Relationships between Seminal Plasma Metabolites, Semen Characteristics and Sperm Kinetics in Donkey (Equus asinus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Semen Collection
2.4. Experimental Design
2.5. Sperm Kinetics
2.6. Mitochondrial Membrane Potential (MMP)
2.7. Lipid Peroxidation (LPO)
2.8. Anti-LPO Potential
2.9. Nitroblue Tetrazolium (NBT) Assay
2.10. 1H NMR Sample Preparation
2.11. 1H NMR Analysis
2.12. Statistical Analysis
3. Results
3.1. Experiment 1. Azoospermic vs. Normospermic Stallions
3.2. Experiment 2. Repeated Semen Collections
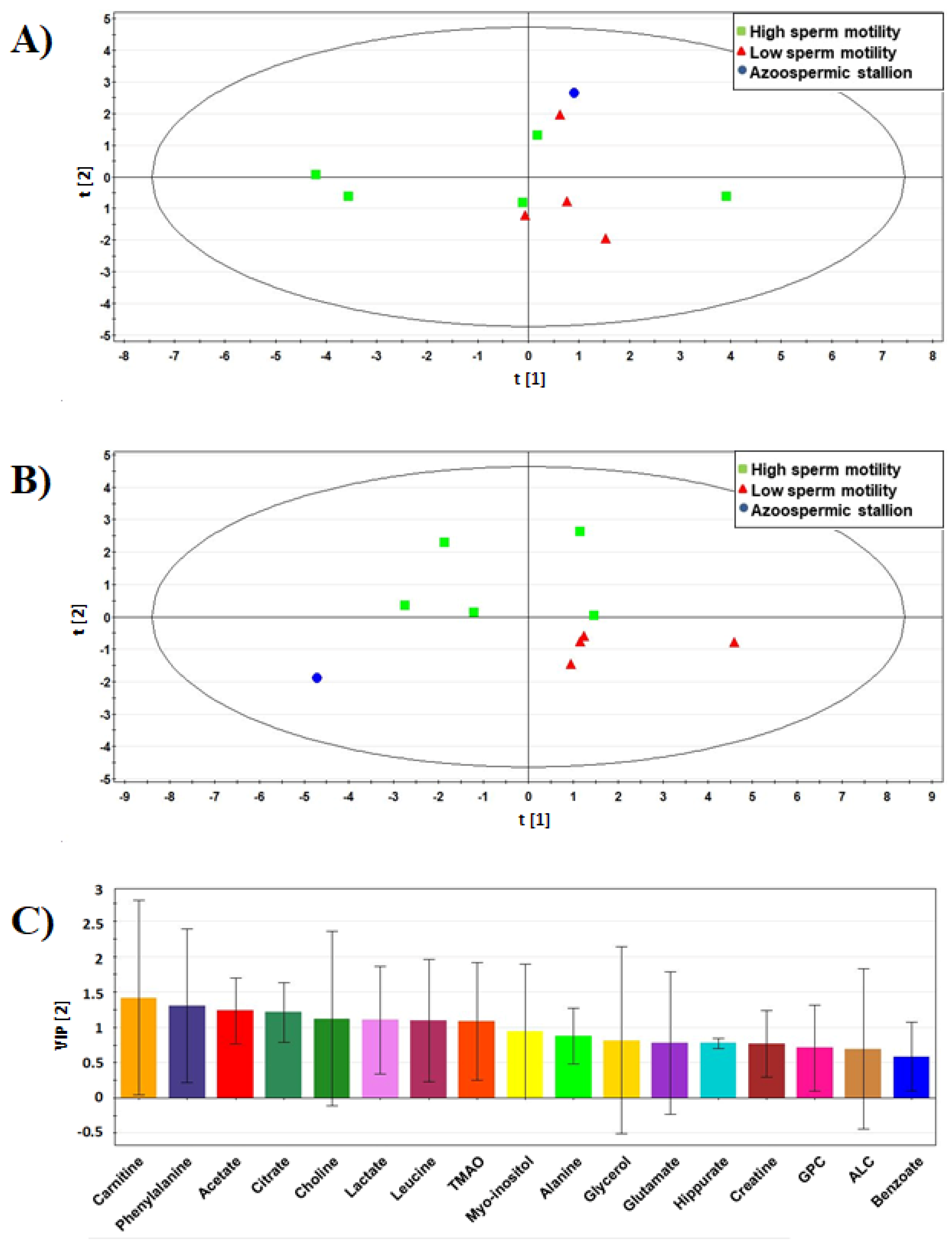
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosiniak, K. Characteristics of the successive jets of ejaculated semen of stallions. J. Reprod. Fertil. 1975, 23, 59–61. [Google Scholar]
- Weber, J.A.; Woods, G.L. Ultrasonographic measurement of stallion accessory sex glands and excurrent ducts during seminal emission and ejaculation. Biol. Reprod. 1993, 49, 267–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magistrini, M.; Lindeberg, H.; Koskinen, E.; Beau, P.; Seguin, F. Biophysical and 1H magnetic resonance spectroscopy characteristics of fractionated stallion ejaculates. J. Reprod. Fertil. 2000, 56, 101–110. [Google Scholar]
- Miró, J.; Taberner, E.; Rivera, M.; Peña, A.; Medrano, A.; Rigau, T.; Peñalba, A. Effects of dilution and centrifugation on the survival of spermatozoa and the structure of motile sperm cell subpopulations in refrigerated Catalonian donkey semen. Theriogenology 2009, 72, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Magelli, C.; Panzani, D.; Camillo, F. Effect of extender, centrifugation and removal of seminal plasma on cooled-preserved Amiata donkey spermatozoa. Theriogenology 2008, 69, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Serres, C.; Rodriguez, A.; Alvarez, A.; Santiago, I.; Gabriel, J.; Gómez-Cuétara, C.; Mateos, E. Effect of centrifugation and temperature on the motility and plasma membrane integrity of Zamorano-Leonés donkey semen. Theriogenology 2002, 58, 329–332. [Google Scholar]
- Pickett, B.; Sullivan, J.; Byers, W.; Pace, M.; Remmenga, E. Effect of centrifugation and seminal plasma on motility and fertility of stallion and bull spermatozoa. Fertil. Steril. 1975, 26, 167–174. [Google Scholar] [CrossRef]
- Miró, J.; Vilés, K.; García, W.; Jordana, J.; Yeste, M. Effect of donkey seminal plasma on sperm movement and sperm–polymorphonuclear neutrophils attachment in vitro. Anim. Reprod. Sci. 2013, 140, 164–172. [Google Scholar] [CrossRef]
- Smith, R.; Vantman, D.; Ponce, J.; Escobar, J.; Lissi, E. Andrology: Total antioxidant capacity of human seminal plasma. Hum. Reprod. 1996, 11, 1655–1660. [Google Scholar] [CrossRef]
- Killian, G.J.; Chapman, D.A.; Rogowski, L.A. Fertility-associated proteins in Holstein bull seminal plasma. Biol. Reprod. 1993, 49, 1202–1207. [Google Scholar] [CrossRef]
- López Rodríguez, A.; Rijsselaere, T.; Beek, J.; Vyt, P.; Van Soom, A.; Maes, D. Boar seminal plasma components and their relation with semen quality. Syst. Biol. Reprod. Med. 2013, 59, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Hollywood, K.; Brison, D.R.; Goodacre, R. Metabolomics: Current technologies and future trends. Proteomics 2006, 6, 4716–4723. [Google Scholar] [CrossRef]
- Lynch, M.; Masters, J.; Pryor, J.; Lindon, J.; Spraul, M.; Foxall, P.; Nicholson, J.K. Ultra high field NMR spectroscopic studies on human seminal fluid, seminal vesicle and prostatic secretions. J. Pharm. Biomed. Anal. 1994, 12, 5–19. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR spectroscopic studies on human seminal plasma: A probative discriminant function analysis classification model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-associated metabolites in bull seminal plasma and blood serum: 1H nuclear magnetic resonance analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef]
- Magistrini, M.; Seguin, F.; Beau, P.; Akoka, S.; Le Pape, A.; Palmer, E. 1H nuclear magnetic resonance analysis of stallion genital tract fluids and seminal plasma: Contribution of the accessory sex glands to the ejaculate. Biol. Reprod. 1995, 52, 599–607. [Google Scholar] [CrossRef]
- Barbacini, S.; Zavaglia, G.; Gulden, P.; Marchi, V.; Necchi, D. Retrospective study on the efficacy of hCG in an equine artificial insemination programme using frozen semen. Equine Vet. Educ. 2000, 12, 312–332. [Google Scholar] [CrossRef]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Contri, A.; Gloria, A.; Robbe, D.; Sfirro, M.P.; Carluccio, A. Effect of sperm concentration on characteristics of frozen-thawed semen in donkeys. Anim. Reprod. Sci. 2012, 136, 74–80. [Google Scholar] [CrossRef]
- Di Palma, T.; Cecchini, S.; Macchia, G.; Pasolini, M.P.; Cocchia, N.; Boni, R. Kinematic, bioenergetic and oxidative evaluations of donkey sperm preserved at +4 °C. Zygote 2020, 28, 300–307. [Google Scholar] [CrossRef]
- Orhan, H.; Gurer-Orhan, H.; Vriese, E.; Vermeulen, N.; Meerman, J. Application of lipid peroxidation and protein oxidation biomarkers for oxidative damage in mammalian cells. A comparison with two fluorescent probes. Toxicol. In Vitro 2006, 20, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Thompson, J.; Tremellen, K. Development of the NBT assay as a marker of sperm oxidative stress. Int. J. Androl. 2010, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, A.; Carobene, A.; Kilercik, M.; Serteser, M.; Sandberg, S.; Aarsand, A.K.; Fernandez-Calle, P.; Jonker, N.; Bartlett, W.A.; Díaz-Garzón, J. Within-subject and between-subject biological variation estimates of 21 hematological parameters in 30 healthy subjects. Clin. Chem. Lab. Med. 2018, 56, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Cuviello, F.; Ostuni, A. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: A pilot study. Metabolomics 2019, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Sirard, M.A. Catalase and oviductal fluid reverse the decreased motility of bovine sperm in culture medium containing specific amino acids. J. Androl. 1998, 19, 31–36. [Google Scholar]
- Menezo, Y.; Laviolette, P. Amino constituents of tubal secretions in the rabbit. Zymogram–proteins–free amino acids. Ann. Biol. Anim. Biochim. Biophys. 1972, 12, 383–396. [Google Scholar] [CrossRef][Green Version]
- Tosic, J.; Walton, A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem. J. 1950, 47, 199–212. [Google Scholar] [CrossRef]
- Sangeeta, S.; Arangasamy, A.; Kulkarni, S.; Selvaraju, S. Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels at pre-freeze and post-thawed ram semen. Anim. Reprod. Sci. 2015, 161, 82–88. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Chieffi Baccari, G. D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs. Biomolecules 2019, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi, P.; Usiello, A.; Pinelli, C.; Baccari, G.C. AMPA receptor expression in mouse testis and spermatogonial GC-1 cells: A study on its regulation by excitatory amino acids. J. Cell. Biochem. 2019, 120, 11044–11055. [Google Scholar] [CrossRef] [PubMed]
- Rufo, G.; Singh, J.; Babcock, D.; Lardy, H. Purification and characterization of a calcium transport inhibitor protein from bovine seminal plasma. J. Biol. Chem. 1982, 257, 4627–4632. [Google Scholar] [CrossRef]
- Wallimann, T.; Moser, H.; Zurbriggen, B.; Wegmann, G.; Eppenberger, H.M. Creatine kinase isoenzymes in spermatozoa. J. Muscle Res. Cell. Motil. 1986, 7, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-C.; Granish, K.A.; Suarez, S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002, 250, 208–217. [Google Scholar] [CrossRef]
- Umehara, T.; Kawai, T.; Goto, M.; Richards, J.S.; Shimada, M. Creatine enhances the duration of sperm capacitation: A novel factor for improving in vitro fertilization with small numbers of sperm. Hum. Reprod. 2018, 33, 1117–1129. [Google Scholar] [CrossRef]
- Mongioi, L.; Calogero, A.; Vicari, E.; Condorelli, R.; Russo, G.; Privitera, S.; Morgia, G.; La Vignera, S. The role of carnitine in male infertility. Andrology 2016, 4, 800–807. [Google Scholar] [CrossRef]
- Jeulin, C.; Lewin, L.M. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum. Reprod. Update 1996, 2, 87–102. [Google Scholar] [CrossRef]
- Nery, I.H.; Araújo Silva, R.A.; Souza, H.M.; Arruda, L.C.; Monteiro, M.M.; Seal, D.C.; Silva, G.R.; Silva, T.M.; Carneiro, G.F.; Batista, A.M. Effects of L-Carnitine on Equine Semen Quality During Liquid Storage. Biopreserv. Biobank. 2020, 18, 403–408. [Google Scholar] [CrossRef]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef]
- Gallo, A.; Menezo, Y.; Dale, B.; Coppola, G.; Dattilo, M.; Tosti, E.; Boni, R. Metabolic enhancers supporting 1-carbon cycle affect sperm functionality: An in vitro comparative study. Sci. Rep. 2018, 8, 11769. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.; Harrison, A. The role of citrate in determining the activity of calcium ions in human semen. Int. J. Androl. 1984, 7, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Zöpfgen, A.; Priem, F.; Sudhoff, F.; Jung, K.; Lenk, S.; Loening, S.; Sinha, P. Relationship between semen quality and the seminal plasma components carnitine, alpha-glucosidase, fructose, citrate and granulocyte elastase in infertile men compared with a normal population. Hum. Reprod. 2000, 15, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G. Epididymal Secretion of Glycerophosphocholine (GPC). In The Epididymis, Sperm Maturation and Fertilisation; Springer: Berlin/Heidelberg, Germany, 1986; pp. 174–179. [Google Scholar]
- Vazquez-Levin, M.; Verón, G. Myo-inositol in health and disease: Its impact on semen parameters and male fertility. Andrology 2020, 8, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Colone, M.; Marelli, G.; Unfer, V.; Bozzuto, G.; Molinari, A.; Stringaro, A. Inositol activity in oligoasthenoteratospermia—An In Vitro study. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 891. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Z.; Tang, W.W.; Hazen, S.L. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef]
- Nagy, R.; Homminga, I.; Jia, C.; Liu, F.; Anderson, J.; Hoek, A.; Tietge, U. Trimethylamine-N-oxide is present in human follicular fluid and is a negative predictor of embryo quality. Hum. Reprod. 2020, 35, 81–88. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Dutta, S.; Sengupta, P.; Khorshid, H.R.K.; Esteves, S.; Gilany, K.; Hedayati, M. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J. Assist. Reprod. Genet. 2019, 36, 241–253. [Google Scholar] [CrossRef]
- Bonechi, C.; Collodel, G.; Donati, A.; Martini, S.; Moretti, E.; Rossi, C. Discrimination of human semen specimens by NMR data, sperm parameters, and statistical analysis. Syst. Biol. Reprod. Med. 2015, 61, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mumcu, A.; Karaer, A.; Dogan, B.; Tuncay, G. Metabolomics analysis of seminal plasma in patients with idiopathic Oligoasthenoteratozoospermia using high-resolution NMR spectroscopy. Andrology 2020, 8, 450–456. [Google Scholar] [CrossRef] [PubMed]

| Seminal Plasma Metabolites | Sperm Motility | Azoospermic | |
|---|---|---|---|
| High | Low | ||
| Acetate | 0.153 ± 0.047 | 0.192 ± 0.030 | 0.127 ± 0.045 |
| Alanine | 0.043 ± 0.259 a | 0.039 ± 0.268 a | 0.003 ± 0.001 b |
| Benzoate | 0.013 ± 0.013 | 0.017 ± 0.006 | 0.007 ± 0.010 |
| Carnitine | 0.420 ± 0.115 A | 0.390 ± 0.074 A | 0.012 ± 0.017 B |
| Choline | 0.260 ± 0.024 a | 0.413 ± 0.139 A | 0.037 ± 0.012 Bb |
| Citrate | 0.069 ± 0.029 AB | 0.099 ± 0.021 A | 0.023 ± 0.005 B |
| Creatine | 0.356 ± 0.160 a | 0.400 ± 0.171 a | 0.006 ± 0.002 b |
| Glutamate | 1.815 ± 0.811 a | 2.362 ± 0.558 A | 0.141 ± 0.027 Bb |
| Glycerol | 18.023 ± 8.096 | 19.066 ± 1.650 | 21.928 ± 0.197 |
| GPC | 1.491 ± 0.852 a | 1.935 ± 0.180 A | 0.074 ± 0.004 Bb |
| Hippurate | 0.002 ± 0.003 | 0.008 ± 0.007 | 0.004 ± 0.006 |
| Lactate | 0.589 ± 0.189 a | 0.781 ± 0.053 A | 0.250 ± 0.028 Bb |
| Leucine | 0.043 ± 0.024 a | 0.039 ± 0.007 a | 0.003 ± 0.001 b |
| Myo-inositol | 0.340 ± 0.259 ab | 0.603 ± 0.178 a | 0.040 ± 0.008 b |
| ALC | 0.107 ± 0.028 | 0.199 ± 0.145 | 0.019 ± 0.001 |
| Phenylalanine | 0.009 ± 0.005 a | 0.001 ± 0.002 b | 0.000 ± 0.000 b |
| TMAO | 0.348 ± 0.270 | 0.229 ± 0.050 | 0.001 ± 0.000 |
| Semen parameters | |||
| pH | 7.50 ± 0.08 A | 7.66 ± 0.19 A | 8.45 ± 0.06 B |
| Osmolarity (mOsm) | 282 ± 8.185 | 293 ± 11 | 293 ± 8 |
| Volume (mL) | 48.3 ± 7.638 a | 46.7 ± 18.9 a | 17.5 ± 3.5 b |
| Sperm concentration (106/mL) | 320.7 ± 19.3 | 333.9 ± 3.7 | 0 |
| Sperm production (109) | 15.3 ± 2.5 | 15.7 ± 6.7 | 0 |
| Sperm kinetics at time 0 | |||
| Tot Mot (%) | 93.1 ± 6.1 a | 56.3 ± 17.0 b | 0 |
| Prog (%) | 33.9 ± 6.0 A | 17.7 ± 3.2 B | 0 |
| VCL (µm/s) | 227.3 ± 25.2 | 205.9 ± 31.5 | 0 |
| VSL (µm/s) | 87.0 ± 13.4 | 70.8 ± 7.4 | 0 |
| VAP (µm/s) | 123.5 ± 25.5 | 100.6 ± 7.4 | 0 |
| Sperm kinetics after 24 h storage | |||
| Tot Mot (%) | 64.3 ± 23.4 A | 2.7 ± 3.8 B | 0 |
| Prog (%) | 21.3 ± 14.0 a | 0.5 ± 0.0 b | 0 |
| VCL (µm/s) | 184.1 ± 27.8 A | 73.8 ± 7.4 B | 0 |
| VSL (µm/s) | 74.6 ± 20.3 A | 21.1 ± 8.1 B | 0 |
| VAP (µm/s) | 97.1 ± 22.2 A | 33.3 ± 9.3 B | 0 |
| Seminal Plasma Metabolites | Stallions | ||
|---|---|---|---|
| MF1 | MF2 | RO | |
| Acetate | 0.134 ± 0.060 | 0.084 ± 0.011 | 0.152 ± 0.055 |
| Alanine | 0.200 ± 0.074 | 0.168 ± 0.018 | 0.150 ± 0.063 |
| Benzoate | 0.032 ± 0.017 | 0.078 ± 0.076 | 0.035 ± 0.013 |
| Carnitine | 0.284 ± 0.105 | 0.237 ± 0.028 | 0.191 ± 0.072 |
| Choline | 0.251 ± 0.105 | 0.178 ± 0.028 | 0.165 ± 0.017 |
| Citrate | 0.092 ± 0.092 | 0.043 ± 0.021 | 0.094 ± 0.042 |
| Creatine | 0.088 ± 0.013 | 0.098 ± 0.020 | 0.091 ± 0.032 |
| Glutamate | 0.917 ± 0.364 a | 0.539 ± 0.100 b | 0.447 ± 0.161 b |
| Glycerol | 17.851 ± 1.754 a | 10.893 ± 2.146 b | 15.265 ± 5.009 ab |
| GPC | 0.546 ± 0.164 | 0.581 ± 0.164 | 0.459 ± 0.209 |
| Hippurate | 0.005 ± 0.009 A | 0.048 ± 0.015 B | 0.011 ± 0.014 A |
| Lactate | 0.349 ± 0.142 A | 0.297 ± 0.059 A | 2.226 ± 1.193 B |
| Leucine | 0.042 ± 0.020 a | 0.020 ± 0.005 b | 0.026 ± 0.011 ab |
| Myo-Inositol | 0.262 ± 0.127 | 0.308 ± 0.038 | 0.213 ± 0.106 |
| ALC | 0.049 ± 0.012 | 0.045 ± 0.011 | 0.032 ± 0.009 |
| Phenylalanine | 0.005 ± 0.005 | 0.018 ± 0.016 | 0.008 ± 0.006 |
| TMAO | 0.119 ± 0.018 | 0.110 ± 0.025 | 0.145 ± 0.042 |
| Semen parameters | |||
| pH | 7.64 ± 0.56 a | 7.52 ± 0.14 a | 8.18 ± 0.23 b |
| Osmolarity (mOsm) | 296 ± 10 a | 293 ± 1 A | 314 ± 11 Bb |
| Semen volume (mL) | 23.7 ± 4.7 a | 69.0 ± 33.0 b | 65.2 ± 16.4 b |
| Sperm concentration (106/mL) | 229.0 ± 54.1 a | 182.0 ± 43.0 ab | 129.5 ± 29.6 b |
| Sperm production (109) | 5.25 ± 0.31 | 13.32 ± 8.8 | 8.15 ± 1.23 |
| Sperm kinetics at time 0 | |||
| Tot Mot (%) | 80.5 ± 14.8 | 90.1 ± 8.2 | 69.1 ± 13.6 |
| Prog (%) | 33.2 ± 8.0 | 25.1 ± 8.5 | 22.8 ± 1.4 |
| VCL (µm/s) | 110.0 ± 7.1 a | 127.7 ± 7.9 b | 117.7 ± 0.1 ab |
| VSL (µm/s) | 58.3 ± 6.5 | 59.8 ± 9.5 | 48.5 ± 2.4 |
| VAP (µm/s) | 77.8 ± 10.5 a | 95.9 ± 6.7 b | 82.0 ± 10.1 ab |
| MMP and oxidative status at time 0 | |||
| MMP (FoB/FoA) | 49.8 ± 17.3 | 79.6 ± 24.7 | 78.8 ± 20.9 |
| LPO (FoA/(FoA+FoB)) | 19.3 ± 8.4 | 14.8 ± 1.8 | 14.9 ± 4.2 |
| Anti-LPO (FoB/(FoA+FoB)) | 30.1 ± 9.6 | 27.9 ± 7.3 | 24.3 ± 10.1 |
| NBT (Formazan µg) | 7.78 ± 2.77 | 11.52 ± 5.44 | 5.80 ± 2.80 |
| Sperm kinetics after 24 h storage | |||
| Tot Mot (%) | 75.5 ± 5.3 a | 63.5 ± 11.5 ab | 40.5 ± 20.9 b |
| Prog (%) | 33.5 ± 2.5 A | 22.8 ± 5.9 AB | 10.4 ± 10.7 B |
| VCL (µm/s) | 110.5 ± 6.5 | 101.8 ± 9.4 | 98.7 ± 23.9 |
| VSL (µm/s) | 65.5 ± 4.7 | 50.9 ± 4.4 | 47.3 ± 24.9 |
| VAP (µm/s) | 85.0 ± 6.5 | 71.8 ± 9.8 | 71.1 ± 32.8 |
| MMP and oxidative status after 24 h storage | |||
| MMP (FoB/FoA) | 40.8 ± 25.3 | 75.1 ± 25.9 | 49.4 ± 15.5 |
| LPO (FoA/(FoA+FoB)) | 24.2 ± 15.3 | 14.7 ± 4.1 | 13.9 ± 5.1 |
| Anti-LPO (FoB/(FoA+FoB)) | 35.1 ± 12.0 | 27.5 ± 4.3 | 32.6 ± 8.5 |
| NBT (Formazan µg) | 7.73 ± 3.18 | 7.88 ± 5.11 | 7.44 ± 3.12 |
| Alanine | Carnitine | Choline | Citrate | Creatine | Glutamate | GPC | Leucine | Myo-Inositol | ALC | TMAO | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate | +0.514 | +0.396 | +0.557 | +0.745 ** | +0.469 | +0.596 * | +0.571 | +0.414 | +0.335 | +0.559 | +0.083 |
| Alanine | +0.923 ** | +0.784 ** | +0.544 | +0.969 ** | +0.938 ** | +0.751 ** | +0.852 ** | +0.696 * | +0.754 ** | +0.709 ** | |
| Carnitine | +0.776 ** | +0.622 * | +0.873 ** | +0.874 ** | +0.763 ** | +0.907 ** | +0.658 * | +0.606* | +0.719 ** | ||
| Choline | +0.697 * | +0.781 ** | +0.767 ** | +0.650 * | +0.652 * | +0.902 ** | +0.852** | +0.465 | |||
| Citrate | +0.541 | +0.600 ** | +0.505 | +0.707 ** | +0.513 | +0.450 | +0.345 | ||||
| Creatine | +0.908 ** | +0.666 * | +0.847 ** | +0.781 ** | +0.759 ** | +0.776 ** | |||||
| Glutamate | +0.890 ** | +0.769 ** | +0.625 * | +0.755 ** | +0.527 | ||||||
| GPC | +0.562 | +0.421 | +0.580 | +0.276 | |||||||
| Leucine | +0.614 * | +0.416 | +0.845 ** | ||||||||
| Myo-inositol | +0.746 ** | +0.623 * | |||||||||
| ALC | +0.262 |
| Semen Parameters | |||
|---|---|---|---|
| SP Metabolites | Semen Volume | Sperm Concentration | Sperm Production |
| Acetate | +0.182 | +0.180 | +0.476 |
| Alanine | +0.249 | +0.759 ** | +0.867 ** |
| Benzoate | +0.752 ** | +0.048 | +0.050 |
| Carnitine | +0.432 | +0.886 ** | +0.850 ** |
| Choline | +0.345 | +0.732 ** | +0.700 ** |
| Citrate | +0.575 | +0.424 | +0.593 * |
| Creatine | +0.314 | +0.751 ** | +0.918 ** |
| Glutamate | +0.223 | +0.770 ** | +0.869 ** |
| Glycerol | −0.484 | −0.314 | −0.105 |
| GPC | +0.144 | +0.746 ** | +0.712 ** |
| Hippurate | +0.581 | −0.105 | +0.074 |
| Lactate | +0.320 | −0.062 | −0.068 |
| Leucine | +0.485 | +0.703 ** | +0.812 ** |
| Myo-inositol | +0346 | +0.692 * | +0.687 * |
| ALC | +0.155 | +0.560 | +0.632 * |
| Phenylalanine | +0.540 | +0.224 | −0.109 |
| TMAO | +0.335 | +0.628 | +0.750 ** |
| Semen parameters | |||
| Osmolarity | −0.066 | −0.482 | −0.324 |
| pH | −0.078 | −0.384 | −0.073 |
| Sperm volume | −0.396 | +0.797 ** | |
| Sperm concentration | +0.228 | ||
| Sperm Kinematic Parameters at Time 0 | Sperm Kinematic Parameters after 24 h of Storage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Semen Plasma Metabolites | TotMot | Prog | VCL | VSL | VAP | TotMot | Prog | VCL | VSL | VAP |
| Acetate | −0.340 | −0.744 * | −0.045 | −0.593 | −0.067 | −0.434 | −0.508 | −0.144 | −0.161 | −0.098 |
| Benzoate | −0.029 | −0.095 | +0.556 | +0.441 | +0.689 * | +0.392 | +0.323 | +0.645* | +0.473 | +0.534 |
| Citrate | −0.341 | −0.760 ** | +0.278 | −0.378 | +0.221 | +0.023 | −0.032 | +0.386 | +0.304 | +0.380 |
| Phenylalanine | +0.326 | +0.517 | +0.226 | +0.565 | +0.558 | +0.821 ** | +0.853 ** | +0.847** | +0.902 ** | +0.909 ** |
| Semen parameters | ||||||||||
| Osmolarity | −0.666 * | −0.532 | −0.120 | −0.025 | +0.106 | −0.249 | −0.273 | −0.058 | −0.184 | −0.141 |
| Semen volume | +0.082 | −0.136 | +0.549 | +0.470 | +0.736 * | +0.425 | +0.375 | +0.591 * | +0.484 | +0.555 |
| Sperm kinematic parameters | ||||||||||
| Tot Mot | +0.742 * | +0.599 | +0.476 | +0.457 | +0.951 ** | +0.757 ** | +0.900 ** | +0.892 ** | ||
| Prog | +0.183 | +0.507 | +0.156 | +0.867 ** | +0.916 ** | +0.886 ** | ||||
| VCL | +0.332 | +0.668 * | +0.966 ** | +0.975 ** | ||||||
| VSL | +0.783 ** | +0.994 ** | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiglione Morelli, M.A.; Ostuni, A.; Giangaspero, B.; Cecchini, S.; Carluccio, A.; Boni, R. Relationships between Seminal Plasma Metabolites, Semen Characteristics and Sperm Kinetics in Donkey (Equus asinus). Animals 2021, 11, 201. https://doi.org/10.3390/ani11010201
Castiglione Morelli MA, Ostuni A, Giangaspero B, Cecchini S, Carluccio A, Boni R. Relationships between Seminal Plasma Metabolites, Semen Characteristics and Sperm Kinetics in Donkey (Equus asinus). Animals. 2021; 11(1):201. https://doi.org/10.3390/ani11010201
Chicago/Turabian StyleCastiglione Morelli, Maria Antonietta, Angela Ostuni, Brunella Giangaspero, Stefano Cecchini, Augusto Carluccio, and Raffaele Boni. 2021. "Relationships between Seminal Plasma Metabolites, Semen Characteristics and Sperm Kinetics in Donkey (Equus asinus)" Animals 11, no. 1: 201. https://doi.org/10.3390/ani11010201
APA StyleCastiglione Morelli, M. A., Ostuni, A., Giangaspero, B., Cecchini, S., Carluccio, A., & Boni, R. (2021). Relationships between Seminal Plasma Metabolites, Semen Characteristics and Sperm Kinetics in Donkey (Equus asinus). Animals, 11(1), 201. https://doi.org/10.3390/ani11010201







