Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Conditions of Hen-Keeping and Rooster Semen Collection
2.2. Composition of Media for the Cryopreservation of Rooster Semen
2.3. Semen Freezing and Thawing
2.4. Motility Evaluation of Frozen/Thawed Semen
2.5. Artificial Insemination
2.6. Lifespan Assessment of Frozen/Thawed Sperm in the Genital Tracts of Hens
2.7. Statistical Analysis
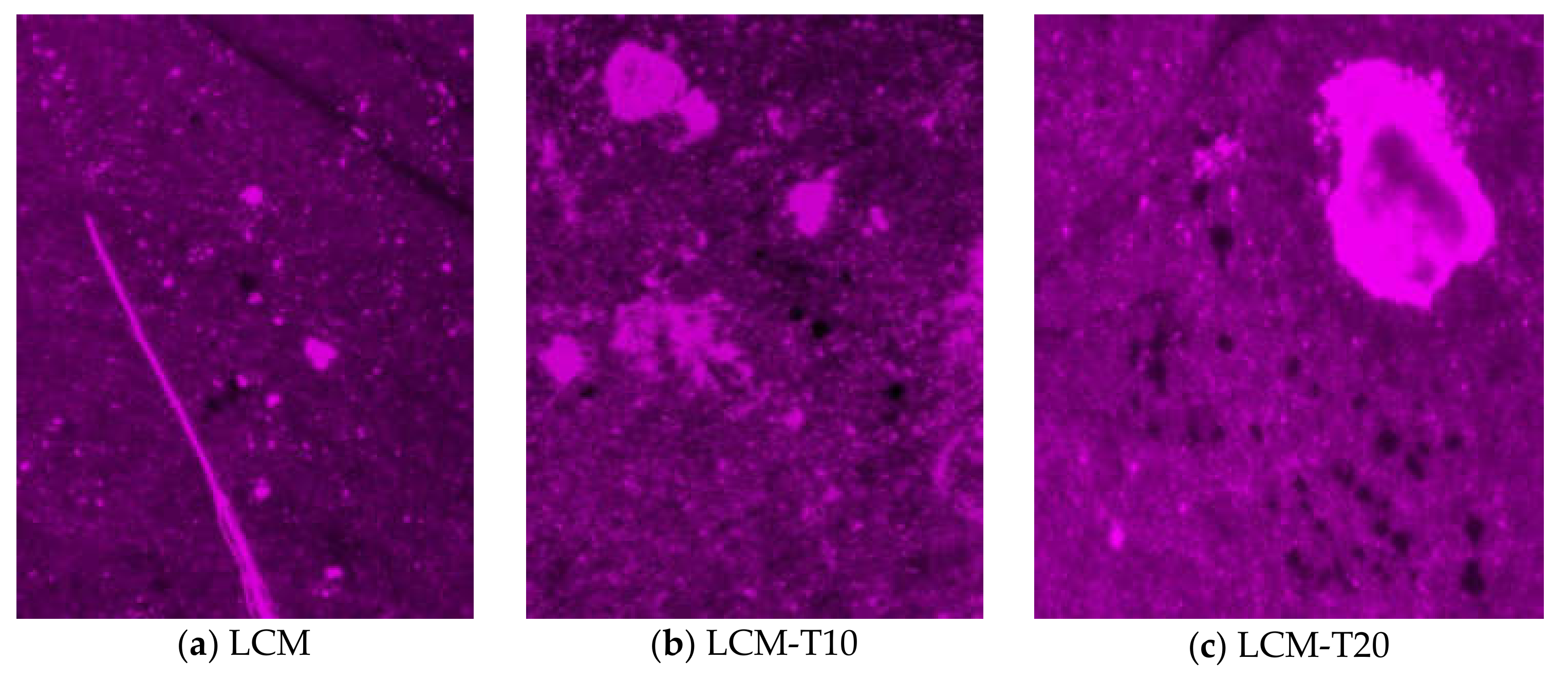
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scherf, B.D.; Pilling, D.; FAO Commission on Genetic Resources for Food and Agriculture Assessments. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture. Rome. 2015. Available online: http://www.fao.org/3/a-i4787e/index.html (accessed on 2015).
- Cavalchini, L.G.; Marelli, S.P.; Strillacci, M.G.; Cozzi, M.C.; Polli, M.; Longeri, M. Heterozygosity analysis of Bionda Piemontese and Bianca di Saluzzo chicken breeds by microsatellites markers: A preliminary study. Ital. J. Anim. Sci. 2007, 6, 63–65. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Hussain, I.; Anwar, M.; Akhter, S.; Blesbois, E. Comparison of extenders for liquid storage of Indian Red Jungle Fowl (Gallus gallus murghi) spermatozoa. Avian Biol. Res. 2016, 9, 207–212. [Google Scholar] [CrossRef]
- Long, J.A.; Bongalhardo, D.C.; Pelaez, J.; Saxena, S.; Settar, P.; O’Sullivan, N.P.; Fulton, J.E. Rooster semen cryopreservation: Effect of pedigree line and male age on post-thaw sperm function. Poult. Sci. 2010, 89, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen Cryopreservation for ex situ Management of Genetic Diversity in Chicken: Creation of the French Avian Cryobank. Poult. Sci. 2007, 86, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, Y.; Aygun, A. Poultry semen cryopreservation technologies. World’s Poult. Sci. J. 2018, 74, 699–710. [Google Scholar] [CrossRef]
- Fulton, J.E. Avian genetic stock preservation: An industry perspective. Poult. Sci. 2006, 85, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Silyukova, Y.; Pleshanov, N.; Stanishevskaya, O. The influence membranes damage andactivity of roosters’ sperm on the fertilization of eggs when using cured cryopreserved sperm. Reprod. Domest. Anim. 2019, 54, 101. [Google Scholar]
- Mosca, F.; Zaniboni, L.; Sayed, A.A.; Madeddu, M.; Iaffaldano, N.; Cerolini, S. Effect of dimethylacetamide and N-methylacetamide on the quality and fertility of frozen/thawed chicken semen. Poult. Sci. 2019, 98, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.J.; Acton, E.; Murray, B.J.; Fonseca, F. Freezing injury: The special case of the sperm cell. Cryobiology 2012, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Thananurak, P.; Chuaychu-Noo, N.; Thélie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019, 98, 4161–4171. [Google Scholar] [CrossRef]
- Smith, A.M.J.; Bonato, M.; Dzama, K.; Malecki, I.A.; Cloete, S.W.P. Mineral profiling of Ostrich (Struthio camelus) seminal plasma and its relationship with semen traits and collection day. Anim. Reprod. Sci. 2018, 193, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef]
- Starciuc, T.; Malfait, B.; Danede, F.; Paccou, L.; Guinet, Y.; Correia, N.T.; Hedoux, A. Trehalose or Sucrose: Which of the Two Should be Used for Stabilizing Proteins in the Solid State? A Dilemma Investigated by In Situ Micro-Raman and Dielectric Relaxation Spectroscopies During and After Freeze-Drying. J. Pharm. Sci. 2019, 109, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Thananurak, P.; Chuaychu-Noo, N.; Phasuk, Y.; Vongpralub, T. Comparison of TNC and standard extender on post-thaw quality and in vivo fertility of Thai native chicken sperm. Cryobiology 2020, 1, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Yee, D.; Nagarajan, N.; Bianchi, F.; Saito, T.; Valenti, V.; Tong, M.; del Re, D.P.; Vecchione, C.; Schirone, L.; et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 1999–2010. [Google Scholar] [CrossRef]
- Attfield, P.V. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 1987, 225, 259–263. [Google Scholar] [CrossRef]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Huang, H.; Wang, H.; Zhao, S.; Dumbleton, J.; Zhao, G.; He, X. Nanoparticle-Mediated Intracellular Delivery Enables Cryopreservation of Human Adipose-Derived Stem Cells Using Trehalose as the Sole Cryoprotectant. ACS Appl. Mater. Interfaces 2015, 7, 5017–5028. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.P.; Mysyakina, I.S.; Usov, A.I.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Microbiology 2014, 83, 184–194. [Google Scholar] [CrossRef]
- Giaever, H.M.; Styrvold, O.B.; Kaasen, I.; Strom, A.R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J. Bacteriol. 1988, 170, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Madeddu, M.; Sayed, A.A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology 2016, 73, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.M.; Gee, G.; Wild, D.E.; Tselutin, K.; Donoghue, A.M. Semen cryopreservation in poultry and non-domestic species: A comparative approach to understanding the fundamentals of avian spermatozoa cryobiology. Br. Poult. Sci. 2000, 41, 6–7. [Google Scholar] [CrossRef]
- Tselutin, K.V.; Tur, B.K. Artificial Insemination and Cryopreservation of Poultry’s Sperm (Roosters, Indica, Gander, Drake); The Russian Academy of Agricultural Sciences: Saint-Petersburg, Russia, 2013; p. 88. [Google Scholar]
- Burrows, W.A.; Quinn, J.P. The method of obtaining spermatozoa from the domestic fowl. Poult. Sci. 1935, 14, 251–254. [Google Scholar] [CrossRef]
- Bakst, M.; Eastridge, J.; Malecki, I. The inner perivitelline layer sperm hole assay: Use of filter paper rings for the isolation of the perivitelline layer overlying the germinal disc and new observations on its morphology. J. Appl. Poult. Res. 2014, 23, 121–128. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Bernal, B.; Iglesias-Cabeza, N.; Sánchez-Rivera, U.; Toledano-Díaz, A.; Castaño, C.; Pérez-Cerezales, S.; Gutiérrez-Adán, A.; López-Sebastián, A.; García-Casado, P.; Gil, M.G.; et al. Effect of supplementation of valine to chicken extender on sperm cryoresistance and post-thaw fertilization capacity. Poult. Sci. 2020, 99, 7133–7141. [Google Scholar] [CrossRef]
- Svoradová, A.; Kuželová, L.; Vašíček, J.; Baláži, A.; Hanusová, E.; Chrenek, P. In vitro effect of various cryoprotectants on the semen quality of endangered Oravka chicken. Zygote 2017, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Madeddu, M.; Sayed, A.A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Data on the positive synergic action of dimethylacetamide and trehalose on quality of cryopreserved chicken sperm. Data Brief 2016, 9, 1118–1121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.H.; Aksan, A.; Menze, M.A.; Hand, S.C.; Toner, M. Trehalose loading through the mitochondrial permeability transition pore enhances desiccation tolerance in rat liver mitochondria. Biochim. Biophys. Acta Biomembr. 2005, 1717, 21–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Rong, J.; Wang, Q.; He, X. The encapsulation and intracellular delivery of trehalose using a thermally responsive nanocapsule. Nanotechnology 2009, 20, 275101. [Google Scholar] [CrossRef] [PubMed]
| Day of Experiment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 14th | 15th | 20th | 25th | |
| Insemination | + | + | + | + | + | ||||||||||
| Collecting eggs for incubation | + | + | + | + | + | + | + | + | + | ||||||
| Collecting eggs for blastodisc assessment | + | + | + | + | |||||||||||
| Semen | Average Semen Motility % | ||
|---|---|---|---|
| LCM-Control * | LCM-T10 (Trehalose 10%) | LCM-T20 (Trehalose 20%) | |
| Native | 85 | 85 | 85 |
| Frozen/thawed | 48 | 48 | 50 |
| Medium | Eggs Laid, psc. | Egg Fertility Rates, % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Experiment | Results for the Entire Period of the Experiment % | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 14 | 15 | 20 | 25 | |||
| LCM-control * | 130 | x | x | 73 | 100 | 100 | 90 | 86 | 91 | 100 | 100 | 100 | 90 | 86 | 20 | 0 | 79 |
| LCM-T10 | 131 | x | x | 76 | 100 | 100 | 90 | 100 | 100 | 92 | 100 | 100 | 95 | 92 | 55 | 0 | 82 |
| LCM-T20 | 139 | x | x | 67 | 100 | 92 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 50 | 15 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanishevskaya, O.; Silyukova, Y.; Pleshanov, N.; Kurochkin, A.; Fedorova, E.; Fedorova, Z.; Perinek, O.; Prituzhalova, A.; Meftakh, I. Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa. Animals 2021, 11, 189. https://doi.org/10.3390/ani11010189
Stanishevskaya O, Silyukova Y, Pleshanov N, Kurochkin A, Fedorova E, Fedorova Z, Perinek O, Prituzhalova A, Meftakh I. Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa. Animals. 2021; 11(1):189. https://doi.org/10.3390/ani11010189
Chicago/Turabian StyleStanishevskaya, Olga, Yulia Silyukova, Nikolai Pleshanov, Anton Kurochkin, Elena Fedorova, Zoya Fedorova, Oksana Perinek, Anna Prituzhalova, and Inessa Meftakh. 2021. "Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa" Animals 11, no. 1: 189. https://doi.org/10.3390/ani11010189
APA StyleStanishevskaya, O., Silyukova, Y., Pleshanov, N., Kurochkin, A., Fedorova, E., Fedorova, Z., Perinek, O., Prituzhalova, A., & Meftakh, I. (2021). Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa. Animals, 11(1), 189. https://doi.org/10.3390/ani11010189





