini, R.A.; Callahan, W.R.; Hastings, A.K.; Parris, D.C.; McCauley, J.D. Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA. Animals 2021, 11, 3067. https://doi.org/10.3390/ani11113067
Pellegrini RA, Callahan WR, Hastings AK, Parris DC, McCauley JD. Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA. Animals. 2021; 11(11):3067. https://doi.org/10.3390/ani11113067
Chicago/Turabian StylePellegrini, Rodrigo A., Wayne R. Callahan, Alexander K. Hastings, David C. Parris, and John D. McCauley. 2021. "Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA" Animals 11, no. 11: 3067. https://doi.org/10.3390/ani11113067
APA StylePellegrini, R. A., Callahan, W. R., Hastings, A. K., Parris, D. C., & McCauley, J. D. (2021). Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA. Animals, 11(11), 3067. https://doi.org/10.3390/ani11113067








