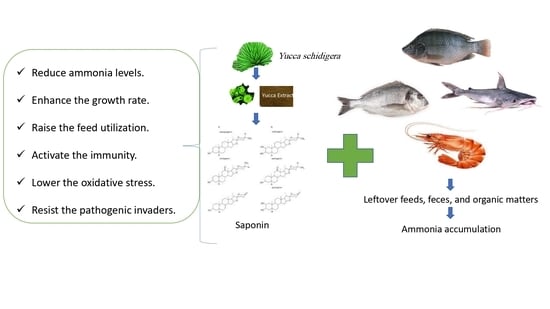
Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability
Abstract
Simple Summary
Abstract
1. Introduction
2. The Nature, Sources, and Composition of Yucca
3. Yucca as a Growth Promotor
4. Yucca as an Immunostimulant
5. Yucca as an Antioxidative Agent
6. Yucca as a Natural Cleaner for Aquatic Water Quality
| Reference | Aquatic Animal sp. | Supplementation Type | Supplementation Source | Trial Period–(BW0) | Applied Doses | Recommended Dose(s) | Impacts |
|---|---|---|---|---|---|---|---|
| Wang et al. [42] | Mirror carp (Cyprinus carpio) | Diet | YSE | 8 weeks–(45.21 ± 0.43 g) | 0, 200, 400 mg/kg | 400 mg/kg | (+) FBW, WG |
| Peterman et al. [46] | Channel catfish (Ictalurus punctatus) | Diet | Commercial blend (One Current™) | 3 months–(11 g) | 0, 1.8, 3.6 g | 3.6 g | (+) WG, SGR, (−) FCR |
| El-Keredy and Naena [47] | Nile tilapia (Oreochromis niloticus) | Diet | YSE | 8 weeks–(20 g) | 0.1, 0.14, 0.2% | 0.1% | (+) WG, FI, FER, PER |
| Bae et al. [49] | Olive flounder (Paralichthys olivaceus) | Diet | YSM | 8 weeks–(5.26 ± 0.17 g) | 1.5 g/kg | 1.5 g/kg | NS |
| Abdel-Tawwab et al. [12] | Nile tilapia (Oreochromis niloticus) | Water | YSE | 8 weeks–(28–32 g) | 0, 1 g/m3 | 1 g/m3 | (+) FBW, WG, SGR, FI |
| Fayed et al. [16] | European seabass (Dicentrarchus labrax) | Water | YSE | 45 days–(5.0 ± 0.5 g) | 0.25, 0.50, 0.75 mg/L | 0.75 mg/L | (+) FBW, WG, (−) FCR |
| Elkhayat et al. [45] | European seabass (Dicentrarchus labrax) | Diet | YSE | 45 days–(5.0 ± 0.5 g) | 0, 0.25, 0.50, 1.00 g/kg | 1.00 g/kg | (+) FBW, WG, SGR, PER, (−) FCR |
| Njagi et al. [13] | Nile tilapia (Oreochromis niloticus) | Diet | YSM | 10 weeks–(3.8 ± 0.05 g) | 0, 0.1, 0.3, 0.5, 1.0, 2.0% | 0.1, 0.14% | (+) WG, SGR, FER |
| Angeles Jr. et al. [51] | Nile tilapia (Oreochromis niloticus) | Diet | YSE-QS | 6 weeks–(1.9 ± 0.08 g) | 150 mg/kg | 150 mg/kg Quillaja saponaria + 150 mg/kg YSE | (+) FBW, WG, SGR |
| Güroy et al. [52] | Striped catfish (Pangasianodon hypophthalmus) | Diet | YSE | 12 weeks–(1.78 ± 0.05 g) | 0, 0.075, 0.1, 0.15% | 0.15% | (+) SGR, (−) FCR |
| Couto et al. [70] | European sea bass (Dicentrarchus labrax) | Diet | Soya-saponin | 59 days–(27± 0.01 g) | 0, 1, 2 g/kg | - | (−) Maltase and alkaline phosphatase activity, (+) intestinal inflammations |
| Couto et al. [69] | Gilthead sea bream (Sparus aurata) | Diet | Soya-saponin | 48 days–(112± 5 g) | 0, 0.1, 0.2% | - | NS |
| Chikwati et al. [67] | Atlantic salmon (Salmo salar) | Diet | Soya-saponin | 80 days–(270 g) | 2 g/kg | - | (−) FI, ADC |
| Chen et al. [48] | Japanese flounder (Paralichthys olivaceus) | Diet | Soya-saponin | 56 days–(2.58 ± 0.01 g) | 0, 0.8, 3.2, 6.4 g/kg | Lower level <0.8 g/kg | High level (6.4 g/kg) resulted in (−) WG, FI, FER |
| Gaber [33] | Nile tilapia (Oreochromis niloticus) | Diet | YSE | 6 months–(14.2 ± 2.9 g) | 0.075 | 0.075 | (+) WG, SGR, ADC, whole-body protein content, (−) lower whole-body lipid content |
| El-Saidy and Gaber [53] | Nile tilapia (Oreochromis niloticus) | Diet | YSE | 30 weeks–(16.82 g) | 0, 250, 500, 750, 1000, 1250, 1500 mg/kg | 750 or 1000 mg/kg | (+) BW, WG, SGR, FER, PER, ADC |
| Kelly and Kohler [43] | Channel Catfish (Ictalurus punctatus) | Diet | YSE | 12 weeks–(post-yolk-sac and 19 g) | 0, 0.5, or 0.1 g/kg diet | 1 g/kg | (+) WG, whole-body protein content, (−) lower whole-body lipid content |
| Francis et al. [44] | Common carp (Cyprinus carpio L.) | Diet | YSE | 8 weeks–(19 g) | 0, 150, 300 mg/kg | 150 mg/kg | (−) FCR, (+) metabolic growth rate |
| Francis et al. [54] | Nile tilapia (Oreochromis niloticus) | Diet | QS | 14 weeks–(50 g) | 0, 150, 300 mg/kg | 300 mg/kg | (+) WG, average values for energy retention, apparent lipid conversion |
| Tidwell et al. [50] | Channel catfish (Ictalurus punctatus) | Diet | YSE | 10 weeks–(1.9 g) | 0, 0.011, 0.11, 1.10 g/kg | Lower levels <1.1 g/kg | (−) WG, (+) FCR |
| Hernández-Acosta et al. [55] | Pacific white shrimp (Litopenaeus vannamei) | Diet | YSE-QS | 40 days–(2.6 g) | 0, 0.25, 0.50, 1.00, 2.00 g/kg | 1.00, 2.00 mg/kg | (+) WG, (−) FCR |
| Yang et al. [11] | Pacific white shrimp (Litopenaeus vannamei) | Diet | YSE | 100 days–(82 ± 0.02 g) | 0, 0.1, 0.2, 0.3% | 0.2% | (+) WG |
| References | Aquatic Animal sp. | Supplementation Type | Supplementation Source | Trial Period–(BW0) | Applied Doses | Recommended Dose(s) | Impacts |
|---|---|---|---|---|---|---|---|
| Wang et al. [42] | Mirror carp (Cyprinus carpio) | Diet | YSE | 8 weeks–(45.21 ± 0.43 g) | 0, 200, 400 mg/kg | 400 mg/kg | (+) LYZ, C3, C4, TGF-β2 mRNA levels, (−) mRNA levels of TNF-α, IL-1β, and IL-6 |
| Peterman et al. [46] | Channel catfish (Ictalurus punctatus) | Diet | Commercial blend (One Current™) | 3 months–(11 g) | 0, 1.8, 3.6 g | 3.6 g | (+) SR% |
| El-Keredy and Naena [47] | Nile tilapia (Oreochromis niloticus) | Diet | YSE | 8 weeks–(20 g) | 0.1, 0.14, 0.2% | 0.1% | (+) SR% |
| Bae et al. [49] | Olive flounder (Paralichthys olivaceus) | Diet | YSM | 8 weeks–(5.26 ± 0.17 g) | 1.5 g/kg | 1.5 g/kg | (+) NBT, CSR |
| Abdel-Tawwab et al. [12] | Nile tilapia (Oreochromis niloticus) | Water | YSE | 8 weeks–(28–32 g) | 0, 1 g/m3 | 1 g/m3 | (+) serum total lipids, TP, albumin, globulin, (−) AST, uric acid, creatinine |
| Fayed et al. [16] | European seabass (Dicentrarchus labrax) | Water | YSE | 45 days–(5.0 ± 0.5 g) | 0.25, 0.50, 0.75 mg/L | 0.75 mg/L | (+) LYZ, WBCs, RBCs, hematocrit, hemoglobin, lymphocytes, neutrophils |
| Reham et al. [63] | Nile tilapia (Oreochromis niloticus) | Water | YSE | 93 days–(51 g) | 1 mg/m3 | 1 mg/L | (+) LYZ, WBCs, hematocrit, hemoglobin, lymphocytes, neutrophils |
| Amoah et al. [62] | Amur catfish (Silurus asotus) | Diet | YSM | 8 weeks–(4.95 ± 0.05 g) | 0.05% | 0.05% | (+) LYZ |
| Njagi et al. [13] | Nile tilapia (Oreochromis niloticus) | Diet | YSM | 10 weeks–(3.8 ± 0.05 g) | 0, 0.1, 0.3, 0.5, 1.0, 2.0% | 0.1, 0.14% | (+) LYZ, NBT |
| Güroy et al. [52] | Striped catfish (Pangasianodon hypophthalmus) | Diet | YSE | 12 weeks–(1.78 ± 0.05 g) | 0, 0.075, 0.1, 0.15% | 0.15% | (+) PCV%, haemoglobin |
| Yang et al. [11] | Pacific white shrimp (Litopenaeus vannamei) | Diet | YSE | 100 days–(82 ± 0.02 g) | 0, 0.1, 0.2, 0.3% | 0.2% | (+) serum protein content, acid phosphatase, alkaline phosphatase, nitric oxide synthase, glutamic–pyruvic transaminase, phenol oxidase |
| Yeh et al. [64] | Giant freshwater prawns (Macrobrachium rosenbergii) | Water | Saponin | 168 h–(17.9 ± 2.7 g) | 0, 0.3, 0.6, 0.9, 1.2 mg/L | 0.9 mg/L | (+) total hemocyte count, (−) phenoloxidase activity, respiratory protein level |
| References | Aquatic Animal sp. | Supplementation Type | Supplementation Source | Trial period–(BW0) | Applied Doses | Recommended Dose(s) | Impacts |
|---|---|---|---|---|---|---|---|
| Wang et al. [42] | Mirror carp (Cyprinus carpio) | Diet | YSE | 8 weeks–(45.21 ± 0.43 g) | 0, 200, 400 mg/kg | 400 mg/kg | (+) TAC, (−) MDA, SOD |
| Bae et al. [49] | Olive flounder (Paralichthys olivaceus) | Diet | YSM | 8 weeks–(5.26 ± 0.17 g) | 1.5 g/kg | 1.5 g/kg | (+) SOD, MPO |
| Abdel-Tawwab et al. [12] | Nile tilapia (Oreochromis niloticus) | Water | YSE | 8 weeks–(28–32 g) | 0, 1 g/m3 | 1 g/m3 | (+) SOD, CAT, GPx, (−) MDA |
| Amoah et al. [62] | Amur catfish (Silurus asotus) | Diet | YSM | 8 weeks–(4.95 ± 0.05g) | 0.05% | 0.05% | (+) SOD |
| Angeles Jr. et al. [51] | Nile tilapia (Oreochromis niloticus) | Diet | YSE + QS | 6 weeks–(1.9 ± 0.08g) | 150 mg/kg | 150 mg/kg | (+) SR post a hypoxia challenge |
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adel, M.; Omidi, A.H.; Dawood, M.A.O.; Karimi, B.; Shekarabi, S.P.H. Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of Persian sturgeon (Acipenser persicus). Aquaculture 2021, 530, 735950. [Google Scholar] [CrossRef] [PubMed]
- FAO. Sustainability in action. In The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- El-Deep, M.H.; Amber, K.A.; Elgendy, S.; Dawood, M.; Zidan, A. In ovo injection of nano-selenium spheres mitigates the hatchability, histopathology image and immune response of hatched chicks. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- El-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Alrashood, S.T.; Khan, H.A.; Sakr, M.S.; Dawood, M. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits’ cecum. Front. Veter Sci. 2020, 7, 579576. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Amber, K.A.; Elgendy, S.; Dawood, M.; Elwakeel, E.M.; Paray, B.A. Oxidative stress, hemato-immunological, and intestinal morphometry changes induced by ochratoxin A in APRI rabbits and the protective role of probiotics. Environ. Sci. Pollut. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Saleh, A.A.; Paray, B.A.; Dawood, M.A.O. Olive cake meal and Bacillus licheniformis impacted the growth performance, muscle fatty acid content, and health status of broiler chickens. Animals 2020, 10, 695. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M. Ángeles Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-Reyes, R.A.; Chien, Y.-H. Ammonia reduction in seawater by Yucca schidigera extract: Efficacy analysis and empirical modelling. Aquac. Res. 2010, 41, 1221–1228. [Google Scholar]
- Adegbeye, M.J.; Elghandour, M.M.; Monroy, J.C.; Abegunde, T.O.; Salem, A.Z.M.; Barbabosa-Pliego, A.; Faniyi, T.O. Potential influence of Yucca extract as feed additive on greenhouse gases emission for a cleaner livestock and aquaculture farming—A review. J. Clean. Prod. 2019, 239, 118074. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Tan, B.-P.; Dong, X.-H.; Chi, S.-Y.; Liu, H.-Y. Effects of different levels of Yucca schidigera extract on the growth and nonspecific immunity of Pacific white shrimp (Litopenaeus vannamei) and on culture water quality. Aquaculture 2015, 439, 39–44. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mounes, H.A.; Shady, S.H.; Ahmed, K.M. Effects of yucca, Yucca schidigera, extract and/or yeast, Saccharomyces cerevisiae, as water additives on growth, biochemical, and antioxidants/oxidant biomarkers of Nile tilapia, Oreochromis niloticus. Aquaculture 2021, 533, 736122. [Google Scholar]
- Njagi, G.W.; Lee, S.; Won, S.; Hong, J.; Bai, S.C.; Hamidoghli, A. Effects of dietary Yucca meal on growth, haematology, non-specific immune responses and disease resistance of juvenile Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Aquac. Res. 2017, 48, 4399–4408. [Google Scholar] [CrossRef]
- ElBialy, Z.I.; Rizk, M.; Al-Hawary, I.I.; Salah, A.S.; Mohammed, R.A.; Assar, D.H.; Almeer, R.; Dawood, M. Yucca schidigera extract mediated the growth performance, hepato-renal function, antioxidative status and histopathological alterations in Nile tilapia (Oreochromis niloticus) exposed to hypoxia stress. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Preetham, E.; Kurian, A.; Lakshmi, S.; Faggio, C.; Esteban, M.A.; Ringø, E. Herbal Immunomodulators in Aquaculture. Rev. Fish. Sci. Aquac. 2020, 1–25. [Google Scholar] [CrossRef]
- Fayed, W.M.; Khalil, R.H.; Sallam, G.R.; Mansour, A.T.; Elkhayat, B.K.; Omar, E.A. Estimating the effective level of Yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and water quality of European seabass juveniles (Dicentrarchus labrax). Aquac. Rep. 2019, 15, 100208. [Google Scholar] [CrossRef]
- Engler, P.; Caillis, P.; Voller, S.; Labrie, L. Dietary Supplementation of a mixture of saponin-rich plants to reduce ammonia-nitrogen excretion in Juvenile Nile tilapia (Oreochromis niloticus). Sustain. Food Prod. 2018, 2, 6–12. [Google Scholar] [CrossRef]
- Fleck, J.D.; Betti, A.H.; da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar]
- Dawood, M.A.O.; Metwally, A.E.S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.R.; Paray, B.A. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Schumann, M.; Brinker, A. Understanding and managing suspended solids in intensive salmonid aquaculture: A review. Rev. Aquac. 2020, 12, 2109–2139. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; Heras, V.D.L.; Calduch-Giner, J. Àlvar; Pérez-Sánchez, J. Tissue-Specific Orchestration of Gilthead Sea Bream Resilience to Hypoxia and High Stocking Density. Front. Physiol. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Metwally, A.E.-S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.R.; Van Doan, H.; Ali, M.A.M. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Kabir, K.A.; Verdegem, M.C.J.; Verreth, J.A.J.; Phillips, M.J.; Schrama, J.W. Effect of dietary protein to energy ratio, stocking density and feeding level on performance of Nile tilapia in pond aquaculture. Aquaculture 2019, 511, 634200. [Google Scholar] [CrossRef]
- Van Doan, H.; Soltani, E.; Ingelbrecht, J.; Soltani, M. Medicinal herbs and plants: Potential treatment of monogenean infections in Fish. Rev. Fish. Sci. Aquac. 2020, 28, 260–282. [Google Scholar] [CrossRef]
- Dawood, M.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar]
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.B.A.; Marzouk, H.S.; El-Hawary, S. Lc–ms/ms and gc–ms profiling as well as the antimicrobial effect of leaves of selected yucca species introduced to egypt. Sci. Rep. 2020, 10, 17778. [Google Scholar] [CrossRef]
- Chase, M.W.; Reveal, J.L.; Fay, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2009, 161, 132–136. [Google Scholar] [CrossRef]
- Tenon, M.; Feuillère, N.; Roller, M.; Birtić, S. Rapid, cost-effective and accurate quantification of Yucca schidigera Roezl. steroidal saponins using HPLC-ELSD method. Food Chem. 2017, 221, 1245–1252. [Google Scholar]
- Thiede, J. Yucca Agavaceae; Springer: Berlin/Heidelberg, Germany, 2020; pp. 363–421. [Google Scholar]
- Su, J.-L.; Shi, B.-L.; Zhang, P.-F.; Sun, D.-S.; Li, T.-Y.; Yan, S.-M. Effects of yucca extract on feed efficiency, immune and antioxidative functions in broilers. Braz. Arch. Biol. Technol. 2016, 59, 16150035. [Google Scholar] [CrossRef]
- Gaber, M.M. The Effects of plant-protein-based diets supplemented with Yucca on growth, digestibility, and chemical composition of Nile tilapia (Oreochromis niloticus, L) Fingerlings. J. World Aquac. Soc. 2006, 37, 74–81. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; Oleszek, W. Saponins and phenolics of Yucca schidigera roezl: Chemistry and bioactivity. Phytochem. Rev. 2005, 4, 177–190. [Google Scholar] [CrossRef]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition bt-Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 241–254. [Google Scholar]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Park, S.-K.; Kang, S.-l.; Kang, H.-C.; Oh, H.-J.; Bae, C.-Y.; Bae, D.-H. Hypocholesterolemic property of Yucca schidigera and Quillaja saponaria extracts in human body. Arch. Pharmacal Res. 2003, 26, 1042–1046. [Google Scholar]
- Makkar, H.P.S.; Sen, S.; Blümmel, M.; Becker, K. Effects of fractions containing saponins from Yucca schidigera, Quillaja saponaria, and Acacia auriculoformis on rumen fermentation. J. Agric. Food Chem. 1998, 46, 4324–4328. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Newbold, C.J.; Rode, L.M.; Cheeke, P.R.; Cheng, K.J. Effects of Yucca schidigera extract on fermentation and degradation of steroidal saponins in the rumen simulation technique (rusitec)1contribution number: 3879739.1. Anim. Feed. Sci. Technol. 1998, 74, 143–153. [Google Scholar]
- Piacente, S.; Montoro, P.; Oleszek, W.; Pizza, C. Yucca schidigera bark: Phenolic constituents and antioxidant activity. J. Nat. Prod. 2004, 67, 882–885. [Google Scholar] [CrossRef]
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Resveratrol and other phenolics from the bark of Yucca schidigera roezl. J. Agric. Food Chem. 2001, 49, 747–752. [Google Scholar]
- Wang, L.; Wu, D.; Fan, Z.; Li, H.; Li, J.; Zhang, Y.; Xu, Q.; Wang, G.; Zhu, Z. Effect of Yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio). Fish Shellfish. Immunol. 2020, 103, 211–219. [Google Scholar]
- Kelly, A.M.; Kohler, C.C. Effects of Yucca shidigera extract on growth, nitrogen retention, ammonia excretion, and toxicity in channel catfish Ictalurus punctatus and hybrid tilapia Oreochromis mossambicus × O. niloticus. J. World Aquac. Soc. 2003, 34, 156–161. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Dietary supplementation with a quillaja saponin mixture improves growth performance and metabolic efficiency in common carp (Cyprinus carpio l.). Aquaculture 2002, 203, 311–320. [Google Scholar] [CrossRef]
- Elkhayat, B.; Mansour, A.; Fayed, W.; Omar, E.; Nour, A.A. The effect of Yucca schidigera extract dietary supplementation on growth performance, feed and protein utilization of European seabass, Dicentrarchus labrax, fingerlings. Egypt. J. Aquac. 2019, 9, 15–31. [Google Scholar] [CrossRef]
- Peterman, B.; Koppien-Fox, J.; Petrie-Hanson, L. A commercial blend of prebiotic fiber, oregano, thyme, cinnamon essential oils and Yucca schidigera (one current™) supplemented feed increased channel catfish fingerling growth and enhanced disease resistance. J. Aquac. Mar. Biol. Ecol. 2020, 2020, 1–16. [Google Scholar]
- El-Keredy, M.S.A.; Naena, N.A.A. Yucca plant as treatment for Pseudomonas aeruginosa infection in Nile tilapia farms with emphasis on its effect on growth performance. Alex. J. Vet. Sci. 2020, 66, 64. [Google Scholar]
- Chen, W.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Zhang, W.; Cai, Y. Effects of dietary soybean saponins on feed intake, growth performance, digestibility and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 2011, 318, 95–100. [Google Scholar]
- Bae, J.; Hamidoghli, A.; Won, S.; Choi, W.; Lim, S.-G.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Bai, S.C. Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 2020, 525, 735333. [Google Scholar]
- Tidwell, J.H.; Webster, C.D.; Clark, J.A.; Yancey, D.H. Effects of Yucca shidigera extract on water quality and fish growth in recirculating-water aquaculture systems. Progress. Fish-Culturist 1992, 54, 196–201. [Google Scholar] [CrossRef]
- Angeles, I.P., Jr.; Gallego, L.M.; Navarro, M.A.M.; Chien, Y.-H. Dietary effects of Quillaja saponaria and Yucca schidigera extract on rearing performance of Nile tilapia Oreochromis niloticus l. And its antioxidant capacity and metabolic response following hypoxic stress. Int. J. Agric. Technol. 2017, 13, 2249–2266. [Google Scholar]
- Güroy, B.; Mantoğlu, S.; Kayalı, S.; Şahin, İ. Effect of dietary Yucca schidigera extract on growth, total ammonia–nitrogen excretion and haematological parameters of juvenile striped catfish Pangasianodon hypophthalmus. Aquac. Res. 2014, 45, 647–654. [Google Scholar] [CrossRef]
- El-Saidy, D.M.S.D.; Gaber, M.M.A. Effect of yucca (Yucca shidigera) on water qualityand growth performances of Nile tilapia (Oreochromis niloticus l.) fingerlings. Egypt. J. Aquat. Biol. Fish. 2004, 8, 33–49. [Google Scholar]
- Francis, G.; Makkar, H.P.; Becker, K. Effects of Quillaja saponins on growth, metabolism, egg production and muscle cholesterol in individually reared Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 129, 105–114. [Google Scholar] [CrossRef]
- Acosta, M.H.; Salazar, G.J.G.; Saenz, F.M.G.; Guzman, G.A.; Alvarez-González, C.A.; Acevedo, E.A.L.; Fitzsimmons, K. The effects of Yucca schidigera and Quillaja saponaria on growth performance and enzymes activities of juvenile shrimp Litopenaeus vannamei cultured in low salinity water. Lat. Am. J. Aquat. Res. 2016, 44, 121–128. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Teiba, I.I.; Zakic, M.A.A.; Alabssawy, A.N.; El-Hais, A.M.; Gabr, A.A.; Dawood, M.A.; Zaineldin, A.I.; Mzengereza, K.; Shadrack, R.S.; et al. Assessing the effectiveness of CoQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2019, 12, 987–1002. [Google Scholar] [CrossRef]
- Capkin, E.; Ozcelep, T.; Kayis, S.; Altinok, I. Antimicrobial agents, triclosan, chloroxylenol, methylisothiazolinone and borax, used in cleaning had genotoxic and histopathologic effects on rainbow trout. Chemosphere 2017, 182, 720–729. [Google Scholar] [CrossRef] [PubMed]
- El Basuini, M.F.; Shahin, S.A.; Teiba, I.I.; Zaki, M.A.A.; El-Hais, A.M.; Sewilam, H.; Almeer, R.; Abdelkhalek, N.; Dawood, M.A.O. The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 531, 735862. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Gewaily, M.S.; Monier, M.N.; Younis, E.M.; Van Doan, H.; Sewilam, H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 2021, 534, 736287. [Google Scholar]
- Amoah, Y.T.; Reddy, A.V.B.; Lee, S.; Bae, J.; Won, S.; Seong, M.; Bai, S.C. Evaluation of different dietary additives based on growth performance, innate immunity and disease resistance in juvenile Amur catfish, Silurus asotus. Int. Aquat. Res. 2017, 9, 351–360. [Google Scholar] [CrossRef]
- Reham, A.A.; Mounes, H.A.M.; Ahmed, K.M. Use of humic acid and yucca extract as a benefactor on water quality and their impact on some hematological and histological parameters of Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2019, 22, 447–460. [Google Scholar]
- Yeh, S.-P.; Liu, C.-H.; Sung, T.-G.; Lee, P.-P.; Cheng, W. Effect of saponin on hematological and immunological parameters of the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture 2006, 261, 1432–1439. [Google Scholar] [CrossRef]
- Kawakami, H.; Yamashita, H.; Sakai, M. Comparative sensitivity of yellowtail Seriola quinqueradiata and goldstriped amberjack S. aureovittata to photobacterium damsela subsp. piscicida. J. World Aquac. Soc. 2000, 31, 213–217. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Chikwati, E.M.; Venold, F.F.; Penn, M.H.; Rohloff, J.; Refstie, S.; Guttvik, A.; Hillestad, M.; Krogdahl, Å. Interaction of soyasaponins with plant ingredients in diets for Atlantic salmon, Salmo salar L. Br. J. Nutr. 2011, 107, 1570–1590. [Google Scholar] [CrossRef]
- Knudsen, D.; Jutfelt, F.; Sundh, H.; Sundell, K.; Koppe, W.; Frøkiær, H. Dietary soya saponins increase gut permeability and play a key role in the onset of soyabean-induced enteritis in Atlantic salmon (Salmo salar l.). Br. J. Nutr. 2008, 100, 120–129. [Google Scholar] [CrossRef]
- Couto, A.; Kortner, T.M.; Penn, M.; Bakke, A.M.; Krogdahl, Å.; Oliva-Teles, A. Effects of dietary soy saponins and phytosterols on gilthead sea bream (Sparus aurata) during the on-growing period. Anim. Feed. Sci. Technol. 2014, 198, 203–214. [Google Scholar] [CrossRef]
- Couto, A.; Kortner, T.M.; Penn, M.; Østby, G.; Bakke, A.M.; Krogdahl, Å.; Oliva-Teles, A. Saponins and phytosterols in diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on growth, intestinal morphology and physiology. Aquac. Nutr. 2015, 21, 180–193. [Google Scholar] [CrossRef]
- Blier, P. Fish health: An oxidative stress perspective. Fish. Aquac. J. 2014, 5, 4172. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2020, 1–34. [Google Scholar] [CrossRef]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A.O. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abdel-Tawwab, M.; Abdel-Latif, H.M.R. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev. Aquac. 2020, 12, 2511–2526. [Google Scholar] [CrossRef]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research: 0; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wu, P.; Liu, Y.; Jiang, W.D.; Jiang, J.; Zhao, J.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: Different change patterns of varied antioxidant enzyme genes and nrf2 signaling factors. PLoS ONE 2017, 12, e0169888. [Google Scholar] [CrossRef]
- Martinez–Alvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Stratev, D.; Zhelyazkov, G.; Noundou, X.S.; Krause, R.W.M. Beneficial effects of medicinal plants in fish diseases. Aquac. Int. 2018, 26, 289–308. [Google Scholar] [CrossRef]
- Mehrinakhi, Z.; Ahmadifar, E.; Sheikhzadeh, N.; Moghadam, M.S.; Dawood, M.A. Extract of grape seed enhances the growth performance, humoral and mucosal immunity, and resistance of common carp (Cyprinus carpio) against Aeromonas hydrophila. Ann. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Van Doan, H.; Ahmed, H.A.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Oregano (Origanum vulgare), st John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 2020, 18, 100445. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Toxic Effects of Ammonia, Nitrite, and Nitrate to Decapod Crustaceans: A Review on Factors Influencing their Toxicity, Physiological Consequences, and Coping Mechanisms. Rev. Fish. Sci. 2013, 21, 1–21. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of total ammonia nitrogen (tan), nitrate and total organic carbon (toc) from aquaculture wastewater using electrochemical technology: A review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, D.; Li, S.; Zhang, Y.; Aweya, J.J. Effects of ammonia on shrimp physiology and immunity: A review. Rev. Aquac. 2020, 12, 2194–2211. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334–337, 12–23. [Google Scholar] [CrossRef]
- Ip, A.Y.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Vatnikov, Y.A.; Kulikov, E.V.; Plushikov, V.G.; Drukovsky, S.G.; Hoseinifar, S.H.; Van Doan, H. The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 2020, 526, 735400. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Qi, X.-Z.; Xue, M.-Y.; Ming-Yang, X.; Zha, J.-W.; Wang, G.-X.; Ling, F. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish. Immunol. 2017, 70, 485–492. [Google Scholar] [CrossRef]
- Yue, F.; Pan, L.; Xie, P.; Zheng, D.; Li, J. Immune responses and expression of immune-related genes in swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 246–251. [Google Scholar] [CrossRef]
- Boyed, C. Water quality for pond aquaculture. Res. Dev. Ser. 1998, 37. [Google Scholar]
- Dawood, M.A.O.; Shukry, M.; Zayed, M.M.; Omar, A.A.; Zaineldin, A.I.; El Basuini, M.F. Digestive enzymes, immunity and oxidative status of Nile tilapia (Oreochromis niloticus) reared in intensive conditions. Slov. Vet. Res. 2019, 56 (Suppl. 22), 99–108. [Google Scholar]
- Headon, D.; Dawson, K. Yucca extract controls atmospheric ammonia levels. Feedstuffs 1990, 62, 15–16. [Google Scholar]
- Kishawy, A.T.Y.; Sewid, A.H.; Nada, H.S.; Kamel, M.A.; El-Mandrawy, S.A.M.; Abdelhakim, T.M.N.; El-Murr, A.E.I.; Nahhas, N.E.; Hozzein, W.N.; Ibrahim, D. Mannanoligosaccharides as a carbon source in Biofloc boost dietary plant protein and water quality, growth, immunity and Aeromonas hydrophila resistance in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A.O. Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability. Animals 2021, 11, 93. https://doi.org/10.3390/ani11010093
Paray BA, El-Basuini MF, Alagawany M, Albeshr MF, Farah MA, Dawood MAO. Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability. Animals. 2021; 11(1):93. https://doi.org/10.3390/ani11010093
Chicago/Turabian StyleParay, Bilal Ahamad, Mohamed F. El-Basuini, Mahmoud Alagawany, Mohammed Fahad Albeshr, Mohammad Abul Farah, and Mahmoud A. O. Dawood. 2021. "Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability" Animals 11, no. 1: 93. https://doi.org/10.3390/ani11010093
APA StyleParay, B. A., El-Basuini, M. F., Alagawany, M., Albeshr, M. F., Farah, M. A., & Dawood, M. A. O. (2021). Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability. Animals, 11(1), 93. https://doi.org/10.3390/ani11010093