Resource Selection by Wild and Ranched White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Season in Florida
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
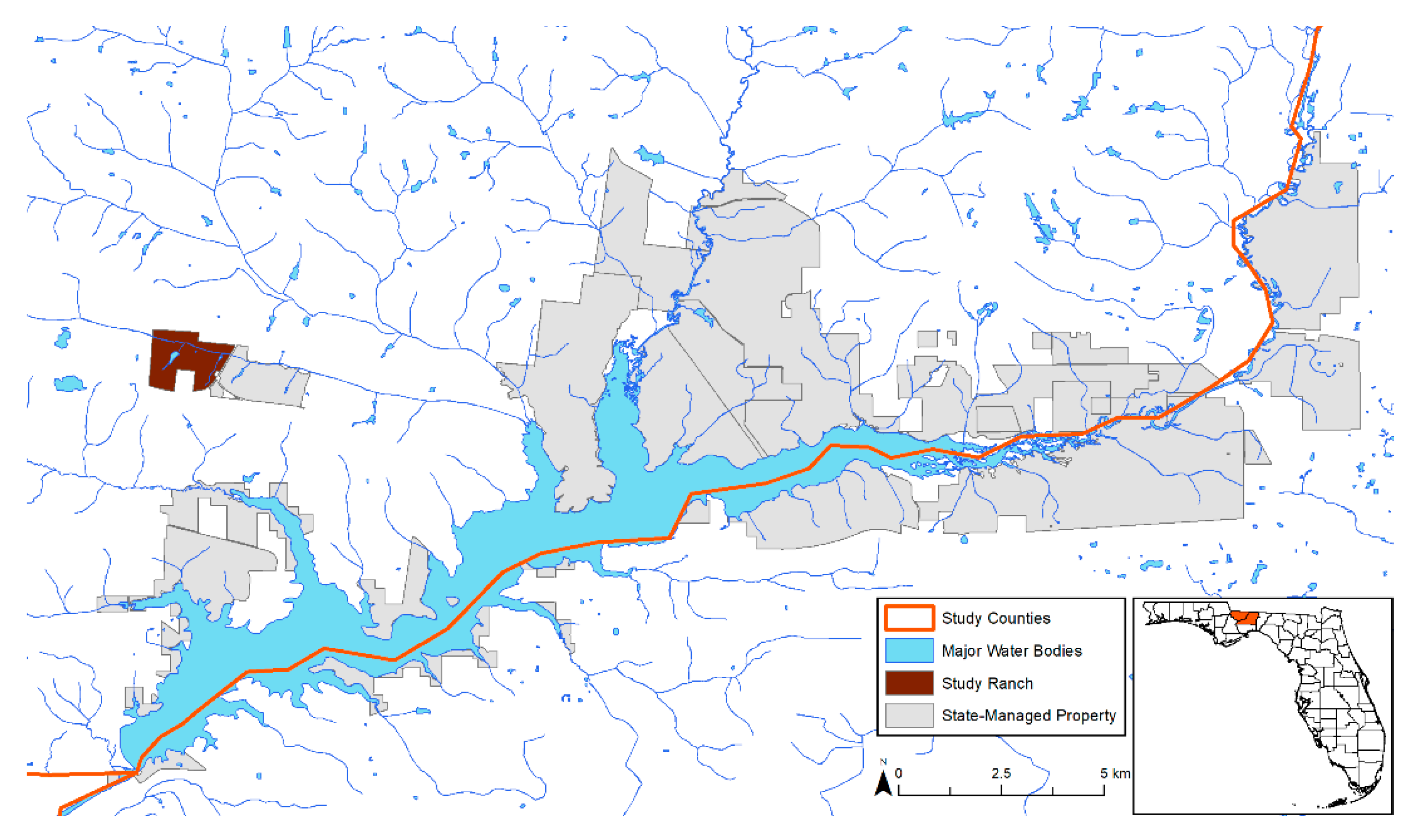
2.1. Study Areas
2.2. Capture, Handling, and Telemetry Data Collection
2.3. Explanatory Environmental Variables
2.4. Resource Selection Model Development
3. Results
3.1. Data Summary
3.2. Resource Selection Models
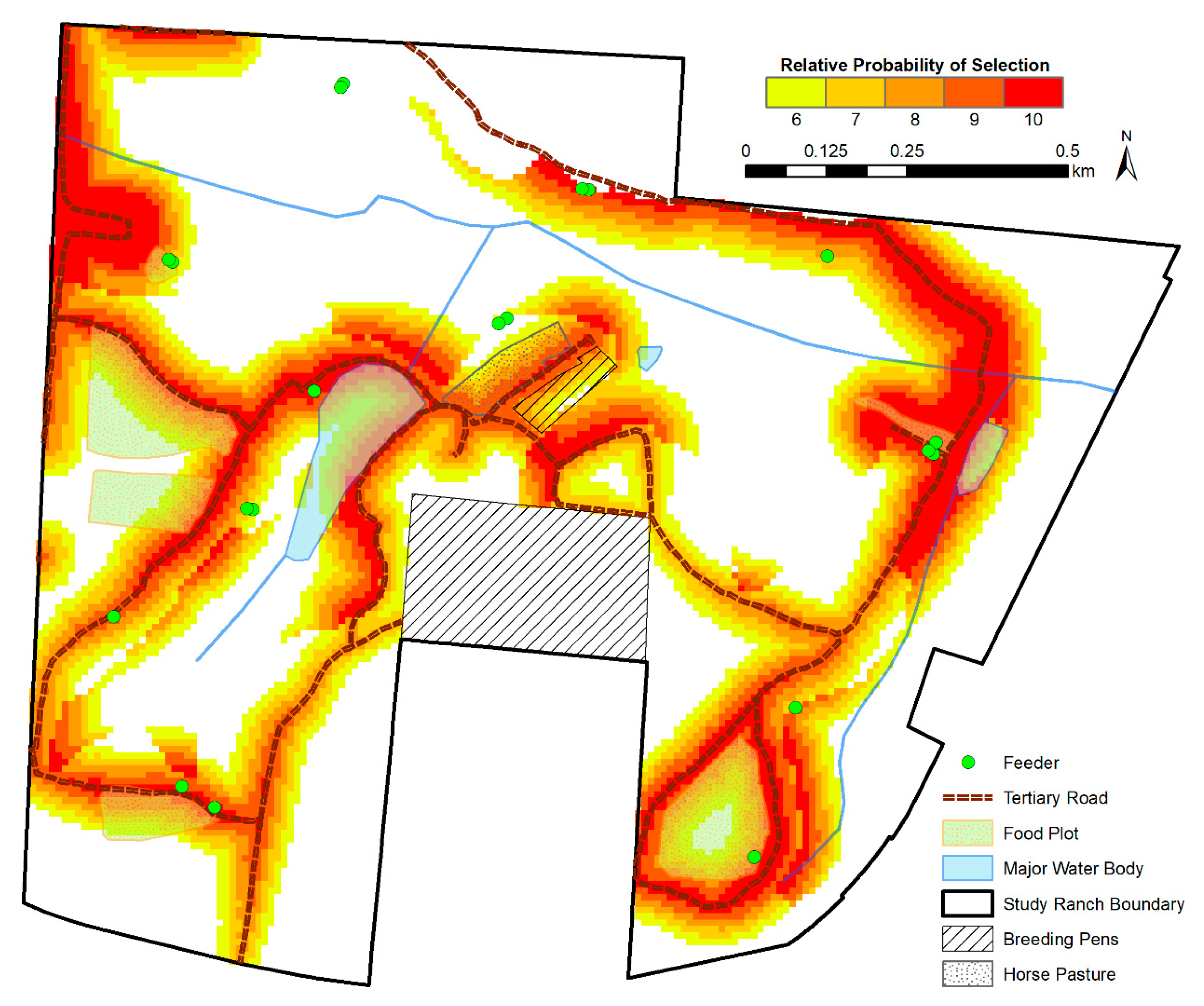
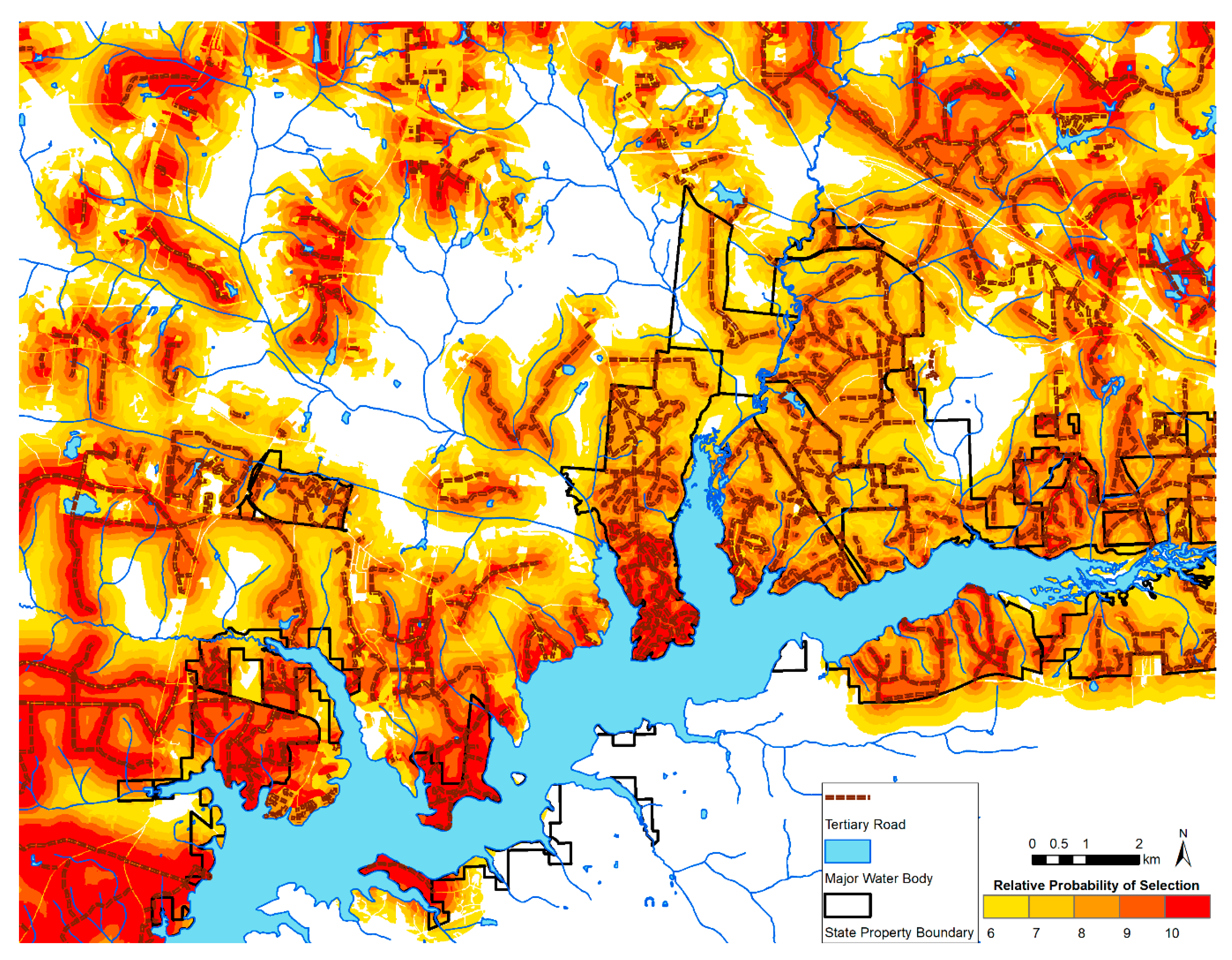
3.3. Spatial Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reisen, W.K. Landscape Epidemiology of Vector-Borne Diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef]
- Morris, L.R.; Proffitt, K.M.; Asher, V.; Blackburn, J.K. Elk Resource Selection and Implications for Anthrax Management in Montana. J. Wildl. Manag. 2016, 80, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, K.M.; Gude, J.A.; Hamlin, K.L.; Garrott, R.A.; Cunningham, J.A.; Grigg, J.L. Elk Distribution and Spatial Overlap with Livestock during the Brucellosis Transmission Risk Period. J. Appl. Ecol. 2011, 48, 471–478. [Google Scholar] [CrossRef]
- Ruder, M.G.; Lysyk, T.J.; Stallknecht, D.E.; Foil, L.D.; Johnson, D.J.; Chase, C.C.; Dargatz, D.A.; Gibbs, E.P.J. Transmission and Epidemiology of Bluetongue and Epizootic Hemorrhagic Disease in North America: Current Perspectives, Research Gaps, and Future Directions. Vector-Borne Zoonotic Dis. 2015, 15, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Couvillion, C.E.; Nettles, V.F.; Davidson, W.R.; Pearson, J.E.; Gustafson, G.A. Hemorrhagic Disease among White-Tailed Deer in the Southeast from 1971 through 1980. In Proceedings of the United States Animal Health Association, St. Louis, MO, USA, 11–16 October 1981; pp. 522–537. [Google Scholar]
- Savini, G.; Afonso, A.; Mellor, P.; Aradaib, I.; Yadin, H.; Sanaa, M.; Wilson, W.; Monaco, F.; Domingo, M. Epizootic Heamorragic Disease. Res. Vet. Sci. 2011, 91, 1–17. [Google Scholar] [CrossRef]
- Southeastern Cooperative Wildlife Disease Study. Hemorrhagic Disease of White-Tailed Deer; College of Veterinary Medicine, University of Georgia: Athens, GA, USA, 2013. [Google Scholar]
- Sayler, K.A.; Dow, C.; Wisely, S.M. Facts about Wildlife Diseases: Hemorrhagic Fever in White-Tailed Deer; University of Florida: Gainesville, FL, USA, 2016. [Google Scholar]
- The Center for Food Security & Public Health; Institute for International Cooperation in Animal Biologics. Diseases Caused by the Epizootic Hemorrhagic Disease Virus Serogroup; Iowa State University, College of Veterinary Medicine: Ames, IA, USA, 2016. [Google Scholar]
- Anderson, D.P.; Outlaw, J.L.; Earle, M.; Richardson, J.W. Economic Impact of U.S. Deer Breeding and Hunting Operations; Agricultural and Food Policy Center, Texas A&M University: College Station, TX, USA, 2017. [Google Scholar]
- Payne, J.M.; Brown, R.D.; Guthery, F.S. Wild Game in Texas. Rangelands 1987, 9, 207–211. [Google Scholar]
- Demarais, S.; Osborn, D.A.; Jackley, J.J. Exotic Big Game: A Controversial Resource. Rangel. Arch. 1990, 12, 121–125. [Google Scholar]
- Harmon, L.E. Permethrin Use on Deer Farms in Florida, Potential for Sequestering in Deer and Influence on Resistance Development in Culicoides; University of Florida: Gainesville, FL, USA, 2019. [Google Scholar]
- Wisely, S.M.; Sayler, K. Autogenous Vaccine Field Trial for Epizootic Hemorrhagic Disease Virus and Bluetongue Virus Does Not Result in High Titer to Homologous Virus Serotypes. Int. J. Infect. Dis. 2016, 53, 149–150. [Google Scholar] [CrossRef]
- Blackburn, J.K.; McNyset, K.M.; Curtis, A.J.; Hugh-Jones, M.E. Modeling the Geographic Distribution of Bacillus Anthracis, the Causative Agent of Anthrax Disease, for the Contiguous United States Using Predictive Ecologic Niche Modeling. Am. J. Trop. Med. Hyg. 2007, 77, 1103–1110. [Google Scholar] [CrossRef]
- Drolet, B.S.; van Rijn, P.; Howerth, E.W.; Beer, M.; Mertens, P.P. A Review of Knowledge Gaps and Tools for Orbivirus Research. Vector-Borne Zoonotic Dis. 2015, 15, 339–347. [Google Scholar] [CrossRef]
- Cauvin, A.; Dinh, E.T.N.; Orange, J.P.; Shuman, R.M.; Blackburn, J.K.; Wisely, S.M. Antibodies to Epizootic Hemorrhagic Disease Virus (EHDV) in Adjacent Farmed and Wild Florida White-Tailed Deer (Odocoileus Virginianus). J. Wildl. Dis. 2020, 56, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, A.I.; Blosser, E.M.; McGregor, B.L.; Runkel, A.E.; Sloyer, K.E.; Erram, D.; Wisely, S.M.; Burkett-Cadena, N.D. Tracking Community Timing: Pattern and Determinants of Seasonality in Culicoides (Diptera: Ceratopogonidae) in Northern Florida. Viruses 2020, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K.A. Evaluating Resource Selection Functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-1-4020-0677-7. [Google Scholar]
- Johnson, D.H. The Comparison of Usage and Availability Measurements for Evaluating Resource Preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- McGregor, B.L.; Stenn, T.; Sayler, K.A.; Blosser, E.M.; Blackburn, J.K.; Wisely, S.M.; Burkett-Cadena, N.D. Host Use Patterns of Culicoides Spp. Biting Midges at a Big Game Preserve in Florida, U.S.A., and Implications for the Transmission of Orbiviruses. Med. Vet. Entomol. 2018, 32, 110–120. [Google Scholar] [CrossRef]
- Miller, M. Resource Selection and Survival of Female White-Tailed Deer in an Agricultural Landscape. Ph.D. Thesis, University of Delaware, Newark, Delaware, 2012. [Google Scholar]
- Little, A.R.; Demarais, S.; Gee, K.L.; Webb, S.L.; Riffell, S.K.; Gaskamp, J.A.; Belant, J.L. Does Human Predation Risk Affect Harvest Susceptibility of White-tailed Deer during Hunting Season? Wildl. Soc. Bull. 2014, 38, 797–805. [Google Scholar] [CrossRef]
- Florida Fish and Wildlife Conservation Commission. Florida Natural Areas Inventory Florida Cooperative Land Cover Map; Version 3.1; Florida Natural Areas Inventory: Tallahassee, FL, USA, 2016. [Google Scholar]
- Florida Natural Areas Inventory. Guide to the Natural Communities of Florida; Florida Natural Areas Inventory: Tallahassee, FL, USA, 2010. [Google Scholar]
- Kilgo, J.C.; Labisky, R.F.; Fritzen, D.E. Influences of Hunting on the Behavior of White-Tailed Deer: Implications for Conservation of the Florida Panther. Conserv. Biol. 1998, 12, 1359–1364. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Meredith, M. Creating a GIS Layer for “Distance from...” in R. Available online: http://www.mikemeredith.net/blog/1212_GIS_layer_for_Distance_from.htm (accessed on 8 November 2017).
- Nekorchuk, D.M.; Morris, L.R.; Asher, V.; Hunter, D.L.; Ryan, S.J.; Blackburn, J.K. Potential Bacillus Anthracis Risk Zones for Male Plains Bison (Bison Bison Bison) in Southwestern Montana, USA. J. Wildl. Dis. 2019, 55, 136. [Google Scholar] [CrossRef]
- ESRI. ArcGIS 10.3.1 for Desktop; ESRI: Redlands, CA, USA, 2014. [Google Scholar]
- Naimi, B. Uncertainty Analysis for Species Distribution Models. R Package. 2017. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 10 January 2021).
- Bates, D.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P. Package “Lme4”. 2018. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 10 January 2021).
- Gillies, C.S.; Hebblewhite, M.; Nielsen, S.E.; Krawchuk, M.A.; Aldridge, C.L.; Frair, J.L.; Saher, D.J.; Stevens, C.E.; Jerde, C.L. Application of Random Effects to the Study of Resource Selection by Animals. J. Anim. Ecol. 2006, 75, 887–898. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Thompson, W.L.; Lee, D.C. Modeling Relationships between Landscape-Level Attributes and Snorkel Counts of Chinook Salmon and Steelhead Parr in Idaho. Can. J. Fish. Aquat. Sci. 2000, 57, 1834–1842. [Google Scholar] [CrossRef]
- Anderson, D.R.; Burnham, K.P.; Thompson, W.L. Null Hypothesis Testing: Problems, Prevalence, and an Alternative. J. Wildl. Manag. 2000, 64, 912–923. [Google Scholar] [CrossRef]
- Sittler, K.L.; Parker, K.L.; Gillingham, M.P. Resource Separation by Mountain Ungulates on a Landscape Modified by Fire: Resource Separation by Stone’s Sheep and Elk. J. Wildl. Manag. 2015, 79, 591–604. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Johnson, S.G. The NLopt Nonlinear-Optimization Package. 2014. Available online: https://nlopt.readthedocs.io/en/latest/Citing_NLopt/ (accessed on 10 January 2021).
- Morris, L.R.; Proffitt, K.M.; Blackburn, J.K. Mapping Resource Selection Functions in Wildlife Studies: Concerns and Recommendations. Appl. Geogr. 2016, 76, 173–183. [Google Scholar] [CrossRef]
- Robinson, H.S.; Ruth, T.; Gude, J.A.; Choate, D.; DeSimone, R.; Hebblewhite, M.; Kunkel, K.; Matchett, M.R.; Mitchell, M.S.; Murphy, K. Linking Resource Selection and Mortality Modeling for Population Estimation of Mountain Lions in Montana. Ecol. Model. 2015, 312, 11–25. [Google Scholar] [CrossRef]
- Wiemers, D.W.; Fulbright, T.E.; Wester, D.B.; Ortega-S, J.A.; Rasmussen, G.A.; Hewitt, D.G.; Hellickson, M.W. Role of Thermal Environment in Habitat Selection by Male White-Tailed Deer during Summer in Texas, USA. Wildl. Biol. 2014, 20, 47–56. [Google Scholar] [CrossRef]
- Beier, P.; McCullough, D.R. Factors Influencing White-Tailed Deer Activity Patterns and Habitat Use. In Wildlife Monographs; Wiley: Hoboken, NJ, USA, 1990; pp. 3–51. [Google Scholar]
- Kie, J.G.; Bowyer, R.T. Sexual Segregation in White-Tailed Deer: Density-Dependent Changes in Use of Space, Habitat Selection, and Dietary Niche. J. Mammal. 1999, 80, 1004–1020. [Google Scholar] [CrossRef]
- Boyce, M.S. Scale for Resource Selection Functions. Divers. Distrib. 2006, 12, 269–276. [Google Scholar] [CrossRef]
- Rouleau, I.; Crête, M.; Ouellet, J.-P. Contrasting the Summer Ecology of White-Tailed Deer Inhabiting a Forested and an Agricultural Landscape. Écoscience 2002, 9, 459–469. [Google Scholar] [CrossRef]
- Dryden, G.M. Quantitative Nutrition of Deer: Energy, Protein and Water. Anim. Prod. Sci. 2011, 51, 292–302. [Google Scholar] [CrossRef]
- Hewitt, D.G. Nutrition. In Biology and Management of White-Tailed Deer; Hewitt, D.G., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 75–106. ISBN 978-1-4398-0651-7. [Google Scholar]
- Stewart, K.M.; Bowyer, R.T.; Weisburg, P.J. Spatial use of landscapes. In Biology and Management of White-Tailed Deer; Hewitt, D.G., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 181–217. ISBN 978-1-4398-0651-7. [Google Scholar]
- McGregor, B.L.; Sloyer, K.E.; Sayler, K.A.; Goodfriend, O.; Krauer, J.M.C.; Acevedo, C.; Zhang, X.; Mathias, D.; Wisely, S.M.; Burkett-Cadena, N.D. Field Data Implicating Culicoides Stellifer and Culicoides Venustus (Diptera: Ceratopogonidae) as Vectors of Epizootic Hemorrhagic Disease Virus. Parasit. Vectors 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Erram, D.; Burkett-Cadena, N. Laboratory Studies on the Oviposition Stimuli of Culicoides Stellifer (Diptera: Ceratopogonidae), a Suspected Vector of Orbiviruses in the United States. Parasit. Vectors 2018, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Erram, D.; Blosser, E.M.; Burkett-Cadena, N. Habitat Associations of Culicoides Species (Diptera: Ceratopogonidae) Abundant on a Commercial Cervid Farm in Florida, USA. Parasit. Vectors 2019, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Blanton, F.S.; Wirth, W.W. The sand flies (Culicoides) of Florida (Diptera: Ceratopogonidae). In Arthropods of Florida and Neighboring Land Areas; Florida Department of Agriculture and Consumer Affairs, Division of Plant Industry: Gainesville, FL, USA, 1979; Volume 10. [Google Scholar]

| Ranched | Equal Area ρx̅ | Equal Area ρσ2 |
|---|---|---|
| Upland mixed hardwood–pine + bottomland mixed hardwood + tertiary roads + water + food | 0.9654 | 0.0002 |
| Upland pine + upland mixed hardwood–pine + bottomland mixed hardwood + tertiary roads + water + food | 0.9715 | 0.0002 |
| Upland mixed hardwood–pine + bottomland mixed hardwood + tertiary roads + water | 0.9782 | 0.0004 |
| Wild | ||
| Upland pine + upland mixed hardwood–pine + bottomland mixed hardwood + tertiary roads + water + food | 0.9438 | 0.0002 |
| Covariate | Ranched Estimate ± SE | Wild Estimate ± SE | Ranched p-Value | Wild p-Value |
|---|---|---|---|---|
| Intercept | −1.7281 ± 0.0612 | −4.3384 ± 0.2899 | <0.001 | <0.001 |
| Upland pine forest | 0.0202 ± 0.0421 | 2.5568 ± 0.1208 | 0.6316 | <0.001 |
| Upland mixed hardwood–coniferous forest | −0.7400 ± 0.0835 | 1.6507 ± 0.1234 | <0.001 | <0.001 |
| Bottomland mixed forest | 0.1925 ± 0.0442 | 2.2778 ± 0.1220 | <0.001 | <0.001 |
| Distance to tertiary roads | −0.3331 ± 0.0151 | −1.1258 ± 0.0389 | <0.001 | <0.001 |
| Distance to permanent water bodies | −0.0825 ± 0.0145 | 0.6075 ± 0.0224 | <0.001 | <0.001 |
| Distance to supplementary food sources | −0.0412 ± 0.0154 | −0.1858 ± 0.0233 | 0.0072 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, E.T.N.; Orange, J.P.; Peters, R.M.; Wisely, S.M.; Blackburn, J.K. Resource Selection by Wild and Ranched White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Season in Florida. Animals 2021, 11, 211. https://doi.org/10.3390/ani11010211
Dinh ETN, Orange JP, Peters RM, Wisely SM, Blackburn JK. Resource Selection by Wild and Ranched White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Season in Florida. Animals. 2021; 11(1):211. https://doi.org/10.3390/ani11010211
Chicago/Turabian StyleDinh, Emily T.N., Jeremy P. Orange, Rebecca M. Peters, Samantha M. Wisely, and Jason K. Blackburn. 2021. "Resource Selection by Wild and Ranched White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Season in Florida" Animals 11, no. 1: 211. https://doi.org/10.3390/ani11010211
APA StyleDinh, E. T. N., Orange, J. P., Peters, R. M., Wisely, S. M., & Blackburn, J. K. (2021). Resource Selection by Wild and Ranched White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Season in Florida. Animals, 11(1), 211. https://doi.org/10.3390/ani11010211






