Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Cooled Perch Design and Experimental Treatments
2.1. Engineering Design of the Thermal Perch System
2.2. Experimental Treatments and Birds
3. Thermal Perch System Improves Adaption to Heat Stress in Laying Hens
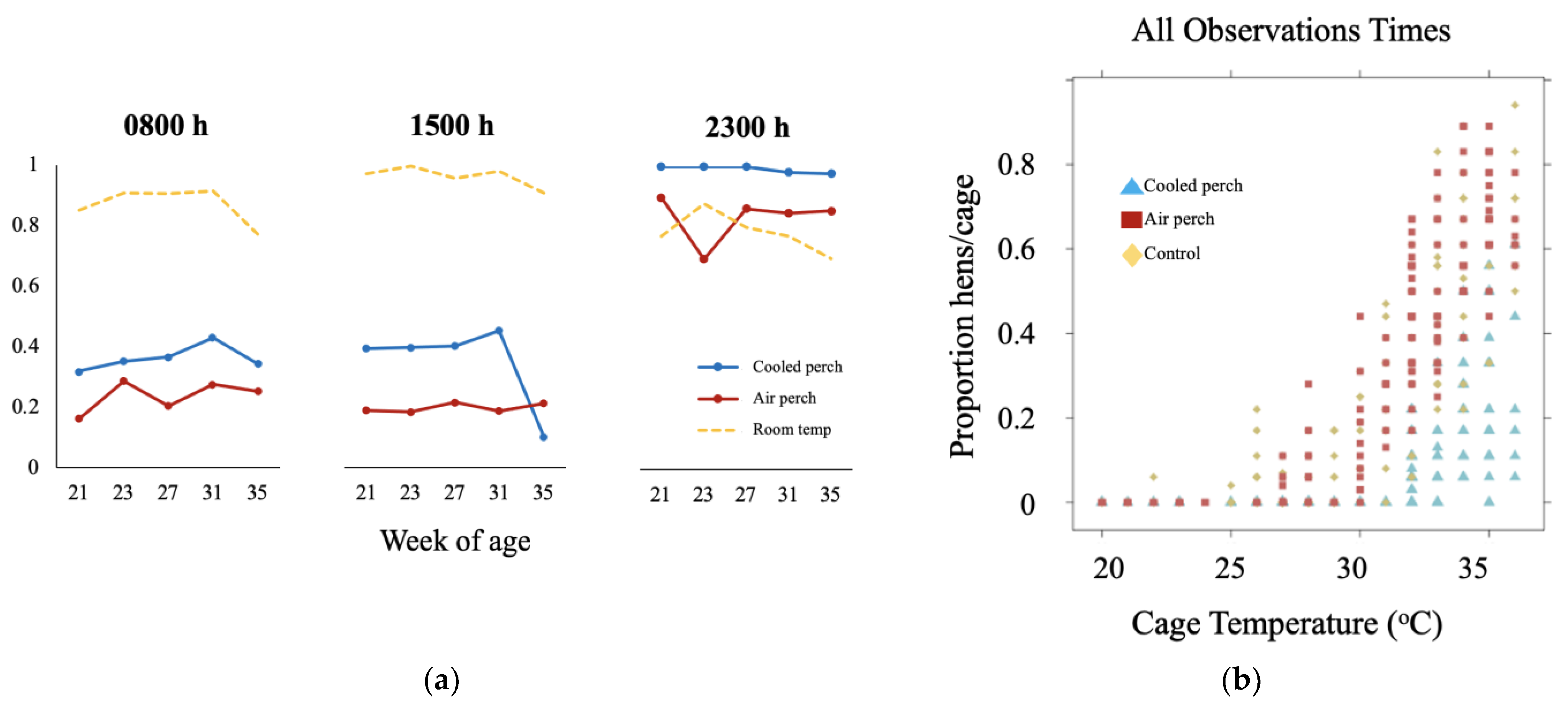
3.1. Behavioral Adaption
3.2. Mortality and Production Traits
3.3. Physical Conditions
3.4. Physiological and Immunological Changes
3.5. Induced Molting under Hot Ambient Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, S.; Sillmann, J.; Sterl, A. Humid heat waves at different warming levels. Sci. Rep. 2017, 7, 7477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; DiGiacomo, K.; Osei-Amponsah, R.; Chauhan, S.S. Impacts of heat stress on meat quality and strategies for amelioration: A review. Int. J. Biometeorol. 2020, 64, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ratwan, P.; Dahiya, S.P.; Nehra, A.K. Climate change and heat stress: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J. Therm. Biol. 2021, 97, 102867. [Google Scholar] [CrossRef] [PubMed]
- Cianconi, P.; Betrò, S.; Janiri, L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front. Psychiatry 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Sahin, N.; Kucuk, O.; Hayirli, A.; Prasad, A.S. Role of dietary zinc in heat-stressed poultry: A review. Poult. Sci. 2009, 88, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S. Alleviating heat stress in domestic fowl: Different strategies. Worlds Poult. Sci. J. 2009, 65, 719–732. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018, 76, 101–106. [Google Scholar] [CrossRef]
- Barrett, N.W.; Rowland, K.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F.; Ashwell, C.M.; Persia, M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019, 98, 6684–6692. [Google Scholar] [CrossRef]
- U.S. Heat Wave Frequency and Length are Increasing. Available online: https://www.globalchange.gov/browse/indicators/us-heat-waves (accessed on 17 August 2021).
- United Egg Producers. Animal Husbandry Guidelines for U.S. Egg Laying-Flocks; United Egg Producers: Atlanta, GA, USA, 2020. [Google Scholar]
- Yahav, S. Regulation of Body Temperature: Strategies and Mechanisms, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124071605. [Google Scholar]
- Franco-Jimenez, D.J.; Scheideler, S.E.; Kittok, R.J.; Brown-Brandl, T.M.; Robeson, L.R.; Taira, H.; Beck, M.M. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 2007, 16, 628–634. [Google Scholar] [CrossRef]
- Akbari Moghaddam Kakhki, R.; Mousavi, Z.; Anderson, K.E. An appraisal of moulting on post-moult egg production and egg weight distribution in white layer hens; meta-analysis. Br. Poult. Sci. 2018, 59, 278–285. [Google Scholar] [CrossRef]
- Han, G.P.; Lee, K.C.; Kang, H.K.; Oh, H.N.; Sul, W.J.; Kil, D.Y. Analysis of excreta bacterial community after forced molting in aged laying hens. Asian-Australas. J. Anim. Sci. 2019, 32, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Ferket, P.R.; Gernat, A.G. Factors That Affect Feed Intake of Meat Birds: A Review. Int. J. Poult. Sci. 2006, 5, 905–911. [Google Scholar]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; van Kempen, T.A.T.G.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Smits, C.; Fink-Gremmels, J. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol. 2019, 48, 582–601. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.M.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Perini, F.; Cendron, F.; Rovelli, G.; Castellini, C.; Cassandro, M.; Lasagna, E. Emerging genetic tools to investigate molecular pathways related to heat stress in chickens: A review. Animals 2021, 11, 46. [Google Scholar]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional manipulation to combat heat stress in poultry—A comprehensive review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef] [PubMed]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S.; Goldfeld, S.; Plavnik, I.; Hurwitz, S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995, 20, 245–253. [Google Scholar] [CrossRef]
- Chepete, H.J.; Xin, H. Cooling laying hens by intermittent partial surface sprinkling. Trans. Am. Soc. Agric. Eng. 2000, 43, 965–971. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef]
- Olsson, I.A.S.; Keeling, L.J. Night-time roosting in laying hens and the effect of thwarting access to perches. Appl. Anim. Behav. Sci. 2000, 68, 243–256. [Google Scholar] [CrossRef]
- Olsson, I.A.S.; Keeling, L.J. The push-door for measuring motivation in Hens: Laying hens are motivated to perch at night. Anim. Welf. 2002, 11, 11–19. [Google Scholar]
- Appleby, M.C.; Walker, A.W.; Nicol, C.J.; Lindberg, A.C.; Freire, R.; Hughes, B.O.; Elson, H.A. Development of furnished cages for laying hens. Br. Poult. Sci. 2002, 43, 489–500. [Google Scholar] [CrossRef]
- Hester, P.Y. The effect of perches installed in cages on laying hens. Worlds Poult. Sci. J. 2014, 70, 247–264. [Google Scholar] [CrossRef]
- Hillman, P.E.; Scott, N.R.; Van Tienhoven, A. Vasomotion in chicken foot: Dual innervation of arteriovenous anastomoses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1982, 11, R582–R590. [Google Scholar] [CrossRef]
- Hillman, P.E.; Scott, N.R. Energy budget of the chicken foot. J. Therm. Biol. 1989, 14, 205–217. [Google Scholar] [CrossRef]
- Muiruri, H.K.; Harrison, P.C. Effect of roost temperature on performance of chickens in hot ambient environments. Poult. Sci. 1991, 70, 2253–2258. [Google Scholar] [CrossRef]
- Reilly, W.M.; Koelkebeck, K.W.; Harrison, P.C. Performance evaluation of heat-stressed commercial broilers provided water-cooled floor perches. Poult. Sci. 1991, 70, 1699–1703. [Google Scholar] [CrossRef]
- Facchi, J.C.; de Lima, T.A.L.; de Oliveira, L.R.; de Oliveira Costermani, H.; Miranda, G.D.S.; de Oliveira, J.C. Perinatal programming of metabolic diseases: The role of glucocorticoids. Metabolism 2020, 104, 154047. [Google Scholar] [CrossRef]
- Okelo, P.O.; Carr, L.E.; Harrison, P.C.; Douglass, L.W.; Byrd, V.E.; Wabeck, C.W.; Schreuders, P.D.; Wheaton, F.W.; Zimmermann, N.G. Effectiveness of a novel method to reduce heat stress in broilers: A cool roost system. Trans. Am. Soc. Agric. Eng. 2003, 46, 1675–1683. [Google Scholar] [CrossRef]
- Zhao, J.P.; Jiao, H.C.; Jiang, Y.B.; Song, Z.G.; Wang, X.J.; Lin, H. Cool perches improve the growth performance and welfare status of broiler chickens reared at different stocking densities and high temperatures. Poult. Sci. 2013, 92, 1962–1971. [Google Scholar] [CrossRef]
- Zhao, J.P.; Jiao, H.C.; Jiang, Y.B.; Song, Z.G.; Wang, X.J.; Lin, H. Cool perch availability improves the performance and welfare status of broiler chickens in hot weather. Poult. Sci. 2012, 91, 1775–1784. [Google Scholar] [CrossRef]
- Xiong, Y.; Gates, R.S.; Hu, J.Y.; Hester, P.Y.; Cheng, H.W. Design and performance of an experimental cooled perch system for heat stress relief of laying hens. Trans. ASABE 2020, 63, 1109–1121. [Google Scholar] [CrossRef]
- Hu, J.Y.; Hester, P.Y.; Makagon, M.M.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Effect of cooled perches on performance, plumage condition, and foot health of caged White Leghorn hens exposed to cyclic heat. Poult. Sci. 2019, 98, 2705–2718. [Google Scholar] [CrossRef]
- Hu, J.Y.; Hester, P.Y.; Makagon, M.M.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Effect of cooled perches on physiological parameters of caged White Leghorn hens exposed to cyclic heat. Poult. Sci. 2019, 98, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Altan, Ö.; Pabuçcuoǧlu, A.; Altan, A.; Konyalioǧlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Fujita, M.; Nakahara, M.; Kuwahara, T.; Kawakami, S.I.; Bungo, T. Effect of high environmental temperature on egg production, serum lipoproteins and follicle steroid hormones in laying hens. J. Poult. Sci. 2011, 48, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Jiao, H.C.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Biggs, P.E.; Persia, M.E.; Koelkebeck, K.W.; Parsons, C.M. Further evaluation of nonfeed removal methods for molting programs. Poult. Sci. 2004, 83, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Mazzuco, H.; Hester, P.Y. The effect of an induced molt and a second cycle of lay on skeletal integrity of white leghorns. Poult. Sci. 2005, 84, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Etches, R.J.; John, T.M.; Verrinder Gibbins, A.M. Behavioural, physiological, neuroendocrine and molecular responses to heat stress. In Poultry Production in Hot Climates, 2nd ed.; CABI: Wallingford, UK, 2008; pp. 48–79. ISBN 9781845932589. [Google Scholar]
- Comito, R.W.; Reece, W.O.; Trampel, D.W.; Koehler, K.J. Acid-base balance of the domestic turkey during thermal panting. Poult. Sci. 2007, 86, 2649–2652. [Google Scholar] [CrossRef]
- Sandercock, D.A.; Hunter, R.R.; Nute, G.R.; Mitchell, M.A.; Hocking, P.M. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: Implications for meat quality. Poult. Sci. 2001, 80, 418–425. [Google Scholar] [CrossRef]
- Felver-Gant, J.N.; Mack, L.A.; Dennis, R.L.; Eicher, S.D.; Cheng, H.W. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult. Sci. 2012, 91, 1542–1551. [Google Scholar] [CrossRef]
- Makagon, M.M.; Cussen, V.A.; Gates, R.S.; Hester, P.Y.; Cheng, H.W. Effects of cooled perch access during chronic heat stress on the behavior of White Leghorn hens. Poult. Sci. 2015, 94, 79. [Google Scholar]
- McNabb, F.M.A. Avian thyroid development and adaptive plasticity. Gen. Comp. Endocrinol. 2006, 147, 93–101. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Kahl, S.; Elsasser, T.H.; Rhoads, R.P.; Collier, R.J.; Baumgard, L.H. Environmental heat stress modulates thyroid status and its response to repeated endotoxin challenge in steers. Domest. Anim. Endocrinol. 2015, 52, 43–50. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Goel, A.; Ncho, C.M.; Choi, Y.H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 11. [Google Scholar] [CrossRef]
- Dukay, B.; Csoboz, B.; Tóth, M.E. Heat-shock proteins in neuroinflammation. Front. Pharmacol. 2019, 10, 920. [Google Scholar] [CrossRef] [Green Version]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [Green Version]
- Wein, Y.; Shira, E.B.; Friedman, A. Increased serum levels of advanced glycation end products due to induced molting in hen layers trigger a proinflammatory response by peripheral blood leukocytes. Poult. Sci. 2020, 99, 3452–3462. [Google Scholar] [CrossRef]
- Biggs, P.E.; Douglas, M.W.; Koelkebeck, K.W.; Parsons, C.M. Evaluation of nonfeed removal methods for molting programs. Poult. Sci. 2003, 82, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Mejia, L.; Meyer, E.T.; Studer, D.L.; Utterback, P.L.; Utterback, C.W.; Parsons, C.M.; Koelkebeck, K.W. Evaluation of limit feeding varying levels of distillers dried grains with solubles in non-feed-withdrawal molt programs for laying hens. Poult. Sci. 2011, 90, 321–327. [Google Scholar] [CrossRef]
- Koch, J.M.; Lay, D.C.; McMunn, K.A.; Moritz, J.S.; Wilson, M.E. Motivation of hens to obtain feed during a molt induced by feed withdrawal, wheat middlings, or melengestrol acetate. Poult. Sci. 2007, 86, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Denbow, D.M. Food intake control in birds. Neurosci. Biobehav. Rev. 1985, 9, 223–232. [Google Scholar] [CrossRef]
- Hu, J.Y.; Hester, P.Y.; Xiong, Y.; Gates, R.S.; Makagon, M.M.; Cheng, H.W. Effect of cooled perches on the efficacy of an induced molt in White Leghorn laying hens previously exposed to heat stress. Poult. Sci. 2019, 98, 4290–4300. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.; Thaxton, P. Physiological changes in caged layers during a forced molt. 2. Gross changes in organs. Poult. Sci. 1979, 58, 707–716. [Google Scholar] [CrossRef]
- Andrews, D.K.; Berry, W.D.; Brake, J. Effect of lighting program and nutrition on feather replacement of molted single comb White Leghorn hens. Poult. Sci. 1987, 66, 1635–1639. [Google Scholar] [CrossRef]
- Gongruttananun, N.; Kochagate, P.; Poonpan, K.; Yu-nun, N.; Aungsakul, J.; Sopa, N. Effects of an induced molt using cassava meal on body weight loss, blood physiology, ovarian regression, and postmolt egg production in late-phase laying hens. Poult. Sci. 2017, 96, 1925–1933. [Google Scholar] [CrossRef]




| Treatment 1 | Cumulative Mortality (%) | Egg Production and Eggshell Quality 2 | Feed Usage 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Hen-Day Eggs (%) | Marketable Egg (%) | Egg Weight (g) | Breaking Force (N) | Eggshell Thickness (mm) | Feed Utilization (g Hen−1 d−1) | Feed Efficiency (kg Feed per Dozen Eggs) | ||
| NP | 10.19 b | 72.6 c | 97.67 | 59.6 b | 35.0 b | 0.33 | 100.56 b | 1.84 |
| AP | 3.70 ab | 74.9 b | 97.80 | 60.0 b | 34.9 b | 0.33 | 98.28 b | 1.58 |
| CP | 2.78 a | 77.6 a | 98.26 | 61.1 a | 36.3 a | 0.34 | 103.02 a | 1.57 |
| n 3 | 264 | 384 | 384 | 1320 | 1320 | 1320 | 264 | 264 |
| SEM | 1.93 | 0.5 | 0.23 | 0.1 | 0.3 | 0.002 | 0.82 | 0.12 |
| p value | ||||||||
| ptreatment | 0.02 | <0.0001 | 0.55 | <0.0001 | <0.0001 | 0.44 | 0.0002 | 0.23 |
| page | - | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ptreatment × age | - | <0.0001 | 0.17 | <0.0001 | 0.01 | 0.33 | 0.04 | 0.19 |
| Treatment 1 | Mean Feather Score 1 | Foot Health 2 | ||
|---|---|---|---|---|
| Hyperkeratosis Score 2 | Mean Nail Length (cm) | Number of Broken Toenails | ||
| NP | 1.95 | 3.83 | 1.8 | 1.13 ab |
| AP | 2.46 | 3.91 | 2.16 | 1.58 a |
| CP | 2.02 | 3.82 | 2.46 | 0.96 b |
| n 3 | 24 | 24 | 24 | 24 |
| SEM | 0.27 | 0.24 | 0.23 | 0.18 |
| p value | 0.36 | 0.96 | 0.13 | 0.04 |
| Treatment 1 | RT (°C) | PCV | H/L (ratio) | IL-6 (pg/mL) | IL-10 (pg/mL) | IFN-Υ (pg/mL) | IgY (ng/mL) | T3 (ng/mL) | T4 (μg/mL) | T3/T4 (ratio) | HSP70 (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| First heating episode (35 weeks of age) | |||||||||||
| NP | 41.9 a | 27.1 | 0.81 | 34 | 62 | 72 | 340 | 184 | 6.44 | 0.029 | 294 b |
| AP | 41.9 a | 25.3 | 0.80 | 40 | 76 | 88 | 322 | 218 | 6.84 | 0.031 | 243 ab |
| CP | 41.7 b | 27.8 | 0.72 | 33 | 49 | 66 | 342 | 204 | 6.59 | 0.031 | 224 a |
| n 2 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| SEM | 0.10 | 1.2 | 0.08 | 4 | 7 | 12 | 28 | 9 | 0.12 | 0.003 | 20 |
| p-value | 0.02 | 0.34 | 0.48 | 0.54 | 0.08 | 0.40 | 0.86 | 0.11 | 0.07 | 0.13 | 0.04 |
| Second heating episode (80 weeks of age) | |||||||||||
| NP | 41.4 a | 30.3 ab | 1.24 a | 25 | 45 | 24 | 214 | 245 b | 7.74 | 0.032 b | 234 |
| AP | 41.4 a | 29.4 b | 1.20 a | 25 | 43 | 48 | 168 | 269 ab | 7.88 | 0.034 ab | 215 |
| CP | 41.1 b | 31.3 a | 0.83 b | 29 | 39 | 48 | 157 | 288 a | 7.69 | 0.038 a | 166 |
| n 2 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| SEM | 0.15 | 0.4 | 0.08 | 4 | 7.5 | 7 | 37 | 12 | 0.17 | 0.001 | 23 |
| p-value | 0.02 | 0.02 | 0.01 | 0.78 | 0.78 | 0.43 | 0.51 | 0.002 | 0.48 | 0.0006 | 0.10 |
| Treatment 1 | RT 2 (°C) | Body Weight | Feed Intake (g Hen−1 d−1) | H/L (Ratio) | CORT (ng mL−1) | T3 (ng mL−1) | T4 (μg mL−1) | T3/T4 (Ratio) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial (kg) | Post-Molting (kg) | Loss (%) 3 | ||||||||
| NP | 41.9 a | 1.57 | 1.35 | 13.3 b | 53.7 b | 1.38 a | 6.47 | 230 | 4.70 | 0.051 |
| AP | 41.8 ab | 1.60 | 1.27 | 19.8 ab | 52.6 b | 1.62 a | 6.27 | 213 | 4.84 | 0.047 |
| CP | 41.6 b | 1.58 | 1.25 | 22.0 a | 58.6 a | 0.91 b | 4.71 | 223 | 4.81 | 0.048 |
| SEM | 0.12 | 0.05 | 0.04 | 2.2 | 1.5 | 0.08 | 0.86 | 10 | 0.17 | 0.003 |
| n 4 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| p-value | 0.01 | 0.94 | 0.11 | 0.02 | 0.02 | 0.01 | 0.38 | 0.53 | 0.82 | 0.71 |
| Treatment 1 | 50% Egg Production (d) | Hen-Day Egg Production 1 (%) | Egg Weight 2 (g) | Breaking Force 2 (N) | Proportion of Eggshell 2 (%) | Eggshell Thickness 2 (mm) |
|---|---|---|---|---|---|---|
| NP | 47.6 | 51.4 | 69.3 | 35.4 | 8.67 a | 0.350 |
| AP | 48.2 | 54.4 | 69.7 | 33.2 | 8.32 b | 0.341 |
| CP | 48.8 | 59.3 | 69.9 | 34.3 | 8.62 a | 0.347 |
| SEM | 0.7 | 2.8 | 0.5 | 1.2 | 0.09 | 0.001 |
| n 3 | 12 | 264 | 240 | 240 | 240 | 240 |
| p-value | ||||||
| ptreatment | 0.49 | 0.29 | 0.66 | 0.45 | 0.03 | 0.09 |
| page | - | <0.0001 | <0.0001 | <0.0001 | 0.04 | 0.04 |
| ptreatment × age | - | <0.0001 | 0.01 | 0.50 | 0.61 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Xiong, Y.; Gates, R.S.; Cheng, H.-W. Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review. Animals 2021, 11, 3026. https://doi.org/10.3390/ani11113026
Hu J, Xiong Y, Gates RS, Cheng H-W. Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review. Animals. 2021; 11(11):3026. https://doi.org/10.3390/ani11113026
Chicago/Turabian StyleHu, Jiaying, Yijie Xiong, Richard S. Gates, and Heng-Wei Cheng. 2021. "Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review" Animals 11, no. 11: 3026. https://doi.org/10.3390/ani11113026
APA StyleHu, J., Xiong, Y., Gates, R. S., & Cheng, H.-W. (2021). Perches as Cooling Devices for Reducing Heat Stress in Caged Laying Hens: A Review. Animals, 11(11), 3026. https://doi.org/10.3390/ani11113026








