Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plants and Their Enrichment in Tannins and Saponins by Extraction
2.2. In Vitro Rumen Fermentation
2.3. Chemical Composition, Fermentation Product and Microbial Analyses
2.4. Statistical Analysis and Calculations
3. Results and Discussion
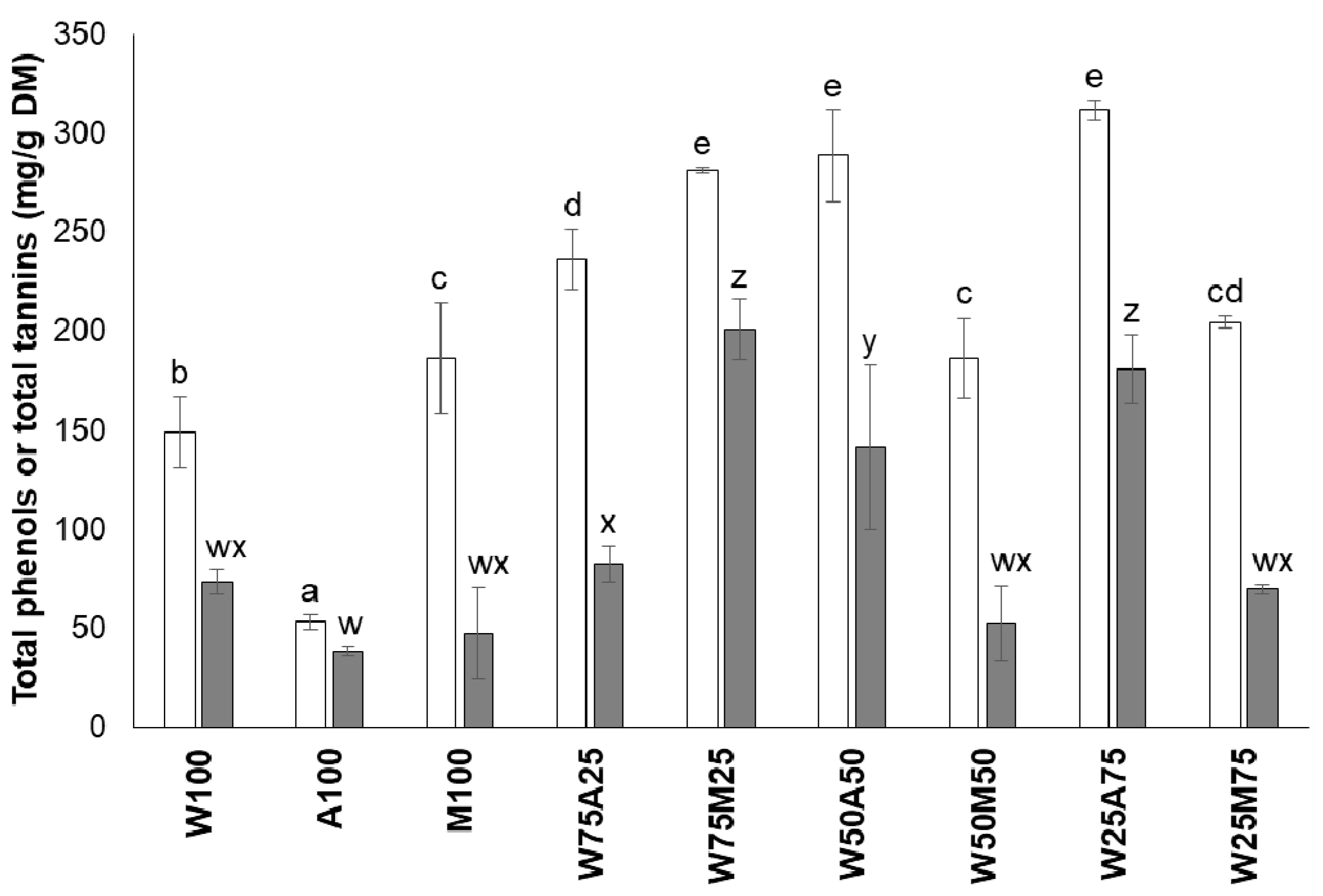
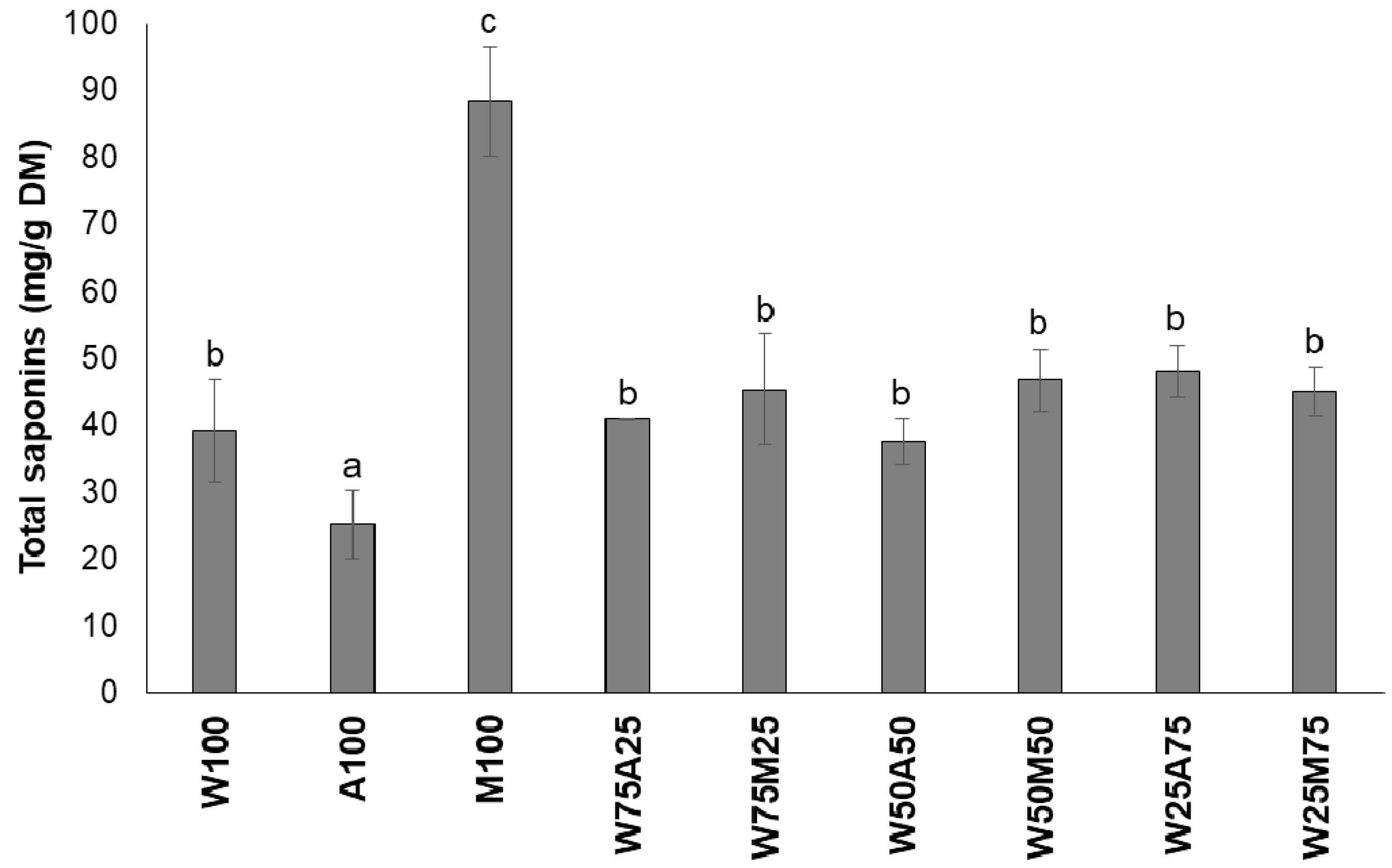
3.1. Effect of Type of Solvent on the Efficiency of Extraction of Tannins and Saponins
3.2. Effects of Basal Diet Type
3.3. Effects of Plant Extracts Rich in Tannins and Saponins and Their Interaction with Diet Type
3.4. Effects of Combining Plant Extracts Rich in Tannins and Saponins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Shibata, M.; Terada, F. Factors affecting methane production and mitigation in ruminants. Anim. Sci. J. 2010, 81, 2–10. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Dangal, S.R.S.; Tian, H.; Zhang, B.; Pan, S.; Lu, C.; Yang, J. Methane emission from global livestock sector during 1890–2014: Magnitude, trends and spatiotemporal patterns. Glob. Chang. Biol. 2017, 23, 4147–4161. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Cottle, D.J.; Nolan, J.V.; Wiedemann, S.G. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 2011, 51, 491–514. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Inventories: Agriculture, forestry and other land use. In Emissions from Livestock and Manure Management; Institute of Global Environmental Strategies: Kanagawa, Japan, 2006; Volume 4, pp. 10.1–10.87. [Google Scholar]
- Dijkstra, J.; Oenema, O.; Bannink, A. Dietary strategies to reducing N excretion from cattle: Implications for methane emissions. Curr. Opin. Environ. Sustain. 2011, 3, 414–422. [Google Scholar] [CrossRef]
- Mitsumori, M.; Enishi, O.; Shinkai, T.; Higuchi, K.; Kobayashi, Y.; Takenaka, A.; Nagashima, K.; Mochizuki, M.; Kobayashi, Y. Effect of cashew nut shell liquid on metabolic hydrogen flow on bovine rumen fermentation. Anim. Sci. J. 2014, 85, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Pirondini, M.; Colombini, S.; Malagutti, L.; Rapetti, L.; Galassi, G.; Zanchi, R.; Crovetto, G.M. Effects of a selection of additives on in vitro ruminal methanogenesis and in situ and in vivo NDF digestibility. Anim. Sci. J. 2015, 86, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.D.; Kreuzer, M.; Diaz, T.E.; Lascano, C.E.; Carulla, J.E.; Soliva, C.R.; Machmüller, A. Saponin rich tropical fruits affect fermentation and methanogenesis in faunated and defaunated rumen fluid. Anim. Feed Sci. Technol. 2003, 109, 79–94. [Google Scholar] [CrossRef]
- Hess, H.D.; Beuret, R.A.; Loetscher, M.; Hindrichsen, I.K.; Machmüller, A.; Carulla, J.E.; Lascano, C.E.; Kreuzer, M. Ruminal fermentation, methanogenesis and nitrogen utilization of sheep receiving tropical grass hay-concentrate diets offered with Sapindus saponaria fruits and Cratylia argentea foliage. Anim. Sci. 2004, 79, 177–189. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Wina, E.; Takahashi, J. Meta-analysis on methane mitigating properties of saponin-rich sources in the rumen in vitro: Influence of addition levels and plant sources. Asian Australas. J. Anim. Sci. 2014, 27, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Stochmal, A.; Pecio, L.; Oleszek, W.; Pers-Kamczyc, E.; Szczechowiak, J.; Nowak, A.; Szumacher-Strabel, M. Rumen antimethanogenic effect of Saponaria officinalis L. phytochemicals in vitro. J. Agric. Sci. 2014, 152, 981–993. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Soliva, C.R.; Machmüller, A.; Kreuzer, M. Efficacy of plant extracts rich in secondary constituents to modify rumen fermentation. Anim. Feed Sci. Technol. 2002, 101, 101–114. [Google Scholar] [CrossRef]
- Sinz, S.; Marquardt, S.; Soliva, C.R.; Braun, U.; Liesegang, A.; Kreuzer, M. Phenolic plant extracts are additive in their effects against in vitro ruminal methane and ammonia formation. Asian-Australas. J. Anim. Sci. 2019, 32, 966–976. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T.; Chen, D.; Zhang, N.; Si, B.; Deng, K.; Tu, Y.; Diao, Q. Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in Dorper crossbred ewe. Animals 2019, 9, 29. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blummel, M.; Becker, K. In vitro effects of and interactions between tannins and saponins and fate of tannins in the rumen. J. Sci. Food Agric. 1995, 69, 481–493. [Google Scholar] [CrossRef]
- Wina, E.; Muetzel, S.; Hoffmann, E.; Makkar, H.P.S.; Becker, K. Saponins containing methanol extract of Sapindus rarak affect microbial fermentation, microbial activity and microbial community structure in vitro. Anim. Feed Sci. Technol. 2005, 121, 159–174. [Google Scholar] [CrossRef]
- Jayanegara, A.; Marquardt, S.; Wina, E.; Kreuzer, M.; Leiber, F. In vitro indications for favourable non-additive effects on ruminal methane mitigation between high-phenolic and high-quality forages. Br. J. Nutr. 2013, 109, 615–622. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Hiai, S.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Yanez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. Camb. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Federation of Animal Science Societies. Guidelines for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Savoy, IL, USA, 2010; Available online: https://aaalac.org/about/Ag_Guide_3rd_ed.pdf (accessed on 2 June 2019).
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Fievez, V.; Babayemi, O.J.; Demeyer, D. Estimation of direct and indirect gas production in syringes: A tool to estimate short chain fatty acid production that requires minimal laboratory facilities. Anim. Feed Sci. Technol. 2005, 123–124, 197–210. [Google Scholar] [CrossRef]
- Nocek, J.E.; Hart, S.P.; Polan, C.E. Rumen ammonia concentration as influenced by storage time, freezing and thawing, acid preservative, and method of ammonia determination. J. Dairy Sci. 1987, 70, 601–607. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-ul-haq, M.; Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Anele, U.Y.; Refat, B.; Swift, M.L.; Zhao, Y.L.; Doublier, C.; McAllister, T.A.; Yang, W.Z. In vitro ruminal fermentation of ground and dry-rolled barley grain differing in starch content. Anim. Feed Sci. Technol. 2015, 203, 88–94. [Google Scholar] [CrossRef]
- Lovett, D.; Lovell, S.; Stack, L.; Callan, J.; Finlay, M.; Conolly, J.; O’Mara, F.P. Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers. Livest. Prod. Sci. 2003, 84, 135–146. [Google Scholar] [CrossRef]
- Grandl, F.; Zeitz, J.O.; Clauss, M.; Furger, M.; Kreuzer, M.; Schwarm, A. Evidence for increasing digestive and metabolic efficiency of energy utilization with age of dairy cattle as determined in two feeding regimes. Animal 2018, 12, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Olijhoek, D.W.; Løvendahl, P.; Lassen, J.; Hellwing, A.L.F.; Höglund, J.K.; Weisbjerg, M.R.; Noel, S.J.; McLean, F.; Højberg, O.; Lund, P. Methane production, rumen fermentation, and diet digestibility of Holstein and Jersey dairy cows being divergent in residual feed intake and fed at 2 forage-to-concentrate ratios. J. Dairy Sci. 2018, 101, 9926–9940. [Google Scholar] [CrossRef]
- Machmüller, A.; Soliva, C.R.; Kreuzer, M. Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion. Br. J. Nutr. 2003, 90, 529–540. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Becker, K. Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J. Appl. Microbiol. 2008, 105, 770–777. [Google Scholar] [CrossRef]
- Narvaez, N.; Wang, Y.; McAllister, T. Effects of extracts of Humulus lupulus (hops) and Yucca schidigera applied alone or in combination with monensin on rumen fermentation and microbial populations in vitro. J. Sci. Food Agric. 2013, 93, 2517–2522. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Liu, J.X.; Lu, Y.; Zhu, W.Y.; Denman, S.E.; McSweeney, C.S. Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen micro-organisms. Lett. Appl. Microbiol. 2008, 47, 421–426. [Google Scholar] [CrossRef]
- Wallace, R.J.; Arthaud, L.; Newbold, C.J. Influence of Yucca schidigera extract on ruminal ammonia concentrations and ruminal microorganisms. Appl. Environ. Microbiol. 1994, 60, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McAllister, T.A.; Yanke, L.J.; Cheeke, P.R. Effect of steroidal saponin from Yucca schidigera extract on ruminal microbes. J. Appl. Microbiol. 2000, 88, 887–896. [Google Scholar] [CrossRef] [PubMed]
| Item | Napier Grass | Concentrate | HF Diet | HC Diet |
|---|---|---|---|---|
| Organic matter | 881 | 942 | 899 | 924 |
| Crude protein | 90 | 184 | 118 | 156 |
| Neutral detergent fiber | 656 | 270 | 540 | 386 |
| Acid detergent fiber | 447 | 117 | 348 | 216 |
| Acid detergent lignin | 94 | 50 | 81 | 63 |
| Diet | Extract | Total Gas (mL/g DM) | Methane (mL/L Total Gas) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| HF | C | 186 b | 233 a | 277 d | 286 d |
| T100 | 182 a,b | 242 b | 221 a,b | 239 b,c | |
| S100 | 173 a | 233 a | 232 b,c | 233 a,b | |
| T25S75 | 179 a,b | 245 b | 218 a | 226 a | |
| T50S50 | 184 b | 247 b | 221 a,b | 229 a,b | |
| T75S25 | 187 b | 243 b | 221 a,b | 229 a,b | |
| HC | C | 236 d | 275 c | 238 c | 247 c |
| T100 | 232 d | 281 c,d | 218 a | 229 a,b | |
| S100 | 229 c,d | 281 c,d | 227 a,b,c | 236 a,b | |
| T25S75 | 221 c | 277 c,d | 223 a,b | 230 a,b | |
| T50S50 | 235 d | 287 e | 225 a,b | 232 a,b | |
| T75S25 | 236 d | 285 d,e | 223 a,b | 232 a,b | |
| SEM | 2.8 | 2.4 | 2.1 | 2.0 | |
| p-value | |||||
| Diet | <0.001 | <0.001 | 0.003 | 0.002 | |
| Extract | 0.001 | <0.001 | <0.001 | <0.001 | |
| Diet × extract | 0.442 | 0.182 | <0.001 | <0.001 | |
| Diet | Extract | IVDMD | IVOMD | Ammonia | Bacteria | Protozoa |
|---|---|---|---|---|---|---|
| (mg/g) | (mg/g) | (mmol/L) | (log/mL) | (log/mL) | ||
| HF | C | 629 d | 704 f | 24.4 f | 8.56 | 6.04 e |
| T100 | 463 a | 449 a,b | 20.2 d | 8.55 | 6.03 d,e | |
| S100 | 520 b | 505 c | 20.2 d | 8.48 | 5.24 a | |
| T25S75 | 47 9 a | 483 b,c | 18.7 c | 8.53 | 5.68 b | |
| T50S50 | 470 a | 444 a | 16.6 a | 8.47 | 5.62 b | |
| T75S25 | 467 a | 477 a,b,c | 16.5 a | 8.38 | 5.94 c,d | |
| HC | C | 674 e | 695 f | 26.2 g | 8.23 | 5.98 c,d,e |
| T100 | 526 b | 548 d | 19.2 c | 8.54 | 6.01 c,d,e | |
| S100 | 604 c,d | 609 e | 21.5 e | 8.65 | 5.26 a | |
| T25S75 | 598 c | 608 e | 20.7 d | 8.41 | 5.61 b | |
| T50S50 | 549 b | 569 d | 18.8 c | 8.53 | 5.68 b | |
| T75S25 | 550 b | 564 d | 17.6 b | 8.58 | 5.92 c | |
| SEM | 8.4 | 13.2 | 0.32 | 0.034 | 0.041 | |
| p-value | ||||||
| Diet | <0.001 | <0.001 | <0.001 | 0.964 | 0.394 | |
| Extract | <0.001 | <0.001 | <0.001 | 0.790 | <0.001 | |
| Diet × extract | 0.033 | <0.001 | <0.001 | 0.277 | 0.223 |
| Diet | Extract | Total VFA | C2 | C3 | C4 | isoC4 | C5 | isoC5 | C2/C3 |
|---|---|---|---|---|---|---|---|---|---|
| (mmol/L) | (%) | (%) | (%) | (%) | (%) | (%) | |||
| HF | C | 84.6 b,c | 54.9 b,c | 26.2 a | 9.80 d,e | 4.40 c | 1.54 d,e | 3.20 c | 2.10 d,e |
| T100 | 80.8 a,b | 55.7 b,c | 27.9 a,b | 8.82 a,b,c | 4.15 b,c | 1.30 b,c,d,e | 2.12 a | 2.00 c,d,e | |
| S100 | 77.7 a,b | 52.6 a,b | 32.5 e | 8.15 a | 3.36 a | 1.06 a,b,c | 2.05 a | 1.63 a | |
| T25S75 | 83.4 a,b,c | 55.2 b,c | 30.3 c,d | 8.10 a | 3.49 a,b | 0.96 a,b | 1.92 a | 1.84 a,b,c | |
| T50S50 | 91.4 b,c | 56.8 c | 28.9 b,c | 8.08 a | 3.50 a,b | 0.85 a | 1.85 a | 1.97 c,d,e | |
| T75S25 | 63.1 a | 56.0 c | 29.8 b,c,d | 8.32 a,b | 3.76 a,b,c | 0.96 a,b | 2.08 a | 1.90 c,d | |
| HC | C | 79.9 a,b | 55.4 b,c | 26.0 a | 10.3 e | 3.83 a,b,c | 1.45 c,d,e | 3.00 b,c | 2.14 e |
| T100 | 76.0 a,b | 55.4 b,c | 27.8 a,b | 9.26 c,d | 3.76 a,b,c | 1.14 a,b,c,d | 2.38 a,b | 2.00 c,d,e | |
| S100 | 102 c | 51.4 a | 31.4 d,e | 8.52 a,b,c | 3.53 a,b | 1.69 e | 2.33 a | 1.66 a,b | |
| T25S75 | 84.8 b,c | 53.8 a,b,c | 29.7 b,c,d | 9.08 b,c,d | 3.67 a,b | 1.28 b,c,d,e | 2.46 a,b | 1.82 a,b,c | |
| T50S50 | 90.9 b,c | 52.6 a,b | 29.2 b,c | 9.31 c,d | 3.89 a,b,c | 1.32 b,c,d,e | 2.51 a,b | 1.88 b,c,d | |
| T75S25 | 86.7 b,c | 53.6 a,b,c | 28.8 b,c | 9.17 c,d | 3.89 a,b,c | 1.17 a,b,c,d | 2.40 a,b | 1.78 a,b,c | |
| SEM | 2.41 | 0.380 | 0.402 | 0.139 | 0.075 | 0.048 | 0.076 | 0.036 | |
| p-value | |||||||||
| Diet | 0.133 | 0.016 | 0.334 | <0.001 | 0.999 | 0.003 | 0.013 | 0.429 | |
| Extract | 0.356 | 0.022 | <0.001 | <0.001 | 0.029 | 0.007 | <0.001 | <0.001 | |
| Diet × extract | 0.046 | 0.258 | 0.917 | 0.491 | 0.185 | 0.031 | 0.385 | 0.724 |
| Diet | Extract | Gas 24 h (mL/g DM) | Gas 48 h (mL/g DM) | CH4 24 h (mL/L gas) | CH4 48 h (mL/L gas) | IVDMD 48 h (mg/g) | IVOMD 48 h (mg/g) | Total VFA 48 h (mmol/L) | Ammonia 48 h (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| HF | T25S75 | 4.4 | 4.1 | −4.9 ** | −3.2 * | −5.6 | −1.7 | 7.8 | −8.2 *** |
| T50S50 | 3.9 | 3.0 | −2.0 | −1.9 | −4.6 | −6.9 | −21.1 * | −16.3 *** | |
| T75S25 | 3.7 * | 0.9 | 1.3 | 0.1 | −2.2 | 3.0 | 1.3 | −19.6 *** | |
| HC | T25S75 | −4.6 | −2.1 | −0.6 | −1.8 | 2.2 | 2.3 | 8.1 | −1.5 |
| T50S50 | 2.4 | 3.0 ** | 1.0 | −0.1 | −3.0 | −1.8 | 12.2 | −6.8 ** | |
| T75S25 | 2.1 | 2.0 | 1.3 | 0.4 | 0.9 | 0.2 | 11.7 | −13.5 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayanegara, A.; Yogianto, Y.; Wina, E.; Sudarman, A.; Kondo, M.; Obitsu, T.; Kreuzer, M. Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation. Animals 2020, 10, 1531. https://doi.org/10.3390/ani10091531
Jayanegara A, Yogianto Y, Wina E, Sudarman A, Kondo M, Obitsu T, Kreuzer M. Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation. Animals. 2020; 10(9):1531. https://doi.org/10.3390/ani10091531
Chicago/Turabian StyleJayanegara, Anuraga, Yogianto Yogianto, Elizabeth Wina, Asep Sudarman, Makoto Kondo, Taketo Obitsu, and Michael Kreuzer. 2020. "Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation" Animals 10, no. 9: 1531. https://doi.org/10.3390/ani10091531
APA StyleJayanegara, A., Yogianto, Y., Wina, E., Sudarman, A., Kondo, M., Obitsu, T., & Kreuzer, M. (2020). Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation. Animals, 10(9), 1531. https://doi.org/10.3390/ani10091531






