Effects of In Vitro Interactions of Oviduct Epithelial Cells with Frozen–Thawed Stallion Spermatozoa on Their Motility, Viability and Capacitation Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Culture Media
2.3. Cryopreserved Stallion Sperm Handling and Processing
2.3.1. Effect of Incubation Time, Sample Concentration and Centrifugation Time on Sperm Motility
2.3.2. Sperm Motility Assay
2.4. Oviducts Collection
2.4.1. Oviductal Cell Collection and Cultures
2.4.2. E-Cadherin and Cytokeratin-7 MRNA Expression in OEC Primary Cultures
2.4.3. Cytokeratin and E-Cadherin Distribution in OEC Primary Cultures
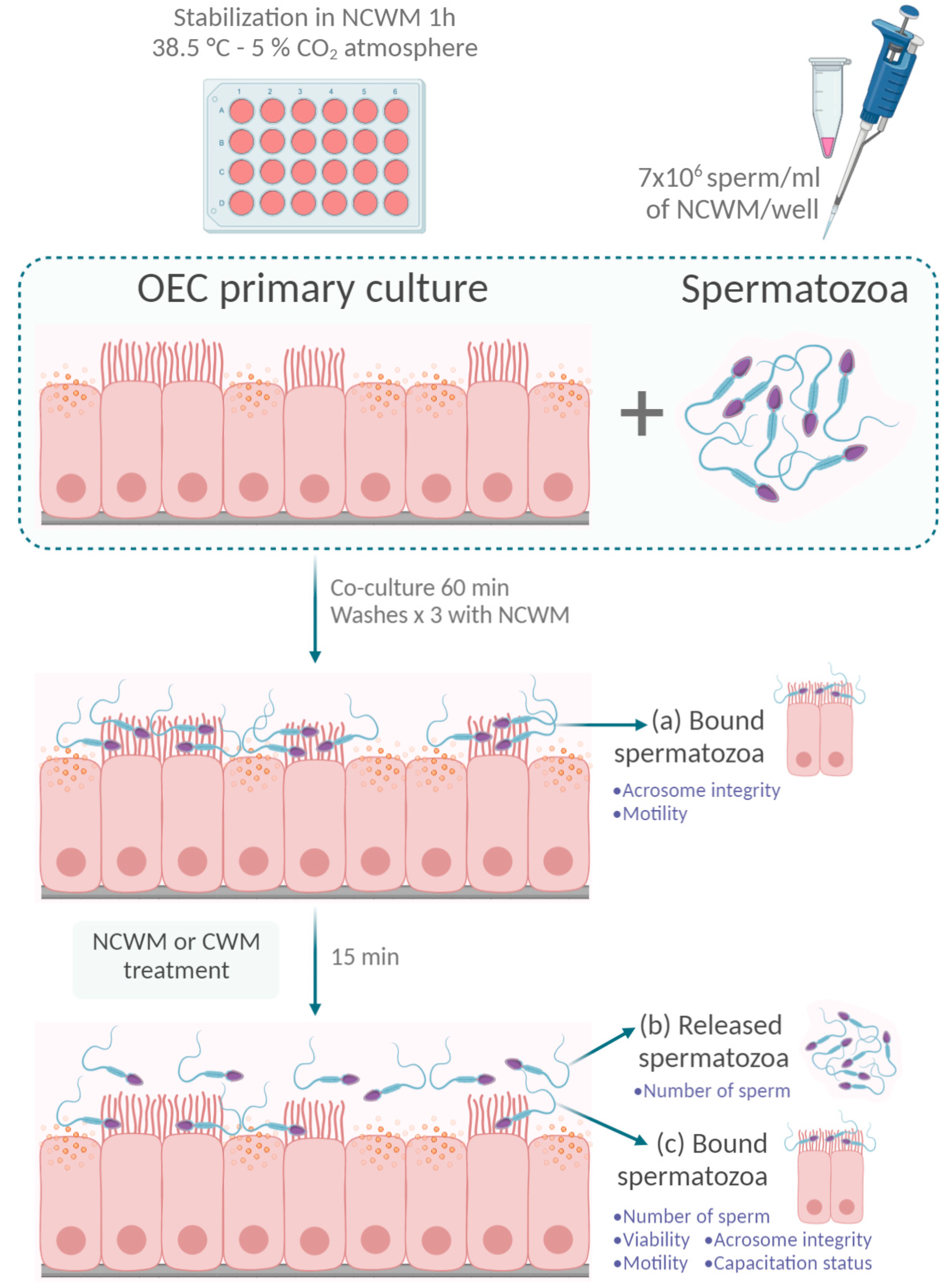
2.5. Co-Cultures of OECs and Spermatozoa
Evaluation of Acrosome Status of Sperm Bound to OEC Primary Cultures
2.6. Retrieval of the Released and Bound Sperm Population after the Incubation of the Co-Cultures under Capacitating Conditions
2.6.1. Evaluation of the Number of Sperm Bound to the OECs after Capacitating Treatment
2.6.2. Evaluation of the Number of Sperm Released from the OECs under Capacitating Conditions
2.6.3. Evaluation of Viability of the Released Sperm Population from the OECs under Capacitating Conditions
2.6.4. Evaluation of Acrosome Status of the Released Sperm Population from the OECs under Capacitating Conditions
2.6.5. Evaluation of Total Motility of the Released Sperm Population from the OECs under Capacitating Conditions
2.6.6. Evaluation of Capacitation Status of the Released Sperm Population from the OECs under Capacitating Conditions
2.7. Statistical Analysis
3. Results
3.1. Effect of Different Processing Conditions on Sperm Motility
3.2. OEC Primary Culture Characterization
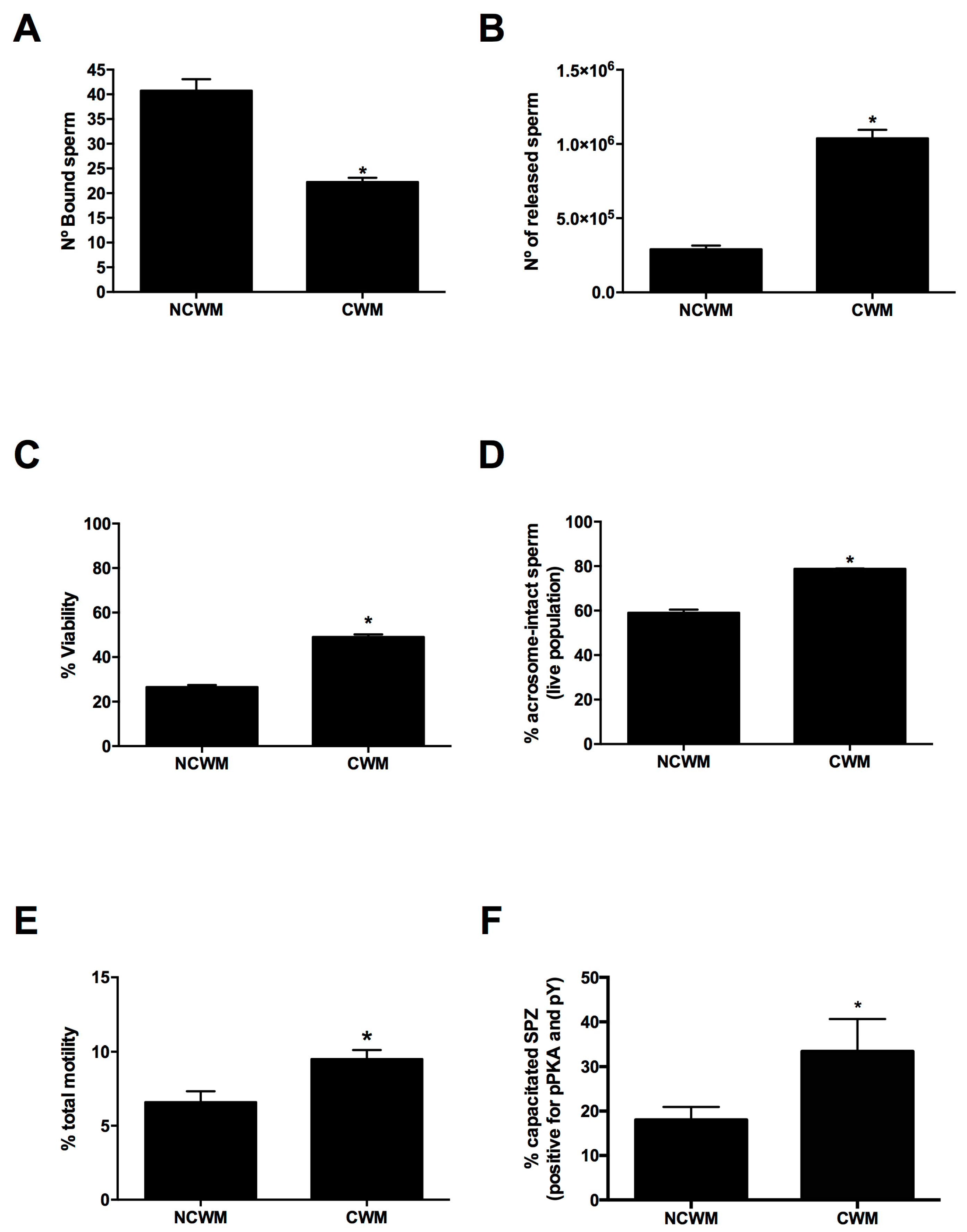
3.3. Characterization of Cryopreserved Semen Bound to OEC Primary Cultures
3.4. Characterization of Cryopreserved Sperm Released after Co-Culture with OECs under Capacitating Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tecirlioglu, R.T.; Trounson, A.O. Embryonic stem cells in companion animals (horses, dogs and cats): Present status and future prospects. Reprod. Fertil. Dev. 2007, 19, 740–747. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.; Léguillette, R.; Richard, E.A.; Couetil, L.; Lavoie, J.P.; Martin, J.G.; Pirie, R.S. Equine asthma: Integrative biologic relevance of a recently proposed nomenclature. J. Vet. Intern. Med. 2018, 32, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, S.M.; Ellis, M.W.; Luo, J. Large Animal Models for the Clinical Application of Human Induced Pluripotent Stem Cells. Stem Cells Dev. 2019, 28, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.G.; Betts, D.H. Stem cell therapy for joint problems using the horse as a clinically relevant animal model. Expert Opin. Biol. Ther. 2007, 7, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.G.G.; Hasler, J.F.F. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Treulen, F.; Aguila, L.; Arias, M.E.; Jofré, I.; Felmer, R. Impact of post-thaw supplementation of semen extender with antioxidants on the quality and function variables of stallion spermatozoa. Anim. Reprod. Sci. 2019, 201, 71–83. [Google Scholar] [CrossRef]
- Ball, B.A.; Vo, A.T.; Baumber, J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001, 62, 508–515. [Google Scholar] [CrossRef]
- Sieme, H.; Harrison, R.A.P.; Petrunkina, A.M. Cryobiological determinants of frozen semen quality, with special reference to stallion. Anim. Reprod. Sci. 2008, 107, 276–292. [Google Scholar] [CrossRef]
- Vidament, M.; Dupere, A.M.; Julienne, P.; Evain, A.; Noue, P.; Palmer, E. Equine frozen semen: Freezability and fertility field results. Theriogenology 1997, 48, 907–917. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; Papa, F.O.; Ramires Neto, C. Advances in Stallion Semen Cryopreservation. Vet. Clin. N. Am. Equine Pract. 2016, 32, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, S.; Bollwein, H.; Siuda, M.; Handler, J. Effects of Different Freezing Protocols on Motility, Viability, Mitochondrial Membrane Potential, Intracellular Calcium Level, and DNA Integrity of Cryopreserved Equine Epididymal Sperm. J. Equine Vet. Sci. 2019, 82. [Google Scholar] [CrossRef] [PubMed]
- Allen, W. The Development and Application of the Modern Reproductive Technologies to Horse Breeding. Reprod. Domest. Anim. 2005, 40, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in the horse? The equine oviduct as a microenvironment for capacitation/fertilization. Reproduction 2016, 152, R233–R245. [Google Scholar] [CrossRef]
- Leemans, B.; Stout, T.A.E.; De Schauwer, C.; Heras, S.; Nelis, H.; Hoogewijs, M.; Van Soom, A.; Gadella, B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction 2019, 157, R181–R197. [Google Scholar] [CrossRef]
- Hinrichs, K. Assisted reproductive techniques in mares. Reprod. Domest. Anim. 2018, 53, 4–13. [Google Scholar] [CrossRef]
- Bedford, S.J.; Kurokawa, M.; Hinrichs, K.; Fissore, R.A. Patterns of Intracellular Calcium Oscillations in Horse Oocytes Fertilized by Intracytoplasmic Sperm Injection: Possible Explanations for the Low Success of This Assisted Reproduction Technique in the Horse. Biol. Reprod. 2004, 70, 936–944. [Google Scholar] [CrossRef]
- Morris, L.H.A. The development of in vitro embryo production in the horse. Equine Vet. J. 2018, 50, 712–720. [Google Scholar] [CrossRef]
- Squires, E.L. Integration of future biotechnologies into the equine industry. Anim. Reprod. Sci. 2005, 89. [Google Scholar] [CrossRef]
- Holt, W.V.; Elliott, R.M.; Fazeli, A.; Sostaric, E.; Georgiou, A.S.; Satake, N.; Prathalingam, N.; Watson, P.F. Harnessing the biology of the oviduct for the benefit of artificial insemination. Soc. Reprod. Fertil. Suppl. 2006, 62, 247–259. [Google Scholar] [PubMed]
- Li, X.; Morris, L.H.A.; Allen, W.R. Influence of co-culture during maturation on the developmental potential of equine oocytes fertilized by intracytoplasmic sperm injection (ICSI). Reproduction 2001, 121, 925–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ellington, J.E.; Ignotz, G.G.; Varner, D.D.; Marcucio, R.S.; Mathison, P.; Ball, B.A. In vitro interaction between oviduct epithelial and equine sperm. Arch. Androl. 1993, 31, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Aldarmahi, A.; Elliott, S.; Russell, J.; Klonisch, T.; Hombach-Klonisch, S.; Fazeli, A. Characterisation of an in vitro system to study maternal communication with spermatozoa. Reprod. Fertil. Dev. 2012, 24, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Dobrinski, I.; Ignotz, G.G.; Thomas, P.G.; Ball, B.A. Role of carbohydrates in the attachment of equine spermatozoa to uterine tubal (oviductal) epithelial cells in vitro. Am. J. Vet. Res. 1996, 57, 1635–1639. [Google Scholar]
- Dobrinski, I.; Suarez, S.S.; Ball, B.A. Intracellular calcium concentration in equine spermatozoa attached to oviductal epithelial cells in vitro. Biol. Reprod. 1996, 54, 783–788. [Google Scholar] [CrossRef]
- Suarez, S.; Redfern, K.; Raynor, P.; Martin, F.; Phillips, D.M. Attachment of boar sperm to mucosal explants of oviduct in vitro: Possible role in formation of a sperm reservoir. Biol. Reprod. 1991, 44, 998–1004. [Google Scholar] [CrossRef]
- Gualtieri, R.; Talevi, R. In vitro-cultured bovine oviductal cells bind acrosome-intact sperm and retain this ability upon sperm release. Biol. Reprod. 2000, 62, 1754–1762. [Google Scholar] [CrossRef]
- Ardón, F.; Helms, D.; Sahin, E.; Bollwein, H.; Töpfer-Petersen, E.; Waberski, D. Chromatin-unstable boar spermatozoa have little chance of reaching oocytes in vivo. Reproduction 2008, 135, 461–470. [Google Scholar] [CrossRef][Green Version]
- Thomas, P.G.A.; Ball, B.A.; Miller, P.G.; Brinsko, S.P.; Southwood, L. A Subpopulation of Morphologically Normal, Motile Spermatozoa Attach to Equine Oviductal Epithelial Cell Monolayers. Biol. Reprod. 1994, 51, 303–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellington, J.E.; Samper, J.C.; Jones, A.E.; Oliver, S.A.; Burnett, K.M.; Wright, R.W. In vitro interactions of cryopreserved stallion spermatozoa and oviduct (uterine tube) epithelial cells or their secretory products. Anim. Reprod. Sci. 1999, 56, 51–65. [Google Scholar] [CrossRef]
- Thomas, P.G.A.; Ball, B.A.; Brinsko, S.P. Interaction of Equine Spermatozoa with Oviduct Epithelial Cell Explants Is Affected by Estrous Cycle and Anatomic Origin of Explant. Biol. Reprod. 1994, 228, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, R.; Suarez, S.S. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol. Reprod. 1996, 54, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A.; Duncan, A.E.; Watson, P.F.; Holt, W. V Sperm-oviduct interaction: Induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol. Reprod. 1999, 60, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Osycka-Salut, C.E.; Castellano, L.; Fornes, D.; Beltrame, J.S.; Alonso, C.A.I.; Jawerbaum, A.; Franchi, A.; Díaz, E.S.; Perez Martinez, S. Fibronectin From Oviductal Cells Fluctuates During the Estrous Cycle and Contributes to Sperm–Oviduct Interaction in Cattle. J. Cell. Biochem. 2017, 118, 4095–4108. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Rapanelli, M.; Ribeiro, M.L.; Farina, M.; Billi, S.; Franchi, A.M.; Perez Martinez, S. The endocannabinoid system in bull sperm and bovine oviductal epithelium: Role of anandamide in sperm-oviduct interaction. Reproduction 2009, 137, 403–414. [Google Scholar] [CrossRef]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta 2008, 1784, 106–115. [Google Scholar] [CrossRef]
- Visconti, P.E.; Westbrook, V.A.; Chertihin, O.; Demarco, I.; Sleight, S.; Diekman, A.B. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002, 53, 133–150. [Google Scholar] [CrossRef]
- Buffone, M.G.; Wertheimer, E.V.; Visconti, P.E.; Krapf, D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 2014, 1842, 2610–2620. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 1994; pp. 189–317. [Google Scholar]
- McPartlin, L.; Suarez, S.S.; Czaya, C.; Hinrichs, K.; Bedford-Guaus, S.J. Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol. Reprod. 2009, 81, 199–206. [Google Scholar] [CrossRef] [PubMed]
- McPartlin, L.A.; Littell, J.; Mark, E.; Nelson, J.L.; Travis, A.J.; Bedford-Guaus, S.J. A defined medium supports changes consistent with capacitation in stallion sperm, as evidenced by increases in protein tyrosine phosphorylation and high rates of acrosomal exocytosis. Theriogenology 2008, 69, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Galantino-homer, H.L.; Visconti, P.E.; Kopf, G.S. Regulation of Protein Tyrosine Phosphorylation during Bovine Sperm Capacitation by a Cyclic Adenosine 3′, 5′ -Monophosphate-Dependent Pathway. Biol. Reprod. 1997, 719, 707–719. [Google Scholar] [CrossRef]
- Osycka-Salut, C.; Gervasi, M.G.; Pereyra, E.; Cella, M.; Ribeiro, M.L.; Franchi, A.M.; Perez-Martinez, S. Anandamide induces sperm release from oviductal epithelia through nitric oxide pathway in bovines. PLoS ONE 2012, 7, e30671. [Google Scholar] [CrossRef]
- Kinger, S.; Rajalakshmi, M. Assessment of the vitality and acrosomal status of human spermatozoa using fluorescent probes. Int. J. Androl. 1995, 18, 12–18. [Google Scholar] [CrossRef]
- Leemans, B.; Stout, T.A.E.; Van Soom, A.; Gadella, B.M. pH dependent effects of procaine on equine gametes. Biol. Reprod. 2019, 101, 1056–1074. [Google Scholar] [CrossRef]
- Aurich, J.; Kuhl, J.; Tichy, A.; Aurich, C. Efficiency of Semen Cryopreservation in Stallions. Animals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Höfner, L.; Luther, A.M.; Waberski, D. The role of seminal plasma in the liquid storage of spermatozoa. Anim. Reprod. Sci. 2020, 2020, 106290. [Google Scholar] [CrossRef]
- Salazar, J.L.; Teague, S.R.; Love, C.C.; Brinsko, S.P.; Blanchard, T.L.; Varner, D.D. Effect of cryopreservation protocol on postthaw characteristics of stallion sperm. Theriogenology 2011, 76, 409–418. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.M.; Sabeur, K.; Ball, B.A. Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology 2008, 69, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Ellerbrock, R.E.; Honorato, J.; Curcio, B.R.; Stewart, J.L.; Souza, J.A.T.; Love, C.C.; Lima, F.S.; Canisso, I.F. Effect of urine contamination on stallion semen freezing ability. Theriogenology 2018, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dobrinski, I.; Thomas, P.G.A.; Ball, B.A. Cryopreservation Reduces the Ability of Equine Spermatozoa to Attach to Oviductal Epithelial Cells and Zonae Pellucidae In Vitro. J. Androl. 1995, 16, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.S.; Blanchard, T.L.; Brinsko, S.P.; Varner, D.D.; Hinrichs, K.; Love, C.C. The “dilution effect” in stallion sperm. Theriogenology 2015, 83, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Irusta, A.; Dominguez, E.M.; Marín-Briggiler, C.I.; Matamoros-Volante, A.; Lucchesi, O.; Tomes, C.N.; Treviño, C.L.; Buffone, M.G.; Lascano, R.; Losinno, L.; et al. Reactive oxygen species are involved in the signaling of equine sperm chemotaxis. Reproduction 2020, 159, 423–436. [Google Scholar] [CrossRef]
- Martins, H.S.; Filho, O.A.M.; Araujo, M.S.S.; Martins, N.R.; De Albuquerque Lagares, M. Evaluation of fertilizing ability of frozen equine sperm using a bovine zona pellucida binding assay. Cryo-Letters 2018, 39, 298–305. [Google Scholar]
- Al-Essawe, E.M.; Wallgren, M.; Wulf, M.; Aurich, C.; Macías-García, B.; Sjunnesson, Y.; Morrell, J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 2018, 115, 99–107. [Google Scholar] [CrossRef]
- Macías García, B.; Morrell, J.M.; Ortega-Ferrusola, C.; González-Fernández, L.; Tapia, J.A.; Rodriguez-Martínez, H.; Peña, F.J. Centrifugation on a single layer of colloid selects improved quality spermatozoa from frozen-thawed stallion semen. Anim. Reprod. Sci. 2009, 114, 193–202. [Google Scholar] [CrossRef]
- Ruiz-Díaz, S.; Oseguera-López, I.; De La Cuesta-Díaz, D.; García-López, B.; Serres, C.; Sanchez-Calabuig, M.J.; Gutiérrez-Adán, A.; Perez-Cerezales, S. The presence of d-penicillamine during the in vitro capacitation of stallion spermatozoa prolongs hyperactive-like motility and allows for sperm selection by thermotaxis. Animals 2020, 10, 1467. [Google Scholar] [CrossRef]
- Choi, Y.H.; Landim-Alvarenga, F.C.; Seidel, G.E.; Squires, E.L. Effect of capacitation of stallion sperm with polyvinylalcohol or bovine serum albumin on penetration of bovine zona-free or partially zona-removed equine oocytes. J. Anim. Sci. 2003, 81, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Pommer, A.C.; Rutllant, J.; Meyers, S.A. Phosphorylation of protein tyrosine residues in fresh and cryopreserved stallion spermatozoa under capacitating conditions. Biol. Reprod. 2003, 68, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Sabatini, C.; Przybył, A.; Ciaramelli, A.; Panzani, D.; Camillo, F. Post-thaw Addition of Caffeine and/or Pentoxifylline Affect Differently Motility of Horse and Donkey-Cryopreserved Spermatozoa. J. Equine Vet. Sci. 2019, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Marzano, G.; Moscatelli, N.; Di Giacomo, M.; Martino, N.A.; Lacalandra, G.M.; Dell’aquila, M.E.; Maruccio, G.; Primiceri, E.; Chiriacò, M.S.; Zara, V.; et al. Centrifugation force and time alter CASA parameters and oxidative status of cryopreserved stallion sperm. Biology 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef]
- Katila, T. In vitro evaluation of frozen-thawed stallion semen: A review. Acta Vet. Scand. 2001, 42, 199–217. [Google Scholar] [CrossRef]
- Miller, D.J. Review: The epic journey of sperm through the female reproductive tract. Animals 2018, 1–11. [Google Scholar] [CrossRef]
- Suarez, S.S. Formation of a reservoir of sperm in the oviduct. Reprod. Domest. Anim. 2002, 37, 140–143. [Google Scholar] [CrossRef]
- Miller, D.J. Regulation of Sperm Function by Oviduct Fluid and the Epithelium: Insight into the Role of Glycans. Reprod. Domest. Anim. 2015, 50, 31–39. [Google Scholar] [CrossRef]
- Martínez-León, E.; Osycka-Salut, C.; Signorelli, J.; Pozo, P.; Perez, B.; Kong, M.; Morales, P.; Perez-Martinez, S.; Diaz, E.S. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum Reprod 2015, 30, 2138–2151. [Google Scholar] [CrossRef]
- Talevi, R.; Gualtieri, R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology 2010, 73, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Choi, Y.H.; Love, C.C.; Love, L.B.; Varner, D.D.; Brinsko, S.; Hinrichs, K. Developmental competence in vivo and in vitro of in vitro-matured equine oocytes fertilized by intracytoplasmic sperm injection with fresh or frozen-thawed spermatozoa. Reproduction 2002, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, S.; Lagutina, I.; Lazzari, G.; Rodriguez-Martinez, H.; Galli, C.; Morrell, J.M. New Methods for Selecting Stallion Spermatozoa for Assisted Reproduction. J. Equine Vet. Sci. 2011, 31, 536–541. [Google Scholar] [CrossRef]
- Choi, Y.H.; Velez, I.C.; Macías-García, B.; Riera, F.L.; Ballard, C.S.; Hinrichs, K. Effect of clinically-related factors on in vitro blastocyst development after equine ICSI. Theriogenology 2016, 85, 1289–1296. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Use of microfluidics to sort stallion sperm for intracytoplasmic sperm injection. Anim. Reprod. Sci. 2019, 202, 1–9. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Association of equine sperm population parameters with outcome of intracytoplasmic sperm injections. Theriogenology 2018, 119, 114–120. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno, B.F.; Bariani, M.V.; Laiz-Quiroga, L.; Martínez-León, E.; Von-Meyeren, M.; Rey, O.; Mutto, A.Á.; Osycka-Salut, C.E. Effects of In Vitro Interactions of Oviduct Epithelial Cells with Frozen–Thawed Stallion Spermatozoa on Their Motility, Viability and Capacitation Status. Animals 2021, 11, 74. https://doi.org/10.3390/ani11010074
Gimeno BF, Bariani MV, Laiz-Quiroga L, Martínez-León E, Von-Meyeren M, Rey O, Mutto AÁ, Osycka-Salut CE. Effects of In Vitro Interactions of Oviduct Epithelial Cells with Frozen–Thawed Stallion Spermatozoa on Their Motility, Viability and Capacitation Status. Animals. 2021; 11(1):74. https://doi.org/10.3390/ani11010074
Chicago/Turabian StyleGimeno, Brenda Florencia, María Victoria Bariani, Lucía Laiz-Quiroga, Eduardo Martínez-León, Micaela Von-Meyeren, Osvaldo Rey, Adrián Ángel Mutto, and Claudia Elena Osycka-Salut. 2021. "Effects of In Vitro Interactions of Oviduct Epithelial Cells with Frozen–Thawed Stallion Spermatozoa on Their Motility, Viability and Capacitation Status" Animals 11, no. 1: 74. https://doi.org/10.3390/ani11010074
APA StyleGimeno, B. F., Bariani, M. V., Laiz-Quiroga, L., Martínez-León, E., Von-Meyeren, M., Rey, O., Mutto, A. Á., & Osycka-Salut, C. E. (2021). Effects of In Vitro Interactions of Oviduct Epithelial Cells with Frozen–Thawed Stallion Spermatozoa on Their Motility, Viability and Capacitation Status. Animals, 11(1), 74. https://doi.org/10.3390/ani11010074




