Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
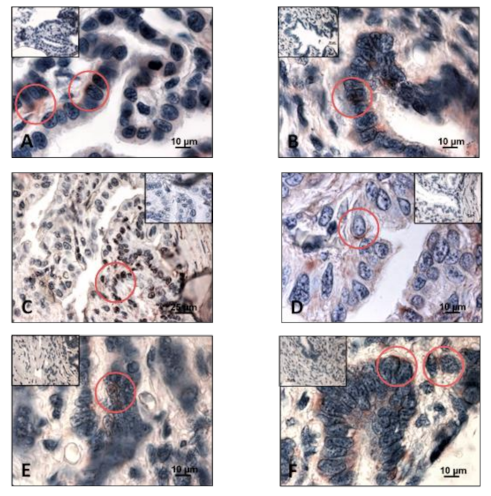
3. Results
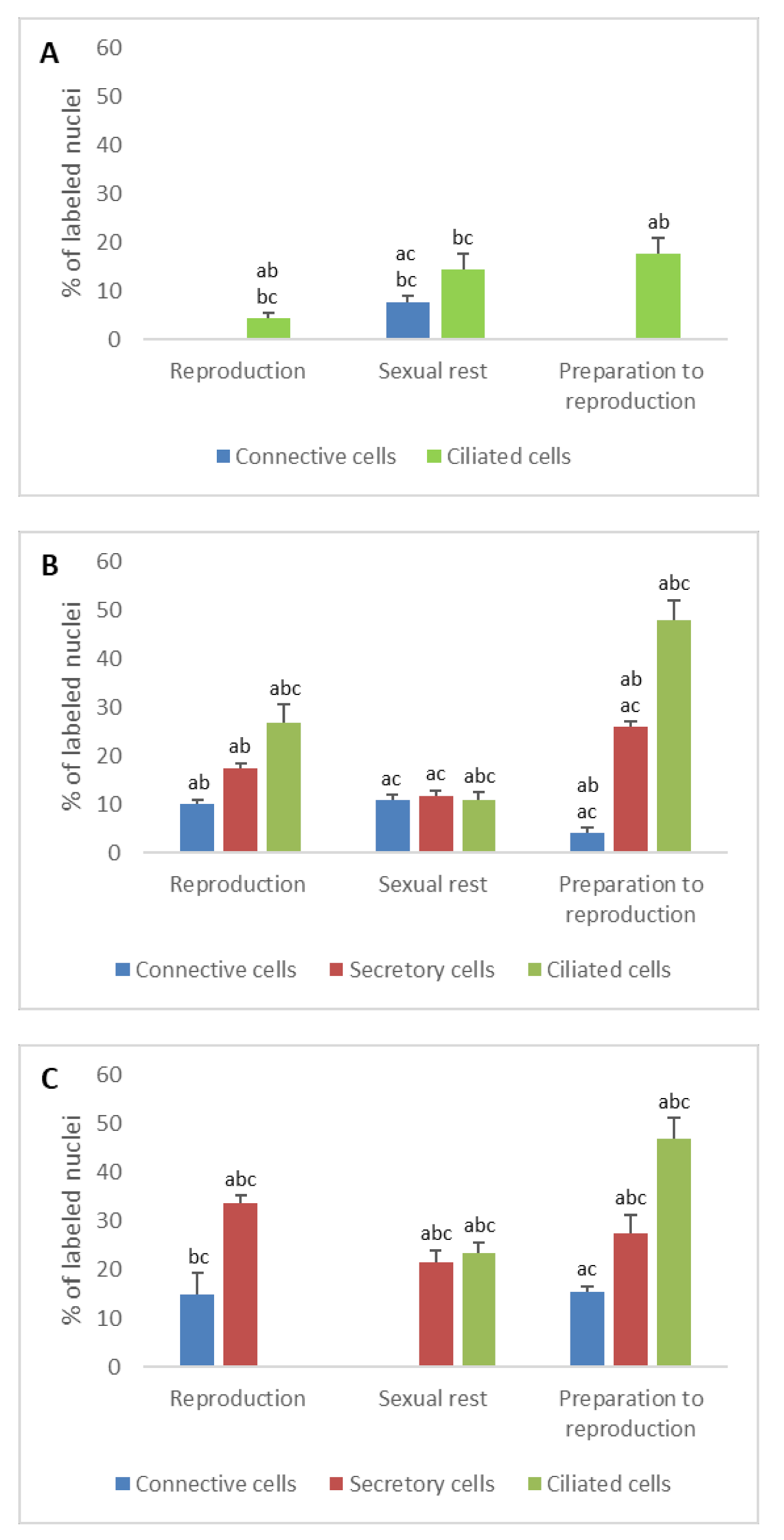
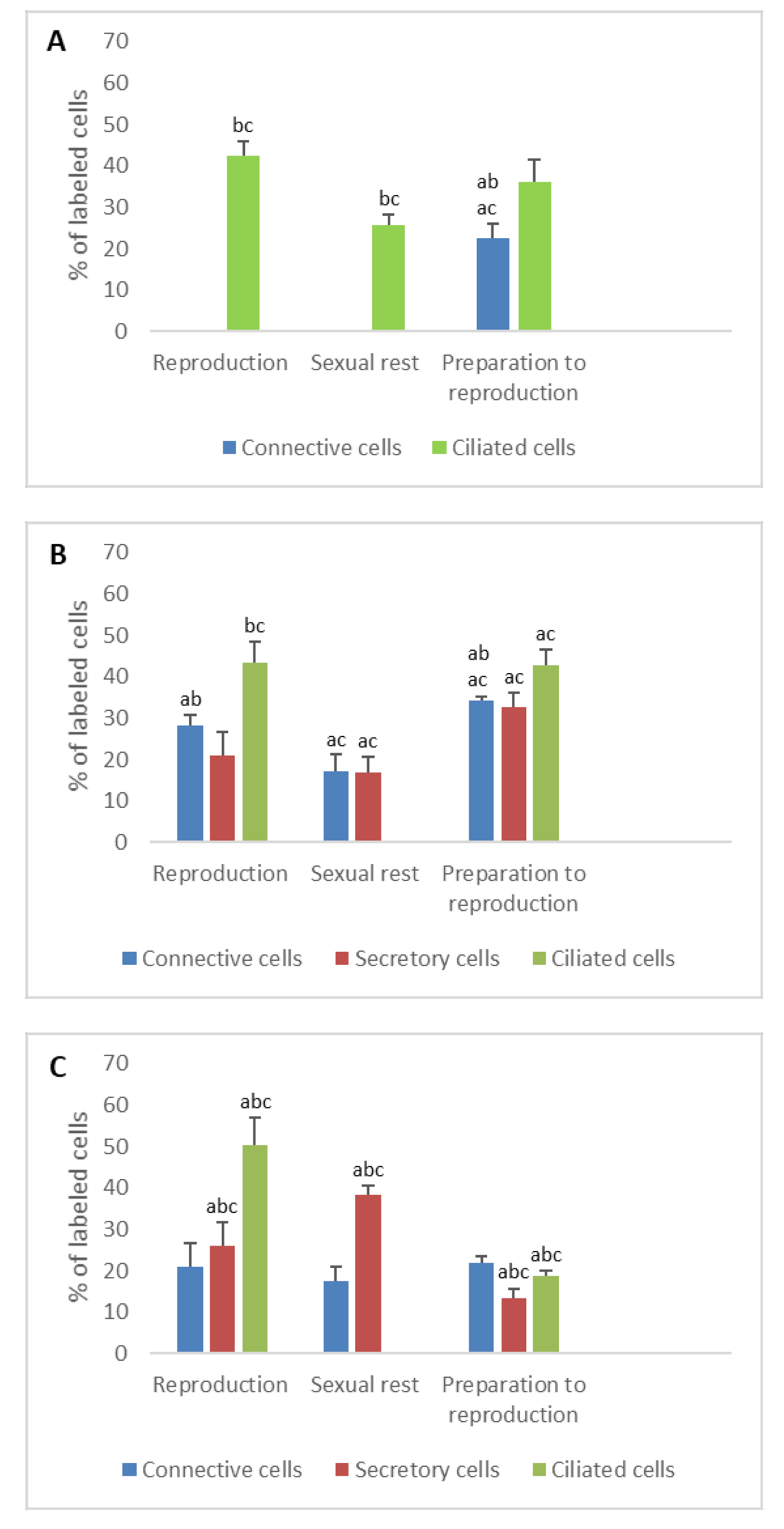
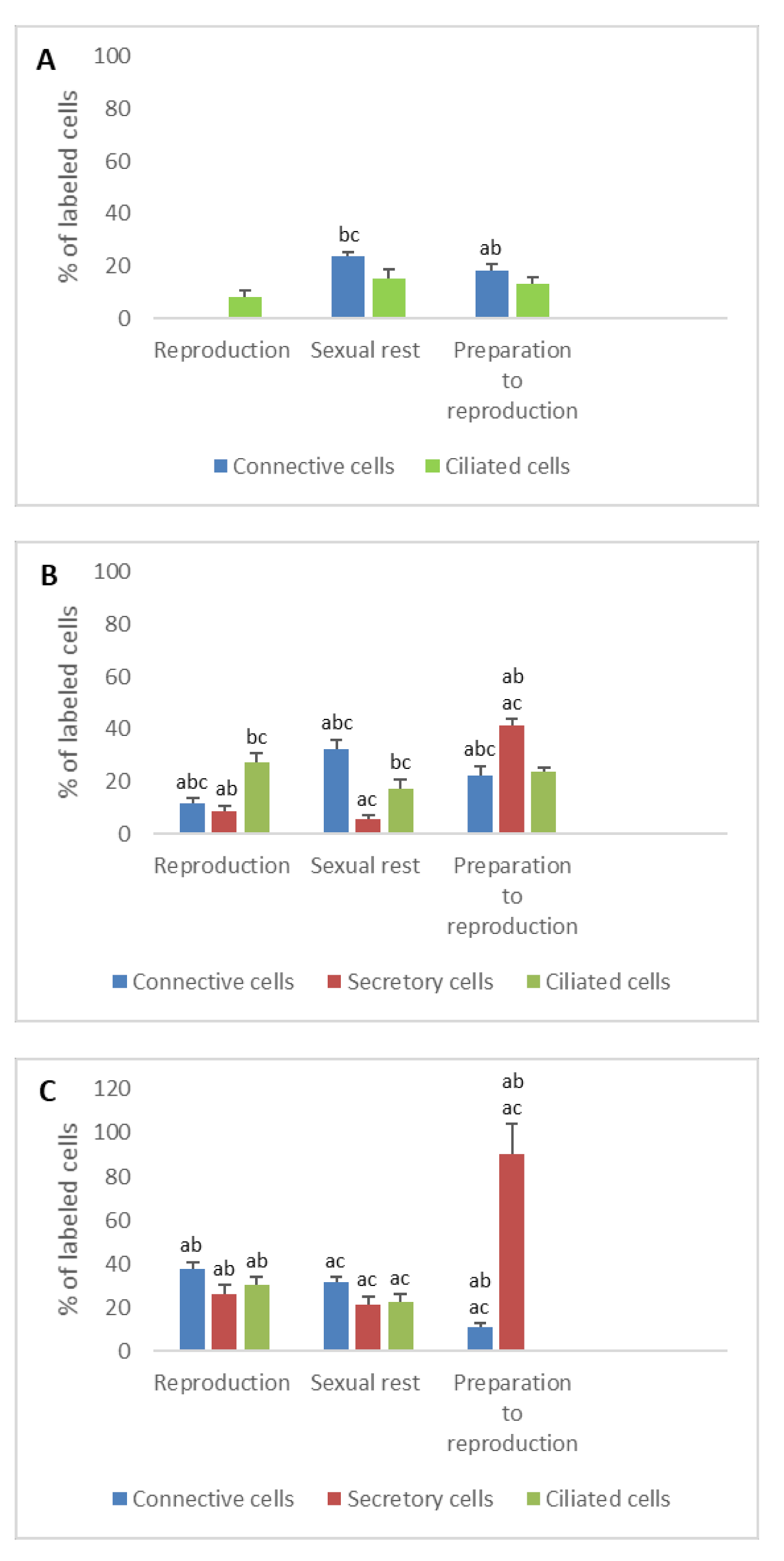
3.1. Detection of Sex Steroid Receptors
3.1.1. Progesterone Receptors (PR)
- In ostium
- In tubal part
- In uterus
3.1.2. Estrogen Receptors (ERα and ERβ)
- In ostium
- In tubal part
- In uterus
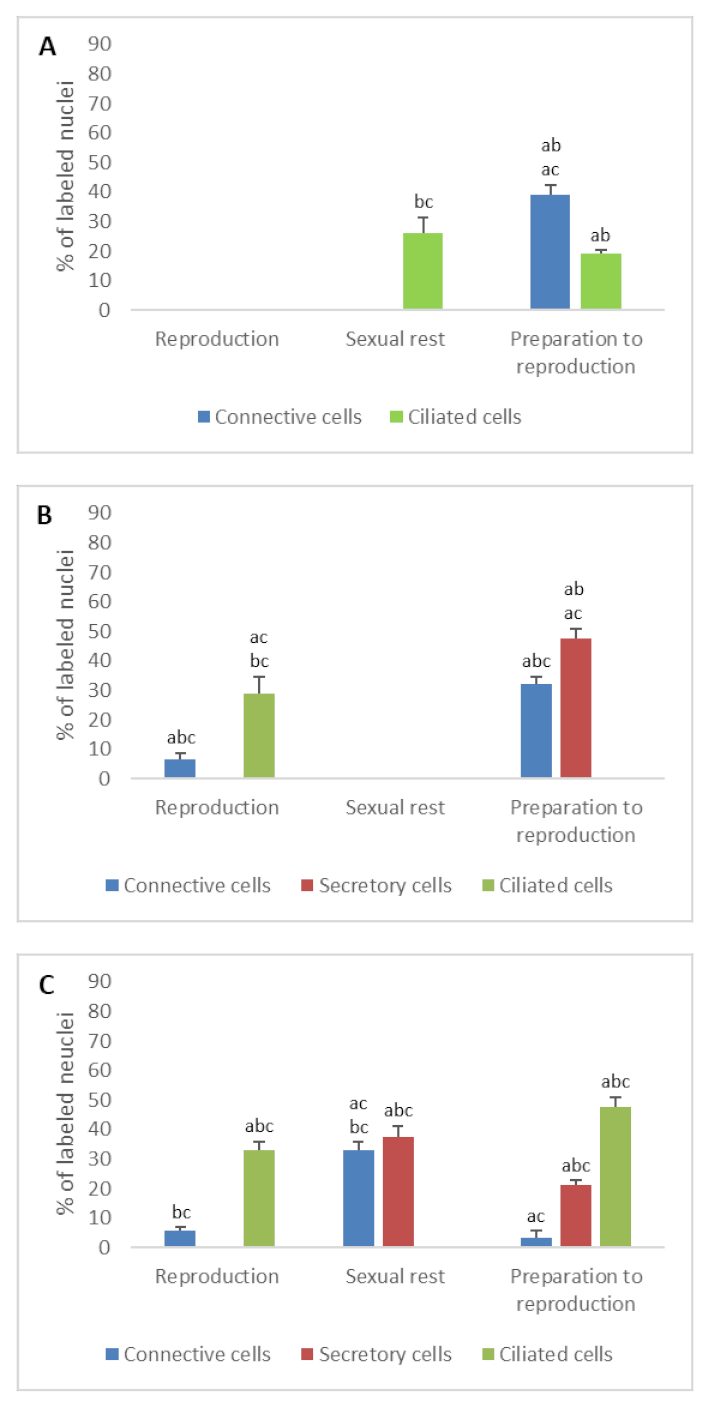
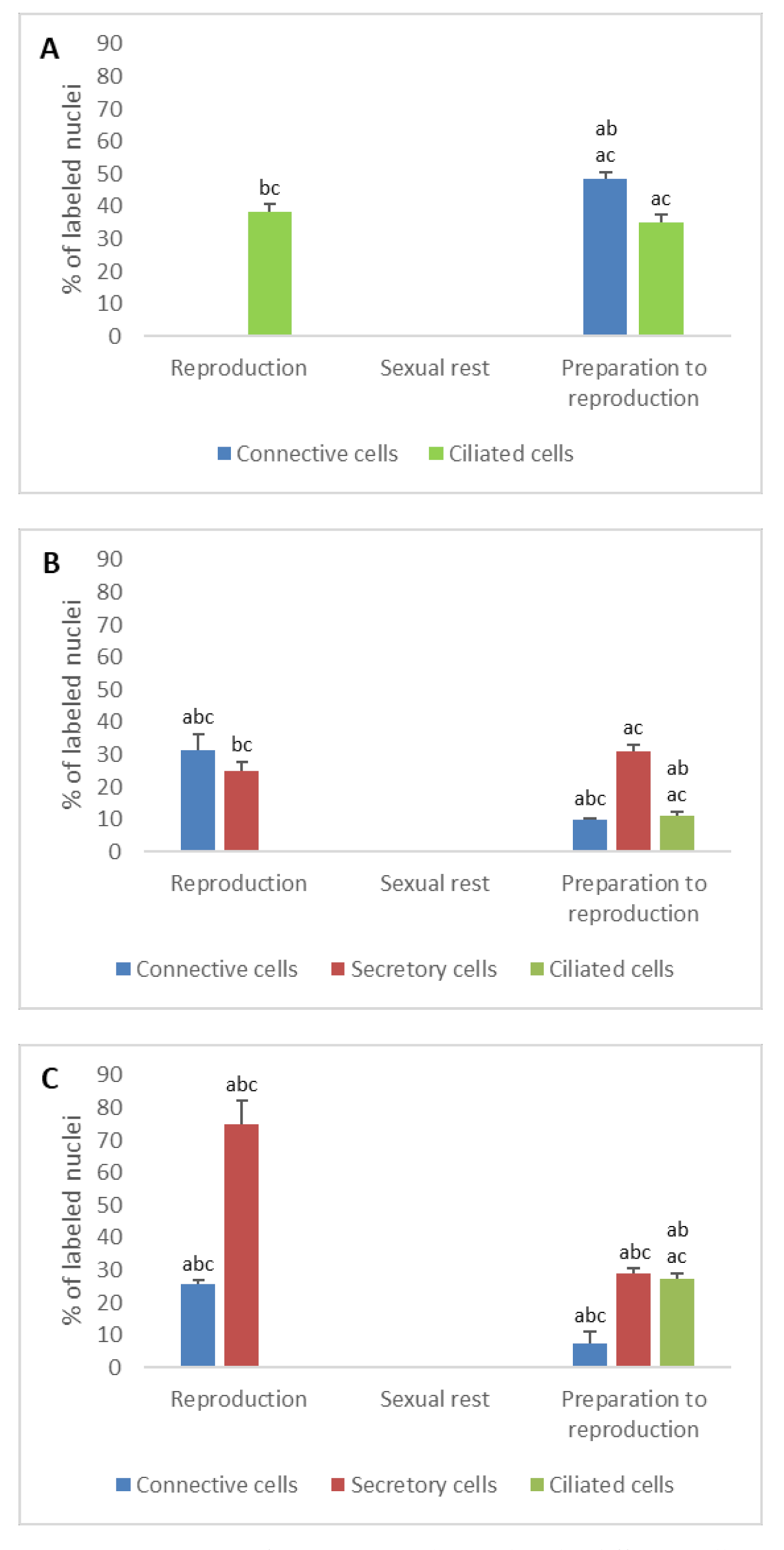
3.2. Detection of Gonadotropin Receptors (FSHR and LHR) and Prolactin Receptor (PRLR)
- In ostium
- In tubal part
- In uterus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, M.E. Steroid receptors and vertebrate evolution. Mol. Cell. Endocrinol. 2019, 496, 110526. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, S.E.; Kettler, A.; Einspanier, R. Expression and localization of estrogen receptor α, estrogen receptor β and progesterone receptor in the bovine oviduct in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2003, 84, 279–289. [Google Scholar] [CrossRef]
- Strosberg, A.D. Structure/function relationship of proteins belonging to the family of receptors coupled to GTP-binding proteins. Eur. J. Biochem. 1991, 196, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.D.; Chen, D.W.; Wang, H.M. Molecular characterization, mRNA expression of prolactin receptor (PRLR) gene during pregnancy, nonpregnancy in the yak (Bos grunniens). Gen. Comp. Endocrinol. 2012, 175, 384–388. [Google Scholar] [CrossRef]
- Rastogi, R.K.; Pinelli, C.; Polese, G.; D’Aniello, B.; Chieffi-Baccariy, D. Hormones and Reproductive Cycles in Anuran Amphibians. In Hormones and Reproduction of Vertebrates, 1st ed.; Norris, D.O., Lopez, K.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 2, pp. 171–186. [Google Scholar]
- Wake, M.H. The reproductive biology of caecilians: An evolutionary perspective. In The Reproductive Biology of Amphibians; Taylor, D.H., Guttman, S.I., Eds.; Springer: New York, NY, USA, 1977; pp. 73–101. [Google Scholar]
- Wake, M.H. Evolution of oviductal gestation in amphibians. J. Exp. Zool. 1993, 266, 394–413. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Premières observations sur le cycle annuel de l’ovaire de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Batracien Apode vivipare. C. R. Séanc Acad. Sci. Paris 1983, 296, 493–498. [Google Scholar]
- Exbrayat, J.-M. Quelques Aspects de la Biologie de la Reproduction chez Typhlonectes compressicaudus (Duméril et Bibron, 1841) Amphibien Apode. Doctor ès. Sciences Thesis, Pierre and Mary Curie University, Paris, France, 1986. [Google Scholar]
- Raquet, M.; Brun, C.; Exbrayat, J.-M. Patterns of apoptosis and proliferation throughout the biennial reproductive cycle of viviparous female Typhlonectes compressicauda (Amphibia, Gymnophiona). Int. J. Mol. Sci. 2017, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Exbrayat, J.-M. Croissance et cycle des voies génitales femelles de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Apode vivipare. Amphibia-Reptilia 1988, 9, 117–134. [Google Scholar] [CrossRef]
- Hraoui-Bloquet, S. Nutrition Embryonnaire et Relations Materno-Foetales chez Typhlonectes compressicaudus (Duméril et Bibron, 1841). Amphibien Gymnophione Vivipare. Ph.D. Thesis, EPHE, Lyon, France, 1995. [Google Scholar]
- Exbrayat, J.-M.; Collenot, G. Quelques aspects de l’évolution de l’ovaire de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Batracien Apode vivipare.—Etude quantitative et histochimique des corps jaunes. Reprod. Nutr. Dévelop. 1983, 23, 889–898. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Reproduction et organes endocrines chez les femelles d’un amphibian gymnophione vivipare Typhlonectes compressicaudus. Bull. Soc. Herp. Fr. 1992, 64, 37–50. [Google Scholar]
- Wake, M.H.; Dickie, R. Oviduct structure and function and reproductive modes in amphibians. J. Exp. Zool. 1998, 282, 477–506. [Google Scholar] [CrossRef]
- Aranzábal, M.C.U. Hormones and the female reproductive system of amphibians. In Hormones and Reproduction of Vertebrates, 1st ed.; Norris, D.O., Lopez, K.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 2, pp. 55–81. [Google Scholar]
- Tsai, P.-S. Neuroendocrine control of reproduction in Amphibians. In Hormones and Reproduction of Vertebrates, 1st ed.; Norris, D.O., Lopez, K.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 2, pp. 21–37. [Google Scholar]
- Moore, F.L. Reproductive endocrinology of amphibians. In Fundamentals of Comparative Vertebrate Endocrinology, 1st ed.; Chester-Jones, I., Ingleton, P.M., Phillips, J.G., Eds.; Springer: New York, NY, USA, 1987; pp. 207–221. [Google Scholar]
- Polzonetti-Magni, A.; Carnevali, O.; Yamamoto, K.; Kikuyama, S. Growth hormone and prolactin in amphibian reproduction. Zool. Sci. 1995, 12, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Exbrayat, J.-M.; Morel, G. The cytological modifications of the distal lobe of the hypophysis in Typhlonectes compressicaudus (Duméril and Bibron, 1841), amphibia gymnophiona, during the cycles of seasonal activity. II: In adult females. Biol. Struct. Morphog. 1990–1991, 3, 129–138. [Google Scholar]
- Exbrayat, J.-M.; Morel, G. Prolactin (PRL)-coding mRNA in Typhlonectes compressicaudus, a viviparous gymnophionan amphibian: An in situ hybridization study. Cell Tissue Res. 1995, 280, 133–138. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. (Ed.) Endocrinology of reproduction. In Reproductive Biology and Phylogeny of Gymnophiona (Caecilians); Series edited by B.G.M. Jamieson; Science Publishers: Enfield, NH, USA, 2006; pp. 183–229. [Google Scholar]
- Exbrayat, J.-M.; Ouhtit, A.; Morel, G. Prolactin (PRL) and prolactin receptor (PRL-R) mRNA expression in Typhlonectes compressicaudus (Amphibia, Gymnophiona) male genital organs. A in situ hybridization study. Ann. Endocrinol. 1996, 57, 55. [Google Scholar]
- Exbrayat, J.-M.; Ouhtit, A.; Morel, G. Visualization of gene expression of prolactin receptors (PRL-R) by in situ hybridization, in Typhlonectes compressicaudus, a gymnophionan amphibian. Life Sci. 1997, 61, 1915–1928. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Genome Visualization by Classic Methods in Light Microscopy; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2000; pp. 1–195. [Google Scholar]
- Nandi, J. Comparative Endocrinology of Steroid Hormones in Vertebrates. Am. Zool. 1967, 7, 115–133. [Google Scholar] [CrossRef]
- Lange, G. Evolution of estrogen functions in vertebrates. J. Steroid Biochem. Mol. Biol. 2002, 83, 219–226. [Google Scholar] [CrossRef]
- Weiler, I.J.; Lew, D.; Shapiro, D.J. The Xenopus laevis estrogen receptor: Sequence homology with human and avian receptors and identification of multiple estrogen receptor messenger ribonucleic acids. Mol. Endocrinol. 1987, 1, 355–362. [Google Scholar] [CrossRef]
- Katsu, Y.; Taniguchi, E.; Urushitani, H.; Miyagawa, S.; Takase, M.; Kubokawa, K.; Tooi, O.; Oka, T.; Santo, N.; Myburgh, J.; et al. Molecular cloning and characterization of ligand-and species-specificity of amphibian estrogen receptors. Gen. Comp. Endocrinol. 2010, 168, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Takase, M.; Iguchi, T. Molecular cloning of two isoforms of Xenopus (Silurana) tropicalis estrogen receptor mRNA and their expression during development. Biochim. Biophys. Acta 2007, 1769, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sanyal, S.; Oh, D.; Kim, J.Y.; Ju, J.W.; Song, K.H.; Kim, J.W.; Kwon, H.B.; Choi, S.H. Molecular cloning and characterization of an amphibian progesterone receptor from Rana dybowskii. Gen. Comp. Endocrinol. 2004, 135, 142–149. [Google Scholar] [CrossRef]
- Suda, M.; Kodama, M.; Oshima, Y.; Yamamoto, K.; Nakamura, Y.; Tanaka, S.; Kikuyama, S.; Nakamura, M. Up-regulation of FSHR expression during gonadal sex determination in the frog Rana rugosa. Gen. Comp. Endocrinol. 2011, 172, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Sengezer-Inceli, M.S.; Kaptan, E.; Sancar, S.; Murathanoglu, O.; Castillo, S.S. Localization of prolactin receptor in the dorsal and ventral skin of the frog (Rana ridibunda). Biologia 2010, 65, 157–163. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. From bony fishes to mammals: Reproductive cycles in vertebrates, hormones and hormone-receptors. Recept. Clin. Investig. 2014, 1, e86. [Google Scholar]
- Hewitt, S.C.; Korach, K.S. Estrogen receptors: New directions in the new millennium. Endocr. Rev. 2018, 39, 664–675. [Google Scholar] [CrossRef]
- Li, S.; O’Neill, S.R.; Zhang, Y.; Holtzman, M.J.; Takemaru, K.-I.; Korach, K.S.; Winuthayanon, W. Estrogen receptor α is required for oviducal transport of embryos. FASEB J. 2017, 31, 1595–1607. [Google Scholar] [CrossRef]
- Iwata, T.; Toyoda, F.; Yamamoto, K.; Kikuyama, S. Hormonal control of urodele reproductive behavior. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 221–229. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brun, C.; Exbrayat, J.-M.; Raquet, M. Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda. Animals 2021, 11, 2. https://doi.org/10.3390/ani11010002
Brun C, Exbrayat J-M, Raquet M. Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda. Animals. 2021; 11(1):2. https://doi.org/10.3390/ani11010002
Chicago/Turabian StyleBrun, Claire, Jean-Marie Exbrayat, and Michel Raquet. 2021. "Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda" Animals 11, no. 1: 2. https://doi.org/10.3390/ani11010002
APA StyleBrun, C., Exbrayat, J.-M., & Raquet, M. (2021). Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda. Animals, 11(1), 2. https://doi.org/10.3390/ani11010002




